Effective anticancer therapy requires effective targeting of the malignant cells. In this issue of Blood, Beers and colleagues demonstrate that rituximab-induced loss of CD20 from the surface of B cells may explain why rituximab is more effective in some B-cell malignancies, such as follicular lymphoma, than in others like CLL.1 They also provide evidence that anti-CD20 mAb, designated type II anti-CD20 mAb, induce considerably less down-modulation of CD20 than rituximab, and therefore could be more effective therapeutically.
Positive clinical trials reported almost yearly have led to expanded clinical indications for rituximab. Most of these trials have been empiric in design. Even with these broadening clinical indications, it remains enigmatic why rituximab-based therapy works better in some subjects than in others, and resistance often develops. The mechanistic explanations for primary or secondary resistance to rituximab remain unclear.
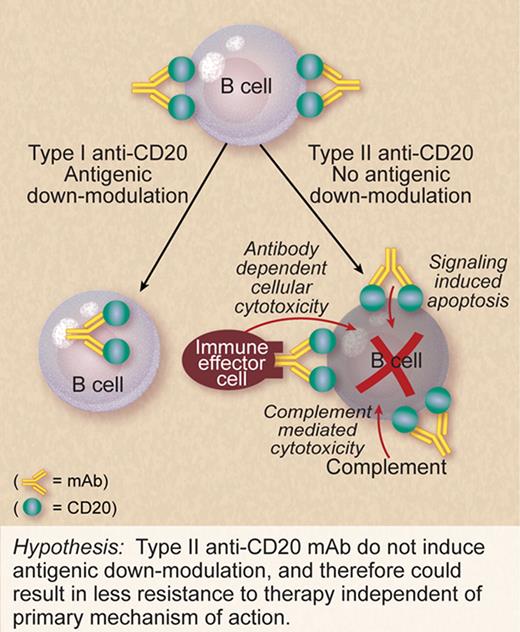
It has generally been accepted that an advantage of CD20 as a target antigen is that it does not down-modulate significantly when bound by monoclonal antibodies (mAb), that is, the anti-CD20/CD20 complex remains on the cell surface long enough for effector mechanisms to kill the target cell. This assumption is based on in vitro observations with a limited number of cell lines using short incubation times. In vivo, there is evidence that this may be different in select scenarios. Beum et al have described a “shaving reaction” in which mAb-CD20 complexes are “shaved” off chronic lymphocytic leukemia (CLL) cells by phagocytes as the malignant cells circulate.2
In this issue of Blood, Beers et al demonstrate in vivo in a mouse model and in vitro using malignant human B cells that rituximab can indeed induce down-modulation of CD20. The mechanism that Beers et al identifies—internalization of the rituximab/CD20 complex into the target cell itself—is distinct from that described by Beum and colleagues as it does not require phagocytes. Beers et al also demonstrate considerable variability in down-modulation based on the target cell type. They find considerable variability even within a given histology. In general, CLL and mantle cell lymphoma showed greater down-modulation of CD20 in response to rituximab than did follicular lymphoma and diffuse large B-cell lymphoma—a pattern that mirrors clinical response to rituximab.
It remains unclear which of the primary mechanisms of action that have been identified in preclinical and correlative studies (target cell killing induced by interaction between rituximab Fc and FcR, complement-induced lysis, or signaling-induced cell death) is responsible for clinical response to rituximab. Indeed, each of these mechanisms may be important in different scenarios based on the type of B-cell malignancy, location of the malignant cells, level of mAb achieved, and use of concomitant therapy such as chemotherapy. Irrespective of which of these mechanisms of action is important in a given scenario, the efficacy of rituximab therapy would be expected to be lost if the malignant cells remain viable after target antigen has been lost from the surface.
If antigen down-modulation of CD20 limits the efficacy of rituximab therapy, is all lost with respect to making a better anti-CD20 mAb? Not according to Beers et al. They previously reported that type II anti-CD20 mAb cross-link CD20 differently than do type I anti-CD20 mAb.3 In this current issue, they report that type II anti-CD20 mAb do not induce modulation to the degree seen with type I anti-CD20 mAb (see figure).
There are a number of next-generation anti-CD20 mAb in development including antibodies with enhanced affinity for FcR4 and enhanced ability to fix complement.5 Most of these are type I anti-CD20 mAb similar to rituximab. Tositumomab is a type II anti-CD20 mAb, first reported 30 years ago.6 It is a murine mAb that has been studied as a component of radioimmunotherapy,7 but not extensively as a single agent or in a humanized form. A humanized type II anti-CD20 mAb, GA101,8 is now in early-phase clinical development. It is too early to know whether it will be more effective than rituximab.
So, when it comes to making a better anti-CD20 mAb, is all lost with loss of surface target antigen? Maybe not if the preclinical findings of Beers et al with type II mAb translate into more efficacious therapy in the clinic.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal