Abstract
Cocaine abuse hastens the neurodegeneration often associated with advanced HIV-1 infection. The mechanisms, in part, revolve around the neuroinflammatory processes mediated by the chemokine monocyte chemotactic protein-1 (MCP-1/CCL2). Understanding factors that modulate MCP-1 and, in turn, facilitate monocyte extravasation in the brain is thus of paramount importance. We now demonstrate that cocaine induces MCP-1 in rodent microglia through translocation of the sigma receptor to the lipid raft microdomains of the plasma membrane. Sequential activation of Src, mitogen-activated protein kinases (MAPKs), and phosphatidylinositol-3′ kinase (PI3K)/Akt and nuclear factor κB (NF-κB) pathways resulted in increased MCP-1 expression. Furthermore, conditioned media from cocaine-exposed microglia increased monocyte transmigration, and thus was blocked by antagonists for CCR2 or sigma receptor. These findings were corroborated by demonstrating increased monocyte transmigration in mice exposed to cocaine, which was attenuated by pretreatment of mice with the sigma receptor antagonist. Interestingly, cocaine-mediated transmigratory effects were not observed in CCR2 knockout mice. We conclude that cocaine-mediated induction of MCP-1 accelerates monocyte extravasation across the endothelium. Understanding the regulation of MCP-1 expression and functional changes by cocaine/sigma receptor system may provide insights into the development of potential therapeutic targets for HIV-1–associated neurocognitive disorders.
Introduction
HIV-1–associated neurocognitive disorders (HANDs) remain a common complication of viral infection despite the advent of antiretroviral therapies (ARTs). One contributing factor is the use of illicit drugs, including but not limited to cocaine. The mechanism by which cocaine augments HANDs has been the subject of intense research.1,2 One possibility rests in the idea that the drug can “open” the blood brain barrier (BBB), which, in turn, can facilitate transmigration of bloodborne inflammatory monocytes into the brain.3,4 Although considerable efforts have been made to best understand the cellular and molecular mechanisms underlying the effects of cocaine on proinflammatory factor secretion and BBB function,5 there exists a paucity of information on the mechanisms by which cocaine influences chemokine secretion and cell migration into and within the central nervous system (CNS).
Common neuropathologic correlates for HANDs include BBB disruption, glial activation, neuroinflammation (proinflammatory factors and chemokines), viral replication, and neuronal aberrations. The key factor mediating monocyte-macrophage transmigration across the BBB is the CC chemokine, monocyte chemoattractant protein-1 (MCP-1/CCL2),6 which mediates its effects by binding to its cognate receptor CCR2.7 Moreover, the best correlate for cognitive impairment remains the numbers of immune competent brain mononuclear phagocytes (MPs; bloodborne macrophages and microglia). Exploration of mechanisms that modulate MCP-1 in the brain is thus of paramount importance to best understand the disease processes.
Among the diverse cell types in the CNS, microglia, the resident brain microglia, play an important role in various neurodenegerative disorders and most notably, HANDs.8 By elaborating a plethora of cytokines and chemokines, microglia exhibit both protective as well a toxic phenotype during injury.8 More recently numbers of activated microglia are significantly increased among cocaine users.9 Several lines of evidence suggest a causal relationship between cocaine abuse and microglial activation that is often accompanied with cytokine production and disruption of host defense.10 The role of microglia in cocaine-induced brain inflammation, however, remains poorly defined. The present study was aimed at exploring the mechanism of cocaine-mediated induction of MCP-1 in brain microglia and its implication in the progression of neuroinflammation associated with HANDs. Understanding the regulation of MCP-1 expression and functional changes induced by cocaine in monocyte migration may provide insights into potential therapeutic targets for HANDs.
Methods
Animals
C57BL/6N mice were purchased from Charles River Laboratories Inc. CCR2 knockout (KO) mice (Taconic) have been backcrossed 10 generations to a C57BL/6N inbred background. All of animals were housed under conditions of constant temperature and humidity on a 12-hour light, 12-hour dark cycle, with lights on at 0700 hours. Food and water were available ad libitum. All animal procedures were performed according to the protocols approved by the Institutional Animal Care and Use Committee of the University of Nebraska Medical Center and the National Institute of Health.
Cell culture and reagents
The BV-2 immortalized cell line and rat primary microglia were cultured after the protocols as previously described.11 Primary human brain microvascular endothelial cells (HBMECs) were cultured in RPMI 1640 containing 10% heat-inactivated fetal bovine serum, 10% Nu-Serum, 2mM glutamine, 1mM pyruvate, penicillin (100 U/mL), streptomycin (100μg/mL), essential amino acids, and vitamins. All cell-culture dishes were coated with rat-tail collagen type I (R&D Systems). MCP-1–neutralizing antibody was purchased from eBioscience. The specific phosphatidylinositol-3′ kinase (PI3K) inhibitor LY294002, MEK1/2 inhibitor U0126, JNK inhibitor SP600125, p38 inhibitor SB 203580, and Src kinase inhibitors PP2 and PP3 were purchased from Calbiochem. CCR2 antagonist RS102895, sigma receptor antagonist BD1047, and lipid raft–disrupting agent methyl-β-cyclodextrin (MβCD) were purchased from Sigma-Aldrich. The concentrations of these inhibitors were based the concentration-curve study and our previous reports.12,13
Mouse cytokine antibody arrays
Levels of cytokines and chemokines were measured using mouse-specific TranSignal Cytokine Antibody Arrays (Ray Biotech Inc) according to the manufacturer's instructions.
MCP-1 protein analysis by ELISA
MCP-1 levels were examined using a MCP-1 enzyme-linked immunosorbent assay (ELISA) kit (R&D Systems). Samples were analyzed for MCP-1 protein in triplicate determined in 3 independent experiments.
Reverse transcription and real-time PCR
The quantitative polymerase chain reaction (qPCR) primers for mouse MCP-1 was obtained from SABiosciences. Total RNA was extracted with TRIzol reagent (Invitrogen) according to the manufacturer's instructions and our previous reports.14
Colocalization of σ-1R with lipid rafts
Lipid rafts in microglia were stained using the Vybrant Lipid raft labeling kit (Molecular Probes) according to the manufacturer's instructions, followed by immunocytochemistry for sigma-1 receptor (σ-1R) as described previously.13
siRNA transfection
BV-2 cells were transfected with short-interfering RNA (siRNA) σ-1R plasmid using the Novagen Ribojuice siRNA transfection reagent (EMD Chemicals Inc) according to the manufacturer's instructions. The knockdown efficiency of siRNAs was determined 2 days after transfection by Western blotting.
Measurement of oxidative stress
BV-2 cells with different treatments were trypsinized and centrifuged, and the resulting cell pellet was stained for 30 minutes with 15μM 5-(and -6)-carboxy-2′,7′-dichlorodihydroflourescein diacetate (carboxy-H2-DCF-DA; Molecular Probes Inc) to assess cytoplasmic reactive oxygen species (ROS).15
Transduction with adenovirus constructs
BV-2 cells were transduced with adenoviral constructs containing either the wild-type (WT) or dominant-negative (DN) forms of Akt. In addition, BV-2 cells were also transduced with recombinant adenovirus vectors coexpressing both green fluorescent protein (GFP) and full-length p65/RelA (RelA FL), or GFP and a transcriptionally inert p65/RelA mutant (RelA1-300) used at a multiplicity of infection (MOI) of 50 as previously described.16
Western blotting
Cocaine-treated BV-2 cells were lysed using the Mammalian Cell Lysis kit (Sigma-Aldrich) and the NE-PER Nuclear and Cytoplasmic Extraction kit (Pierce). Western blots were then probed with antibodies recognizing the phosphorylated forms of Src, ERK1/2, JNK, p38, and Akt (Cell Signaling; 1:200), IκBα, NF-κB p65 (Cell Signaling; 1:1000), and σ-1R as previously described.13
ChIP assay
Chromatin immunoprecipitation (ChIP) assay was performed according to the manufacturer's instructions (Millipore). The purified DNA was subjected to PCR to identify the promoter region containing the NF-κB binding site “GGG RNN YYC C.”17 The sequence of the primers used to identify the MCP-1 promoter bound by transcription factor NF-κB were as follows: sense, 5′-CATCTGGAGCTCACATTCCAGCTA-3′; antisense, 5′-GCG-CAAATGTGAATCATAAACGAA-3′.
Monocyte isolation and transmigration
Monocytes were obtained from HIV-1, HIV-2, and hepatitis B seronegative donor leukopacks, and separated by countercurrent centrifugal elutriation as previously described.18 Monocytes were washed with phosphate-buffered saline (PBS) and fluorescently labeled with 10μM Cell tracker green (Molecular Probes) for 10 minutes at room temperature. Labeled cells (2 × 105 cells) were added to the upper compartments of transwell inserts and allowed to transmigrate at 37°C in a humid atmosphere of 5% CO2 for 24 hours. Transmigrated monocytes were counted by 2 investigators “blinded” to the identity of the treatment group according to a previously published report.19
BMM isolation and cultivation
Both male C57 BL/6 mice (Charles River Laboratory) and CCR2 KO mice (4-5 weeks old) were used as bone marrow–derived monocyte (BMM) donors. Briefly, the femur was removed, and bone marrow cells were dissociated into single-cell suspensions and cultured for 5 days supplemented with 1000 U/mL macrophage colony stimulating factor (MCSF; a generous gift from Wyeth-Pfizer). Cultured BMMs were 98% CD11b+ as evidenced by flow cytometry. For tracking experiments, cells were labeled with the membrane dye PKH26 according to the manufacturer's instructions (Sigma-Aldrich).
In vivo monocyte transmigration assay
Assay of monocyte transmigration into the brain was performed in C57BL/6 mice. Animals were divided into 3 groups (n = 6). These groups were treated with (1) saline, (2) cocaine, or (3) BD1047 and cocaine. Cocaine and BD1047 each were injected intraperitoneally at a dose of 20 mg/kg once daily. In the BD1047 plus cocaine group of mice, BD1047 was first injected for 2 days, followed by cocaine injection for a subsequent 7 days. For CCR2 KO mice, cocaine was injected as described. On the eighth day, animals were injected with PKH 26–labeled BMM at a concentration of 107 cells/100 μL through the tail vein. At 24 hours after cell infusion, the animals were killed and subjected to transcardial perfusion with saline to remove labeled monocytes from tissue blood vessels. Brain tissues were then removed and frozen at −80°C until crysosectioning was carried out. Parenchymal regions were counted in the entire area of 3 coronal brain sections: 1.94 mm, 1.34 mm, and 0.14 mm to bregma according to a previous report using ImageJ 1.43n software.20
Immunofluorescence microscopy
Primary rat microglia seeded in coverlsips were fixed with 4% paraformaldehyde and incubated with Iba-1 antibody (Wako Chemicals) followed by Alexa Fluor 488 goat anti–mouse (Molecular Probes). Mounting medium (Prolong Gold Antifade Reagent; Invitrogen) was applied and coverslips were sealed with clear nail polish. Fluorescent images were acquired at room temperature on a Zeiss Observer. A Z1 inverted microscope was used (40×/0.3 oil objective [for Iba-1, lipid raft staining and for monocyte transmigration], 20×/0.4 objective [for ROS]); images were processed using AxioVs 40 Version 4.8.0.0 software (Carl Zeiss MicroImaging GmbH). Photographs were acquired using an AxioCam MRm digital camera.
Statistical analysis
Statistical analysis was performed using one-way analysis of variance with a post-hoc Student t test. Results were judged statistically significant if P values were less than .05 by analysis of variance.
Results
Cocaine induces MCP-1 mRNA and protein expression in microglia
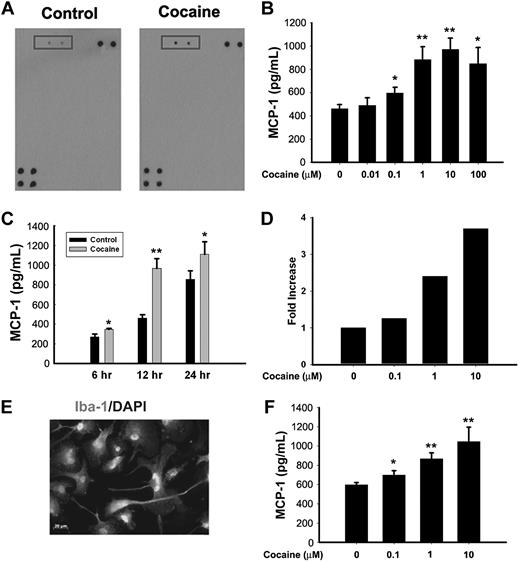
MCP-1 functions as a potent chemokine that is critical for progression of HANDs.6 In the present study, as an initial screening to identify cytokines/chemokines expressed in BV-2 cells exposed to cocaine, supernatant from cocaine-treated BV-2 cells was subject to mouse cytokine antibody array analysis. As shown in Figure 1A, cocaine treatment of BV-2 cells resulted in dramatic induction of MCP-1 expression compared with any of the other mediators examined. To further validate the expression of MCP-1 induced by cocaine, BV-2 cells were treated with various concentrations of cocaine (0.01, 0.1, 1, 10, and 100μM) for 12 hours and assessed for MCP-1 expression by ELISA. As shown in Figure 1B, in BV-2 cells there was a concentration-dependent increase in MCP-1 expression after cocaine treatment, with the maximal expression observed at 10μM cocaine. This concentration of cocaine was therefore chosen for all our further studies. The next step was to examine the time course of MCP-1 induction. Cocaine-mediated induction of MCP-1 was observed as early as 6 hours after treatment and was significantly up-regulated even at 24 hours after treatment in BV-2 cells (Figure 1C). To examine whether cocaine-mediated induction of MCP-1 was also evident at the transcriptional level, cocaine-treated BV-2 cells were assessed for MCP-1 mRNA. As shown in Figure 1D, there was also a concomitant increase in MCP-1 mRNA in the presence of cocaine. These findings were also reproducible in rat primary microglial cells (Figure 1E-F).
Cocaine induces MCP-1 mRNA and protein expression in microglia. (A) Supernatant fluids from cocaine-treated cells were assessed for release of cytokine/chemokines using the mouse cytokine antibody array. Cocaine treatment resulted in induction of MCP-1 expression (rectangles). (B) Concentration curve of cocaine-mediated induction of MCP-1 expression in BV-2 cells. Cells were incubated with various concentrations of cocaine (0.01, 0.1, 1, 10, and 100μM) for 12 hours, followed by collection of media for assay of MCP-1 expression by ELISA. (C) Time dependence of cocaine-mediated induction of MCP-1 expression in BV-2 cells. (D) Cocaine-mediated induction of MCP-1 mRNA expression by real time RT-PCR. (E) Representative picture of Iba-1 (microglia marker) staining in rat primary microglia. (F) Concentration curve of cocaine-mediated (0.1, 1, and 10μM) induction of MCP-1 expression in rat primary cultured microglia. All the data are presented as means ± SD of 4 individual experiments. *P < .05, **P < .01 versus control group.
Cocaine induces MCP-1 mRNA and protein expression in microglia. (A) Supernatant fluids from cocaine-treated cells were assessed for release of cytokine/chemokines using the mouse cytokine antibody array. Cocaine treatment resulted in induction of MCP-1 expression (rectangles). (B) Concentration curve of cocaine-mediated induction of MCP-1 expression in BV-2 cells. Cells were incubated with various concentrations of cocaine (0.01, 0.1, 1, 10, and 100μM) for 12 hours, followed by collection of media for assay of MCP-1 expression by ELISA. (C) Time dependence of cocaine-mediated induction of MCP-1 expression in BV-2 cells. (D) Cocaine-mediated induction of MCP-1 mRNA expression by real time RT-PCR. (E) Representative picture of Iba-1 (microglia marker) staining in rat primary microglia. (F) Concentration curve of cocaine-mediated (0.1, 1, and 10μM) induction of MCP-1 expression in rat primary cultured microglia. All the data are presented as means ± SD of 4 individual experiments. *P < .05, **P < .01 versus control group.
Engagement of σ-1R is critical for cocaine-induced MCP-1 expression in microglial cells
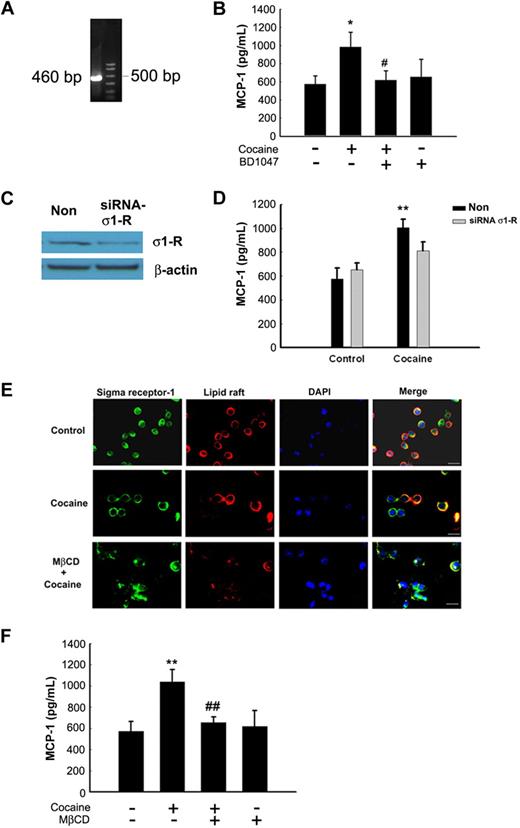
Although originally proposed to belong to a subtype of opioid receptors, σ-1Rs are now confirmed to be nonopioid receptors that bind to diverse classes of psychotropic drugs, including cocaine.21 Because cocaine mediated up-regulation of MCP-1, we next sought to examine whether this process involved the engagement of σ-1R in BV-2 cells. First, we wanted to assess whether BV-2 microglial cells did indeed express σ-1R. These cells expressed both the σ-1R RNA as demonstrated by RT-PCR analysis (Figure 2A) and protein as shown by Western blot analysis (Figure 2C). The next step was to assess the role of σ-1R in cocaine-mediated induction of MCP-1. As shown in Figure 2B, cocaine-mediated up-regulation of MCP-1 expression was significantly attenuated by pretreatment of BV-2 cells with the σ-1R antagonist BD1047 (10μM; Figure 2B). To further validate the involvement of σ-1R in cocaine-induced regulation of MCP-1 expression, we used the knockdown approach by transfecting BV-2 cells with σ-1R siRNA. As shown in Figure 2C, transfection of BV-2 cells with σ-1R siRNA resulted in knock-down of σ-1R expression. In addition, σ-1R siRNA also significantly abrogated cocaine-mediated induction of MCP-1 expression (Figure 2D). These findings thus underscored the role of σ-1R in cocaine-induced expression of MCP-1 in BV-2 cells.
Engagement of σ-1R is critical for cocaine-induced MCP-1 expression in microglia. (A) RNA isolated from cocaine-treated BV-2 cells was subject to RT-PCR analysis using σ-1R primers. (B) Pretreatment of BV-2 cells with σ-1R antagonist BD1047 abolished cocaine-mediated induction of MCP-1 expression in BV-2 cells. (C) Whole-cell lysates from BV-2 cells transfected with either σ-1R or nonsense (Non) siRNAs were subject to Western blot analysis using antibodies specific for σ-1R. (D) σ-1R siRNA, but not Non siRNA, inhibited cocaine-mediated induction of MCP-1 expression. (E) BV-2 cells were treated with cocaine and double-stained using antibodies specific for ganglioside GM1-lipid raft marker (red TRITC fluorescence) or σ-1R (green FITC fluorescence). Overlay images are shown in the right panel. Data are representative from 3 typical experiments. Scale bars all indicate 20 μm. (F) Role of lipid rafts play in cocaine-mediated induction of MCP-1 in BV-2 cells. Cells were pretreated with MβCD (1mM) followed by treatment with cocaine. Supernatant fluids were harvested at 12 hours after cocaine treatment, followed by assessment of MCP-1 expression by ELISA. All the data are presented as means ± SD of 4 individual experiments. *P < .05; **P < .01 versus control group; #P < .05; ##P < .01 versus cocaine-treated group.
Engagement of σ-1R is critical for cocaine-induced MCP-1 expression in microglia. (A) RNA isolated from cocaine-treated BV-2 cells was subject to RT-PCR analysis using σ-1R primers. (B) Pretreatment of BV-2 cells with σ-1R antagonist BD1047 abolished cocaine-mediated induction of MCP-1 expression in BV-2 cells. (C) Whole-cell lysates from BV-2 cells transfected with either σ-1R or nonsense (Non) siRNAs were subject to Western blot analysis using antibodies specific for σ-1R. (D) σ-1R siRNA, but not Non siRNA, inhibited cocaine-mediated induction of MCP-1 expression. (E) BV-2 cells were treated with cocaine and double-stained using antibodies specific for ganglioside GM1-lipid raft marker (red TRITC fluorescence) or σ-1R (green FITC fluorescence). Overlay images are shown in the right panel. Data are representative from 3 typical experiments. Scale bars all indicate 20 μm. (F) Role of lipid rafts play in cocaine-mediated induction of MCP-1 in BV-2 cells. Cells were pretreated with MβCD (1mM) followed by treatment with cocaine. Supernatant fluids were harvested at 12 hours after cocaine treatment, followed by assessment of MCP-1 expression by ELISA. All the data are presented as means ± SD of 4 individual experiments. *P < .05; **P < .01 versus control group; #P < .05; ##P < .01 versus cocaine-treated group.
Having determined the role of σ-1R in cocaine-mediated induction of MCP-1, we next asked whether activation of σ-1R with cocaine involved translocation of these receptors to the lipid raft microdomains because σ-1R can translocate into the lipid domains of plasma membrane.22 Cocaine-treated BV-2 cells were examined for colocalization of σ-1R and lipid raft using antibody specific for σ-1R and lipid raft marker (GM-1). There was a diffuse pattern of σ-1R expression in the cytoplasm of untreated BV-2 cells, which did not colocalize with the lipid raft microdomains (Figure 2E top panels). Treatment with cocaine, on the other hand, induced clustering and polarization of σ-1R with the lipid raft domains resulting in colocalization of the 2 (Figure 2E; middle panels). Further validation of this phenomenon was carried out by pretreating BV-2 cells with MβCD, a cholesterol-depleting agent, which resulted in abrogation of cocaine-induced translocation of σ-1R (Figure 2E bottom panels). These results thus implicate that σ-1R translocation and lipid raft organization are mutually dependent events. Interestingly, pretreatment of BV-2 cells with MβCD (1mM) markedly reduced cocaine-mediated induction of MCP-1 expression compared with control cells (Figure 2F). These results thus underpin the critical role of lipid raft organization, perhaps by acting as a platform for recruitment and/or association of signaling partners, in cocaine-induced downstream expression of MCP-1 in BV-2 cells.
Involvement of ROS and Src kinase in cocaine-mediated MCP-1 expression
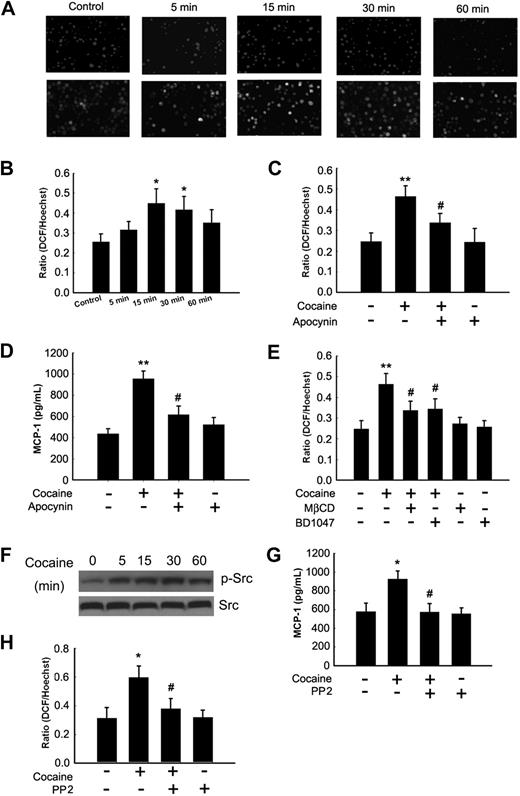
Oxidative stress has been demonstrated to affect signaling pathways and cytokine and chemokine production. We thus sought to determine whether cocaine-mediated release of MCP-1 in BV-2 cells involved generation of ROS. BV-2 cells were exposed to cocaine for more than a period of 60 minutes before staining with H2DCF-DA, followed by assessment of ROS generation. As shown in Figure 3A, cocaine induced a time-dependent increase in the formation of ROS with a peak at 15 minutes after treatment (Figure 3A-B). One of the mechanisms underlying ROS production is through a respiratory burst orchestrated by the activation of NADPH oxidase.15 Based on the recent findings linking NADPH oxidase activity to cytokine/chemokine production in microglia, macrophages, and astrocytes,23,24 we sought to delineate the role of NADPH oxidase in the induction of MCP-1. BV-2 cells were pretreated for 1 hour with the NADPH oxidase inhibitor apocynin (250μM), followed by stimulation with cocaine and subsequent ROS assay. Interestingly, pretreatment of cells with apocynin abrogated cocaine-mediated induction of ROS (Figure 3C) and, consequently, MCP-1 expression (Figure 3D). Consistent with the role of BD1047 in abrogation of MCP-1 expression, ROS production induced by cocaine was also significantly attenuated by pretreatment of BV-2 cells with the σ-1R antagonist (Figure 3E). It has been shown by Zhang et al that lipid raft clustering on the plasma membrane is critical for redox transmembrane signaling mediated by NADPH oxidase.25 To validate the role of lipid rafts as platforms for ROS production, BV-2 cells were pretreated with MβCD before cocaine exposure. This resulted in significant abrogation of cocaine-mediated production of ROS (Figure 3E).
Cocaine-mediated induction of MCP-1 involves generation of ROS and Src kinase activation. (A) Cocaine-induced ROS generation in a time-dependent manner measured by laser-scanning microscopy. (B) BV-2 cells were treated with 10μM cocaine for the indicated time points (0-60 minutes) before incubation with carboxy-H2-DCF-DA and assessed for oxidative stress. Values are displayed as a ratio of the DCF fluorescent value to the Hoechst (nuclear stain) fluorescent value. A respiratory burst culminates after 15 minutes of stimulation. (C) BV-2 cells pretreated with apocynin (250μM) followed by stimulation with the cocaine for 30 minutes. Apocynin pretreatment resulted in abrogation of cocaine-induced respiratory burst. (D) Inhibition of NADPH oxidase by apocynin resulted in abrogation of cocaine-mediated induction of MCP-1. (E) Pretreatment of BV-2 cells with BD1047 and MβCD abrogated cocaine-induced ROS production. (F) Cocaine-induced Src phosphorylation in BV-2 cells. (G) Pretreatment with PP2 abrogated cocaine-induced ROS production. (H) Inhibition of the Src activity by Src inhibitor PP2 resulted in amelioration of cocaine-mediated induction of MCP-1. All the data are presented as means ± SD of 4 individual experiments. *P < .05; **P < .01 versus control group; #P < .05 versus cocaine-treated group.
Cocaine-mediated induction of MCP-1 involves generation of ROS and Src kinase activation. (A) Cocaine-induced ROS generation in a time-dependent manner measured by laser-scanning microscopy. (B) BV-2 cells were treated with 10μM cocaine for the indicated time points (0-60 minutes) before incubation with carboxy-H2-DCF-DA and assessed for oxidative stress. Values are displayed as a ratio of the DCF fluorescent value to the Hoechst (nuclear stain) fluorescent value. A respiratory burst culminates after 15 minutes of stimulation. (C) BV-2 cells pretreated with apocynin (250μM) followed by stimulation with the cocaine for 30 minutes. Apocynin pretreatment resulted in abrogation of cocaine-induced respiratory burst. (D) Inhibition of NADPH oxidase by apocynin resulted in abrogation of cocaine-mediated induction of MCP-1. (E) Pretreatment of BV-2 cells with BD1047 and MβCD abrogated cocaine-induced ROS production. (F) Cocaine-induced Src phosphorylation in BV-2 cells. (G) Pretreatment with PP2 abrogated cocaine-induced ROS production. (H) Inhibition of the Src activity by Src inhibitor PP2 resulted in amelioration of cocaine-mediated induction of MCP-1. All the data are presented as means ± SD of 4 individual experiments. *P < .05; **P < .01 versus control group; #P < .05 versus cocaine-treated group.
Src protein tyrosine kinase is known to be required for cocaine-mediated effects, particularly for the increased expression of the NMDA receptor.26 Specifically, Check et al have demonstrated participation of Src kinase in lipopolysaccharide-induced NADPH oxidase activation.27 We thus sought to examine whether activation of Src played a role in cocaine-mediated activation of NADPH oxidase and for the subsequent induction of MCP-1. As shown in Figure 3F, treatment of BV-2 cells with cocaine resulted in increased phosphorylation of Src. To further ascertain that Src was involved in cocaine-mediated induction of ROS and MCP-1 expression, BV-2 cells were pretreated with the Src tyrosine kinase inhibitor PP2 followed by treatment with cocaine for 24 hours and then assessed for ROS production and MCP-1 expression. Treatment of cells with PP2, but not with PP3, an inactive analog of PP2 (data not shown), significantly inhibited cocaine-induced production of both ROS (Figure 3G) and MCP-1 (Figure 3H).
Cocaine-induced expression of MCP-1 involves ERK1/2, JNK, MAPK, and PI3K/Akt pathways
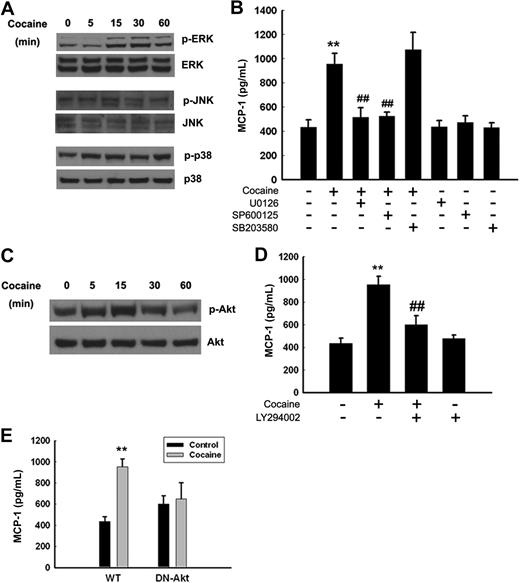
Several studies have implicated the role of MAPKs and PI3K/Akt and PI3K/Akt-dependent pathways in the induction MCP-1.28,29 We therefore sought to examine the involvement of MAPKs and PI3K/Akt pathways in cocaine-mediated induction of MCP-1 expression. Treatment of BV-2 cells with cocaine resulted in a time-dependent increase in phosphorylation of ERK, JNK (Figure 4A), and Akt (Figure 4C), with maximal activation at 15 minutes after treatment. Cocaine exposure also induced a transient and biphasic activation of p38 kinase. The next step was to assess the specificity of MAPKs and PI3K/Akt pathways in cocaine-mediated enhancement of MCP-1 expression. This was determined using a pharmacologic approach. BV-2 cells were pretreated with inhibitors specific for the respective signaling pathways before stimulation with cocaine. As shown in Figure 4B and D, pretreatment with JNK (SP600125; 20μM), MEK1/2 (U0126; 20μM), and PI3K (LY294002; 10μM), but not p38 (SB203580; 20μM) inhibitors, resulted in the amelioration of cocaine-mediated induction of MCP-1 release. Further validation of the involvement of Akt pathway in this process was confirmed by transfecting cells with either the WT or DN constructs of Akt, followed by treatment with cocaine. Cocaine-mediated induction of MCP-1 was attenuated by the DN-Akt construct, but not by the WT-Akt construct. Taken together, these findings underpin the involvement of MAPKs and PI3K/Akt cascade in cocaine-mediated induction of MCP-1 in BV-2 cells.
Cocaine-mediated induction of MCP-1 expression involves MAPKs and PI3K/Akt cell-signaling pathways. (A) Western blot analysis of time-dependent activation of ERK, JNK, and p38 by cocaine. (B) Inhibition of the ERK and JNK pathways by MEK1/2 (U0126) and JNK inhibitor (SP600125) resulted in amelioration of cocaine-mediated induction of MCP-1. (C) Time-dependent activation of Akt in cocaine-treated BV-2 cells. (D) Pretreatment with PI3K inhibitor (LY294002) resulted in inhibition of cocaine-mediated induction of MCP-1 expression. (E) Transduction with DN-Akt and not WT-Akt resulted in abrogation of cocaine-mediated induction of MCP-1. All the data are presented as means ± SD of 4 individual experiments. **P < .01 versus control group; ##P < .01 versus cocaine-treated group.
Cocaine-mediated induction of MCP-1 expression involves MAPKs and PI3K/Akt cell-signaling pathways. (A) Western blot analysis of time-dependent activation of ERK, JNK, and p38 by cocaine. (B) Inhibition of the ERK and JNK pathways by MEK1/2 (U0126) and JNK inhibitor (SP600125) resulted in amelioration of cocaine-mediated induction of MCP-1. (C) Time-dependent activation of Akt in cocaine-treated BV-2 cells. (D) Pretreatment with PI3K inhibitor (LY294002) resulted in inhibition of cocaine-mediated induction of MCP-1 expression. (E) Transduction with DN-Akt and not WT-Akt resulted in abrogation of cocaine-mediated induction of MCP-1. All the data are presented as means ± SD of 4 individual experiments. **P < .01 versus control group; ##P < .01 versus cocaine-treated group.
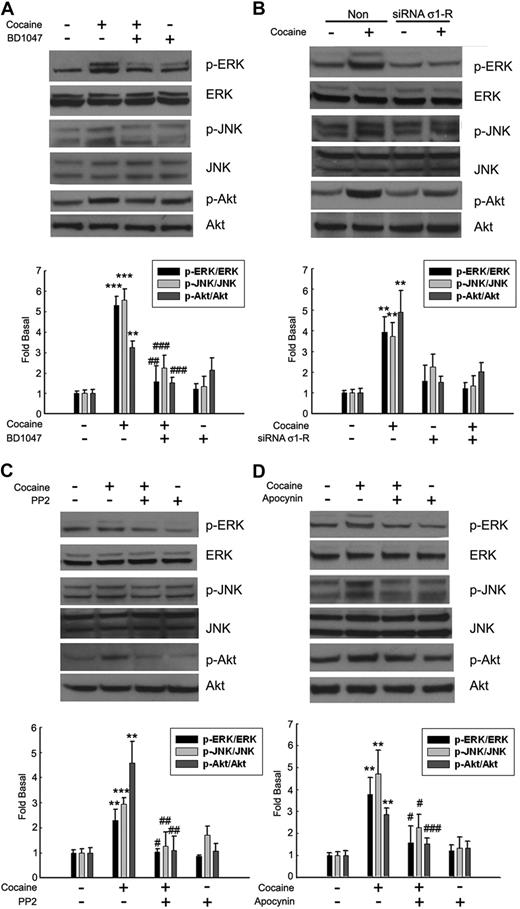
Having determined that cocaine-mediated translocation/activation of the σ-1R to the lipid raft microdomains and its activation of the MAPKs and PI3K/Akt pathways as 2 critical processes involved in the induction of MCP-1, we next sought to link σ-1R activation with the signal transduction pathways. BV-2 cells pretreated with either the σ-1R antagonist or σ-1R siRNA were treated with cocaine, followed by assessment for activation of the MAPK/Akt pathway. Cocaine-mediated activation of both MAPKs (ERK1/2, JNK) and Akt pathways was inhibited by σ-1R antagonist (Figure 5A) and σ-1R siRNA (Figure 5B). Similarly, there was also a link between cocaine-mediated activation of Src and NADPH oxidase with the downstream signaling pathways, as evidenced by the fact that cocaine-mediated activation of both MAPK (ERK1/2, JNK) and Akt proteins was inhibited in cells pretreated with inhibitors specific for either Src kinase (Figure 5C) or NADPH oxidase (Figure 5D).
Involvement of σ-1R, Src kinase, and NADPH oxidase in the regulation of MAPKs and PI3K/Akt cell-signaling pathways. Pretreatment of BV-2 cells with σ-1R antagonist-BD1047 (A), σ-1R siRNA (B), Src inhibitor PP2 (C), or NADPH inhibitor apocynin (D) resulted in inhibition of cocaine-mediated phosphorylation of ERK, JNK, and Akt pathways. Representative immunoblots and the densitometric analyses of pERK/ERK, pJNK/JNK, and p-Akt/Akt from 4 separate experiments are presented. All the data are indicated as means ± SD of 4 individual experiments. **P < .01; ***P < .001 versus control group; #P < .05; ##P < .01; ###P < .001 versus cocaine-treated group.
Involvement of σ-1R, Src kinase, and NADPH oxidase in the regulation of MAPKs and PI3K/Akt cell-signaling pathways. Pretreatment of BV-2 cells with σ-1R antagonist-BD1047 (A), σ-1R siRNA (B), Src inhibitor PP2 (C), or NADPH inhibitor apocynin (D) resulted in inhibition of cocaine-mediated phosphorylation of ERK, JNK, and Akt pathways. Representative immunoblots and the densitometric analyses of pERK/ERK, pJNK/JNK, and p-Akt/Akt from 4 separate experiments are presented. All the data are indicated as means ± SD of 4 individual experiments. **P < .01; ***P < .001 versus control group; #P < .05; ##P < .01; ###P < .001 versus cocaine-treated group.
Cocaine-mediated induction of MCP-1 involves NF-κB activation
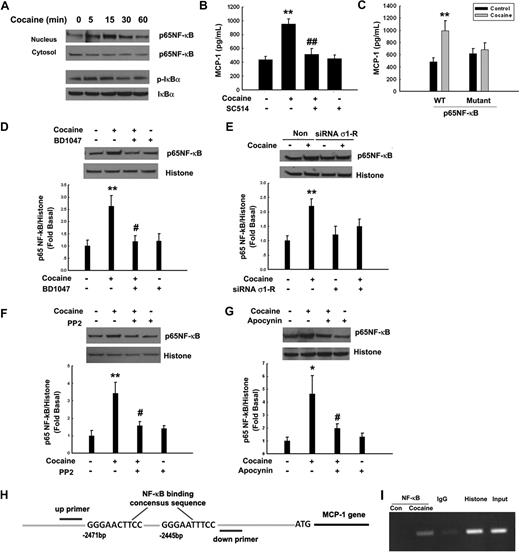
Members of the NF-κB family are considered essential regulators of cellular activities associated with inflammation/chemokine production.30 Thus, we next sought to examine whether cocaine-mediated induction of MCP-1 expression involved activation of NF-κB in BV-2 cells. Cocaine exposure resulted in translocation of NF-κB p65 into the nucleus with a maximal response within 15 minutes (Figure 6A). Normally, NF-κB activation and its nuclear translocation are prevented by inhibitory IκB proteins, which in turn are regulated by their phosphorylation status. Treatment of BV-2 cells with cocaine resulted in increased p65 NF-κB activation, with a concomitant increase in cytosolic IκB phosphorylation (Figure 6A). To further confirm the role of NF-κB p65 in cocaine-induced MCP-1 expression, BV-2 cells were either pretreated with IκB kinase-2 inhibitor-SC514 or were transduced with an adenovirus construct of NF-κB p65. As shown in Figure 6B-C, pretreatment of cells with SC514 (1μM) or a mutant adenovirus construct of NF-κB resulted in amelioration of cocaine-mediated induction of MCP-1, thus underpinning the involvement of NF-κB p65 in this process.
Cocaine-mediated induction of MCP-1 expression involves NF-κB activation. (A) Exposure of BV-2 cells to cocaine resulted in time-dependent increase in phosphorylation of the p65 subunit of NF-κB in the nuclear fraction, with a concomitant decrease in the cytosolic fraction. Reciprocally, cocaine exposure resulted in increased phosphorylation of IκBα in the cytosolic fraction of BV-2 cells. (B) Pretreatment with the IκBα inhibitor SC514 resulted in inhibition of cocaine-mediated induction of MCP-1. (C) Overexpression of the mutant but not the full-length p65/RelA NF-κB construct resulted in abrogation of cocaine-mediated induction of MCP-1. BV-2 cells exposed to cocaine in the presence or absence of σ-1R antagonist BD1047 (D), σ-1R siRNA (E), Src inhibitor PP2 (F), or NADPH inhibitor apocynin (G) were examined for cocaine-mediated translocation of NF-κB. Representative immunoblots and the densitometric analysis of p-P65 NF-κB/histone from 4 separate experiments are presented. All the data are means ± SD of 4 individual experiments. *P < .05; **P < .01 versus control group; #P < .05; ##P < .01 versus cocaine-treated group. (H) Schematic illustration of NF-κB binding consensus sequence on the MCP-1 promoter region. (I) ChIP assay demonstrating cocaine-mediated binding of p65NF-κB to the MCP-1 promoter. The image is representative of 3 independent experiments.
Cocaine-mediated induction of MCP-1 expression involves NF-κB activation. (A) Exposure of BV-2 cells to cocaine resulted in time-dependent increase in phosphorylation of the p65 subunit of NF-κB in the nuclear fraction, with a concomitant decrease in the cytosolic fraction. Reciprocally, cocaine exposure resulted in increased phosphorylation of IκBα in the cytosolic fraction of BV-2 cells. (B) Pretreatment with the IκBα inhibitor SC514 resulted in inhibition of cocaine-mediated induction of MCP-1. (C) Overexpression of the mutant but not the full-length p65/RelA NF-κB construct resulted in abrogation of cocaine-mediated induction of MCP-1. BV-2 cells exposed to cocaine in the presence or absence of σ-1R antagonist BD1047 (D), σ-1R siRNA (E), Src inhibitor PP2 (F), or NADPH inhibitor apocynin (G) were examined for cocaine-mediated translocation of NF-κB. Representative immunoblots and the densitometric analysis of p-P65 NF-κB/histone from 4 separate experiments are presented. All the data are means ± SD of 4 individual experiments. *P < .05; **P < .01 versus control group; #P < .05; ##P < .01 versus cocaine-treated group. (H) Schematic illustration of NF-κB binding consensus sequence on the MCP-1 promoter region. (I) ChIP assay demonstrating cocaine-mediated binding of p65NF-κB to the MCP-1 promoter. The image is representative of 3 independent experiments.
The next logical step was to examine whether there existed a link that could tie together the activation of σ-1R, Src, NADPH oxidase, and NF-κB. Similar to our studies on signaling molecules (Figure 5), BV-2 cells were pretreated with σ-1R antagonist, σ-1R siRNA, Src inhibitor PP2, or the NADPH oxidase inhibitor apocynin, followed by treatment with cocaine. As shown in Figure 6, σ-1R antagonist (Figure 6D), σ-1R siRNA (Figure 6E), Src inhibitor (Figure 6F), and NADPH oxidase inhibitor (Figure 6G) were all able to inhibit cocaine-mediated activation of NF-κB. These findings thus linked cocaine-mediated activation of σ-1R, Src activation, and ROS generation to downstream activation of NF-κB.
To further confirm the involvement of NF-κB in MCP-1 expression, we sought to explore the binding of NF-κB to the MCP-1 promoter in its natural chromatin context to reveal active accessible NF-κB sites. In vivo MCP-1 promoter occupancy was investigated by ChIP assays. These experiments revealed increased binding of NF-κB to the MCP-1 promoter in BV-2 cells (Figure 6H-I) treated with cocaine.
Cocaine-mediated induction of MCP-1 in microglia enhances monocyte transmigration in an in vitro BBB model
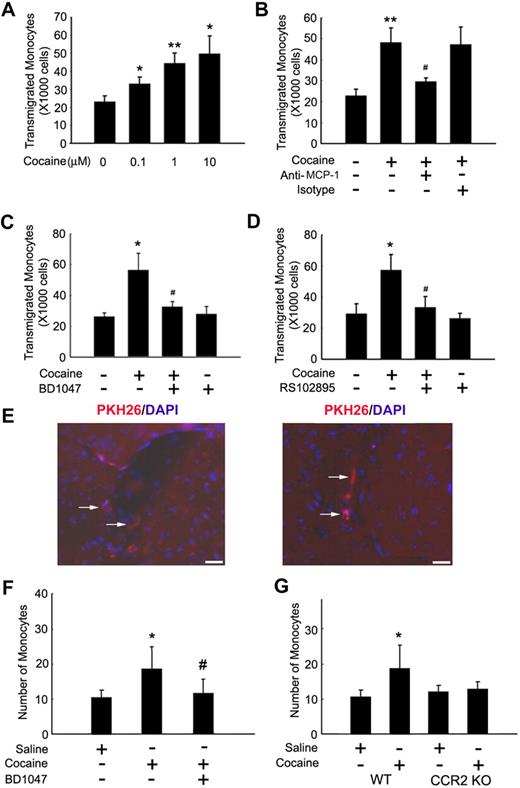
To identify the functional relevance of up-regulation of MCP-1, we next set out to examine the ability of cocaine-stimulated microglial conditioned media (CM) to induce monocyte transmigration in an in vitro model of BBB. As shown in Figure 7A, CM from cocaine-treated BV-2 cells induced monocyte transmigration in a concentration-dependent manner. Furthermore, to confirm the role of MCP-1 in this process, monoctye transmigration was evaluated in the presence of either MCP-1 or isotype control antibodies. MCP-1 antibody significantly restricted monocyte migration compared with the isotype antibody control (Figure 7B), suggesting thereby that MCP-1 was critical for the transmigration of monocytes across the BBB. Having determined the role of σ-1R in cocaine-induced MCP-1 expression in BV-2 cells, we further tested whether CM from BV-2 cells pretreated with σ-1R antagonist BD1047 could inhibit monocyte transmigration. As expected, CM collected from cells treated with the σ-1R antagonist resulted in inhibition of monocyte transmigration (Figure 7C). Because MCP-1 signals through its cognate receptor CCR2, which is expressed on monocytes, we next set out to examine whether pretreatment of monocytes with a CCR2 antagonist, RS-102895, could abrogate monocyte transmigration. As shown in Figure 7D, pretreatment of monocytes with RS-102895 (20μM) significantly inhibited MCP-1–induced monocyte transmigration. These results suggested that MCP-1–mediated monocyte migration also involved complementary expression of CCR2 on the monocytes.
Cocaine-mediated induction of MCP-1 enhances monocyte transmigration both in vitro and in vivo. (A) Concentration-dependent transmigration of monocytes in the presence of CM from cocaine-treated BV-2 cells. (B) Increased monoctye transmigration in the presence of CM from cocaine-treated cells was ameliorated by the MCP-1–blocking antibody (1 μg/mL). (C) Pretreatment with BD1047 ameliorated cocaine-mediated increase in monoctye transmigration. (D) Pretreatment of monocytes with CCR2 antagonist RS102895 ameliorated cocaine-mediated increase in monoctye transmigration. All the data are presented as means ± SD of 4 individual experiments. *P < .05; **P < .01 versus control group; #P < .05 versus cocaine-treated group. (E) Detection of PKH26-labeled monocytes in the brains of mice treated with cocaine. Fluorescence micrographs show monocytes in the perivascular cuff (left panel; arrows) and the parenchymal (right panel; arrows) areas of the brain. (F) Increased monoctye transmigration observed in the cocaine-treated group was ameliorated by pretreatment of mice with BD1047. (G) Increased monocyte transmigration in the cocaine-treated WT but not CCR2 KO mice. *P < .05 versus saline group; #P < .05 versus cocaine group counted from the parenchyma. (F-G) Data are expressed as the mean number of PKH26-labeled cells in the entire area of 3 coronal brain sections ± SD (n = 6 per group).
Cocaine-mediated induction of MCP-1 enhances monocyte transmigration both in vitro and in vivo. (A) Concentration-dependent transmigration of monocytes in the presence of CM from cocaine-treated BV-2 cells. (B) Increased monoctye transmigration in the presence of CM from cocaine-treated cells was ameliorated by the MCP-1–blocking antibody (1 μg/mL). (C) Pretreatment with BD1047 ameliorated cocaine-mediated increase in monoctye transmigration. (D) Pretreatment of monocytes with CCR2 antagonist RS102895 ameliorated cocaine-mediated increase in monoctye transmigration. All the data are presented as means ± SD of 4 individual experiments. *P < .05; **P < .01 versus control group; #P < .05 versus cocaine-treated group. (E) Detection of PKH26-labeled monocytes in the brains of mice treated with cocaine. Fluorescence micrographs show monocytes in the perivascular cuff (left panel; arrows) and the parenchymal (right panel; arrows) areas of the brain. (F) Increased monoctye transmigration observed in the cocaine-treated group was ameliorated by pretreatment of mice with BD1047. (G) Increased monocyte transmigration in the cocaine-treated WT but not CCR2 KO mice. *P < .05 versus saline group; #P < .05 versus cocaine group counted from the parenchyma. (F-G) Data are expressed as the mean number of PKH26-labeled cells in the entire area of 3 coronal brain sections ± SD (n = 6 per group).
In vivo monocyte transmigration
To validate the role of cocaine/σ-1R axis in MCP-1–mediated monocyte transmigration, PKH26-labeled bone marrow–derived mouse monocytes were transplanted by intravenous injection into mice that were either untreated or treated with cocaine. Of particular note, PKH26-labeled monocyte distribution was observed within the perivascular (Figure 7E left panel) and the parenchymal (Figure 7E right panel) regions of the brain. Histologic examination of mouse brain sections revealed increased transmigration of PKH26-labeled monocytes in the perivascular cuffs and in the parenchymal regions of cocaine-treated mice compared with mice not injected with cocaine. This effect was ameliorated by pretreatment of mice with the σ-1R antagonist (Figure 7F). These findings were also corroborated by a genetic approach using the CCR2 KO mice. Unlike the WT mice that demonstrated enhanced monocyte transmigration in response to cocaine compared with the saline controls (Figure 7E), CCR2 KO mice exposed to cocaine failed to show any difference in monocyte transmigration compared with the saline-injected animals (Figure 7G).
Discussion
Although antiviral therapies have had a profound impact on controlling systemic HIV-1 load, thus leading to increased longevity in patients with AIDS, the inability of some of these drugs to cross the BBB actually results in a slow and smoldering infection/cell activation in the CNS. The brain thus becomes a sanctuary for virus-induced toxicity, leading to increased prevalence of HANDs as these patients continue to live longer. Adding fuel to this problem is the increased use of illicit drug abuse that is also a burning issue in patients with HIV-1. Intriguingly, increased use of the psychostimulant cocaine in patients with HIV-1 has been linked to increased HIV seroprevalence and disease progression.2 In cell culture studies, cocaine has been shown to compromise the BBB, thereby increasing emigration of infected/activated monocytes from blood into the brain.31 Cocaine has also been shown to impair the BBB by modulating transcriptional regulation of key cellular functional genes of HBMECs.4 Although many studies are extant on the identification of cellular and molecular mechanisms underlying the effects of cocaine on BBB structure and function,4,32 very few studies have actually explored direct effects of cocaine on the microglia. More recently, numbers of both activated macrophages as well as activated microglia have been shown to be significantly increased among cocaine users.9
Taking into account cocaine-mediated toxicity of the cerebrovascular unit, cocaine has been very aptly implicated as a cofactor in neuroAIDS.2,33 One of the hallmark features of HAND is increased bloodborne macrophage and lymphocyte infiltration of inflammatory cells into the CNS, mediated primarily by chemokines, such as MCP-1. In the present study, we demonstrated that exposure of cocaine to rat microglial cells resulted in induction of MCP-1 at both the transcriptional and translational levels.
Cocaine is a psychostimulant that is known to exhibit moderate affinity for σ-1R expressed in most neuronal cells.34 Intriguingly, systemic exposure to cocaine is known to enhance HIV-1 infection in vivo by activating σ-1R and, subsequently, by modulating the expression of HIV coreceptors.35 Consistent with this finding, cocaine-mediated induction of MCP-1 involved binding to σ-1R, as evidenced by abrogation of cocaine-induced effect in the presence of σ-1R antagonist, BD1047. σ-1Rs that are located in the lipid raft microdomains of the endoplasmic reticulum are known to translocate into the plasma membrane after activation.21,36 In the present findings, we demonstrate cytoplasmic localization of σ-1R in untreated microglia, which is consistent with the previous report that σ-1Rs are expressed in the rat microglia.37,38 However, after treatment with cocaine, colocalization of σ-1R with the lipid raft domains of the plasma membrane occurred, thereby implicating their translocation. This translocation and subsequent MCP-1 production by these cells was abrogated in the presence of lipid raft–disrupting agent. These findings thus underpin the importance of lipid rafts in modulation of inflammatory responses. Similar reports implicating the role of lipid raft formation in ganglioside-stimulated NO production lend credence to our findings.39
Accumulating evidence indicates that membrane lipid rafts and lipid platforms may represent important mechanisms by which redox signals are generated and transmitted in response to various agonists or stimuli.25,40 Our findings clearly demonstrated time-dependent induction by cocaine of NADPH oxidase-generated ROS, which was critical for the induction of MCP-1. Interestingly, cocaine-induced ROS generation was inhibited not only by σ-1R antagonist, but also by the lipid raft disrupter as well as NADPH oxidase inhibitor. These novel findings thus underscore the role of σ-1R/lipid rafts in NADPH-mediated ROS generation. In light of these findings, it can be speculated that lipid rafts serve as “platforms or hubs” for coalescing key signaling molecules triggered by the σ-1R. Along these lines is the recent report by Shin et al emphasizing the role of lipid raft–associated ROS generation downstream of TLR2 in the induction of innate immune responses.41
In our efforts to dissect cocaine-mediated downstream signaling events, we demonstrate participation of Src kinase in this process, a finding that is consistent with the reports showing involvement of Src in both STAT3-mediated expression of MCP-1 in vascular smooth muscle cells42 and in TNF-induced expression of MCP-1 in THP cells.43 Further support of Src kinase activation in microglia has also been demonstrated in the hippocampus after transient forebrain ischemia.44 Another key feature of our findings is that cocaine-induced ROS production is dependent on activation of Src kinase. Inhibition of Src activity with the inhibitor significantly blocked cocaine-induced ROS production. In other words, Src kinase activation appears to be upstream and is required for ROS production stimulated with cocaine. Further dissection of the signaling pathways involved in cocaine-mediated induction of MCP-1 using both the pharmacologic and genetic approaches revealed activation of ERK, JNK, MAPKs, and the PI3K/Akt pathways. These findings are in agreement with the effect of cocaine in neurons in our previous study.12 Our findings demonstrate the involvement of ERK and JNK pathways, but not p38, in cocaine-mediated induction of MCP-1 expression. The role of JNK but not p38 has been reported in Pneumocystis-mediated stimulation of MCP-1 in alveolar epithelial cells.45 However, unlike cocaine-mediated involvement of ERK in our system, there was no involvement of ERK in the epithelial cells. This difference could be attributable to the cell type and/or the stimulant. In addition to the role of MAPK activation, PI3K/Akt was also involved in cocaine-induced MCP-1 expression.
The transcription factor NF-κB is known to play a key role in the production of proinflammatory chemokines. Our results revealed that cocaine-induced MCP-1 expression via NF-κB phosphorylation involved activation of Src, NADPH oxidase, and MAPKs, and the PI3K/Akt cascade. The requirement of NF-κB signaling in MCP-1 expression has been confirmed by previous studies.28,46 Accumulating evidence also indicates that Src and ERK pathways are involved in NF-κB activation in pancreatic acinar AR42J cells.47 Furthermore, in addition to MAPKs, Akt can also transduce its signal via activation of NF-κB.48 Our findings demonstrate the role of p65/RelA nuclear translocation in cocaine-induced MCP-1 expression.
We also demonstrated the functional implication of cocaine-mediated induction of MCP-1 both in in vitro and in in vivo models of monocyte transmigration. Herein, we showed increased ability of CM from cocaine-treated microglia to induce transmigration of monocytes across an in vitro BBB model; furthermore, we showed that this effect was inhibited by CM that had been treated with the neutralizing antibody specific for MCP-1. These findings were also validated in cocaine-injected mice that demonstrated increased transmgiration of PKH26-labeled monocytes in the brain compared with mice not injected with cocaine. This effect was abrogated with the σ-1R antagonist. These findings are in agreement with a recent study investigating the role of MCP-1 in monocyte infiltration in West Nile virus encephalitis.49 It is likely that MCP-1 released from the microglia in response to cocaine binds to the chemokine receptor CCR2 that is expressed on the blood-derived monocytes. Intriguingly, we observed that cocaine induced monocyte transmigration in WT but not in the CCR2 KO mice, as expected, further corroborating the role of the MCP-1 pathway in monocyte transmigration.
In summary, our findings have chalked out a detailed molecular pathway of cocaine-mediated induction of MCP-1 in microglia, involving σ-1R translocation, Src activation, ROS generation, and activation of MAPKs and PI3/Akt pathways, with subsequent activation of NF-κB resulting in increased MCP-1 expression, ultimately leading to recruitment of increased numbers of inflammatory cells into the CNS. These findings have implications for cocaine abusers with HIV-1 who are known to have increased risk of stroke and CNS-associated neuroinflammation. σ-1R antagonists can thus be considered as adjunct therapeutic agents for the treatment of cocaine addicts that have HIV-1.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr T. P. Su (NIDA, NIH) for insightful discussions and for providing σ-1R siRNA and antibody. We thank Dr K. Walsh (Tufts University School of Medicine) for providing the adenovirus vectors expressing either the WT or DN forms of Akt. We also thank Dr S. Maggirwar (University of Rochester Medical Center) for providing us with recombinant adenovirus vectors coexpressing either GFP and full-length RelA (RelAFL), or mutant RelA.
This work was supported by grants MH-068212, DA020392, DA023397, DA024442, and DA027729 from the National Institutes of Health (S.B.) in addition to the Nebraska Tobacco Settlement Biomedical Research.
National Institutes of Health
Authorship
Contribution: H.Y. designed research, performed research, and wrote the manuscript; Y.Y., C.B.-B., and N.G. performed research; K.J.K., K.F., H.E.G., and J.Q.W. provided insightful discussions and vital reagents and reviewed the manuscript; T.-P.S. provided valuable suggestions and reagents and discussed the results; and S.B. planned and designed the research, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Shilpa J. Buch, Department of Pharmacology and Experimental Neuroscience, 985880 Nebraska Medical Center (DRC 8011), University of Nebraska Medical Center, Omaha, NE 68198-5880; e-mail: sbuch@unmc.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal