Abstract
A risk-adapted strategy based on all-trans retinoic acid (ATRA) and anthracycline monochemotherapy (PETHEMA LPA99 trial) has demonstrated a high antileukemic efficacy in acute promyelocytic leukemia. We designed a new trial (LPA2005) with the objective of achieving stepwise improvements in outcome. Between July 2005 and April 2009, low- and intermediate-risk patients (leukocytes < 10 × 109/L) received a reduced dose of mitoxantrone for the second consolidation course, whereas high- risk patients younger than 60 years of age received cytarabine combined with ATRA and idarubicin in the first and third consolidation courses. Of 372 patients attaining complete remission after ATRA plus idarubicin (92.5%), 368 proceeded to consolidation therapy. For low- and intermediate-risk patients, duration of neutropenia and thrombocytopenia and hospital stay were significantly reduced without sacrificing antileukemic efficacy, compared with the previous LPA99 trial. For high-risk patients, the 3-year relapse rate was significantly lower in the LPA2005 trial (11%) than in the LPA99 (26%; P = .03). Overall disease-free survival was also better in the LPA2005 trial (P = .04). In conclusion, the lower dose of mitoxantrone resulted in a significant reduction of toxicity and hospital stay while maintaining the antileukemic activity, and the combination of ATRA, idarubicin, and cytarabine for high-risk acute promyelocytic leukemia significantly reduced the relapse rate in this setting. Registered at http://www.clinicaltrials.gov as NCT00408278.
MedscapeCME Continuing Medical Education online
This activity has been planned and implemented in accordance with the Essential Areas and policies of the Accreditation Council for Continuing Medical Education (ACCME) through the joint sponsorship of Medscape, LLC and the American Society of Hematology. Medscape, LLC is accredited by the ACCME to provide continuing medical education for physicians. Medscape, LLC designates this educational activity for a maximum of 0.75 AMA PRA Category 1 credits™. Physicians should only claim credit commensurate with the extent of their participation in the activity. All other clinicians completing this activity will be issued a certificate of participation. To participate in this journal CME activity: (1) review the learning objectives and author disclosures; (2) study the education content; (3) take the post-test and/or complete the evaluation at http://cme.medscape.com/journal/blood; and (4) view/print certificate. For CME questions, see page 5280.
Disclosures
The authors and Associate Editor Martin S. Tallman declare no competing financial interests. The CME questions author Désirée Lie, University of California, Irvine, CA, served as a nonproduct speaker for “Topics in Health” for Merck Speaker Services.
Learning objectives
Upon completion of this activity, participants will be able to:
Describe the overall differences between the LPA99 (AIDA-ATRA + idarubicin-) and LPA2005 (AIDA + cytarabine in high-risk) treatment protocols for acute promyelocytic leukemia
Identify differences in the treatment of low-risk, intermediate-risk, and high-risk patients in the AIDA and AIDA + cytarabine in high-risk protocols
Compare the rate of differentiation syndrome among patients who received the AIDA and AIDA + cytarabine in high-risk protocols
Compare hospitalization outcomes for patients who received the AIDA versus the AIDA + cytarabine in high-risk protocols
Compare differences in relapse rate among patients with acute promyelocytic leukemia on the AIDA versus the AIDA + cytarabine in high-risk protocol
Introduction
A risk-adapted strategy designed by the cooperative group Programa Español de Tratamientos en Hematología (PETHEMA), which was based on the combination of all-trans retinoic acid (ATRA) and anthracycline monochemotherapy for induction and consolidation (PETHEMA LPA99 trial), has previously demonstrated a high antileukemic efficacy and high protocol compliance coupled with moderate toxicity in patients with acute promyelocytic leukemia (APL).1,2
A new trial (LPA2005 trial) to improve the outcome of this strategy was designed with the primary objective of decreasing the relapse rate in high-risk patients (ie, presenting white blood cell [WBC] counts > 10 × 109/L)3 younger than 60 years of age. To accomplish this objective, cytarabine was added to the combination of ATRA and idarubicin in consolidation therapy. In addition, the protocol intended to reduce toxicity during consolidation therapy in low- and intermediate-risk patients (ie, presenting WBC counts < 10 × 109/L) by a dose reduction of mitoxantrone. Although the potential benefit of the addition of cytarabine has been suggested in previous studies,4 the specific use of this agent for high-risk patients was mainly supported by a joint study of the PETHEMA and the European APL groups5 and a study of the Gruppo Italiano Malattie Ematologiche dell'Adulto (GIMEMA).6 The authors of both studies have suggested a benefit in terms of reduction of relapse risk with the use of cytarabine in consolidation in patients with high-risk disease. We report here the results obtained in 402 consecutive patients with newly diagnosed APL who were enrolled in the LPA 2005 trial. The outcome was compared with that achieved in 561 patients treated with the previous LPA99 trial.
Methods
Eligibility
Patients enrolled in the consecutive PETHEMA LPA99 and LPA2005 trials were required to have a diagnosis of de novo APL with demonstration of the t(15;17) or PML/RARA rearrangements, normal hepatic and renal function, no cardiac contraindications to anthracycline chemotherapy, and an Eastern Cooperative Oncology Group (ECOG) performance status of less than 4. Informed consent was obtained from all patients. According to the Declaration of Helsinki, the protocol was approved by the Research Ethics Board of each participating hospital. The trial is registered at http://www.ClinicalTrials.gov (NCT00408278).
Induction therapy
Induction therapy consisted of oral ATRA (45 mg/m2/d) divided into 2 daily doses, which was maintained until complete hematologic remission and idarubicin (12 mg/m2/d) given as an intravenous bolus on days 2, 4, 6, and 8 (ATRA and idarubicin [AIDA] regimen), except for patients older than 70 years of age who received only the 3 first doses of idarubicin. For patients 20 years of age or younger, the ATRA was adjusted to 25 mg/m2/d. Induction therapy was identical for both LPA2005 and LPA99 trials.
Postremission therapy
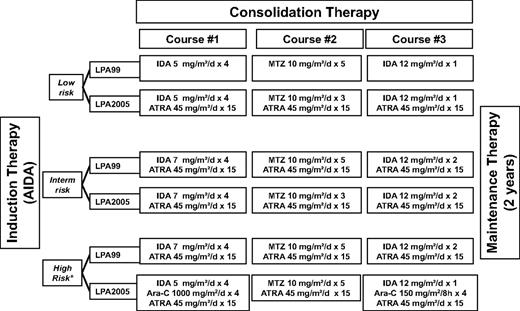
Following a risk-adapted strategy, patients who achieved complete remission (CR) received 3 monthly consolidation courses with ATRA and anthracycline-based chemotherapy. Figure 1 summarizes the ATRA and chemotherapy dose and schedule of consolidation therapy in both LPA2005 and LPA99 trials. In brief, compared with the LPA99 trial that has been described elsewhere,1 the changes implemented in the LPA2005 were the following: (1) For low-risk patients, ATRA was combined to chemotherapy in the 3 consolidation courses; in addition, mitoxantrone was reduced from 5 to 3 days in the second course of consolidation. (2) For intermediate-risk patients, mitoxantrone was also reduced from 5 to 3 days in the second course of consolidation. (3) For high-risk patients, cytarabine was added together with a slight reduction of idarubicin in the first and third consolidation course. High-risk patients older than 60 years did not receive cytarabine and were treated as intermediate-risk patients. Maintenance therapy was identical in both PETHEMA LPA99 and LPA2005 trials as was described elsewhere.1
Treatment schedule of the LPA99 and LPA2005 trials. Ara-C indicates cytarabine; IDA, idarubicin; and MTZ, mitoxantrone. High-risk patients older than 60 years did not receive cytarabine and were treated as intermediate-risk patients.
Treatment schedule of the LPA99 and LPA2005 trials. Ara-C indicates cytarabine; IDA, idarubicin; and MTZ, mitoxantrone. High-risk patients older than 60 years did not receive cytarabine and were treated as intermediate-risk patients.
Supportive measures
Platelet transfusions, fresh-frozen plasma, and cryoprecipitate for the management of coagulopathy in the LPA99 and LPA2005 trials were given as previously described.7 In the LPA2005 trial, tranexamic acid prohylaxis was not used. The management of differentiation syndrome (DS) also was similar in both trials,1 except for prophylaxis. In the LPA99 trial, DS prophylaxis with prednisone (0.5 mg/kg/d orally for 15 days) was given to all patients, whereas in the LPA2005 trial, only patients with WBC count greater than 5 × 109/L at presentation or achieved during the 2 first weeks of ATRA therapy received dexamethasone (2.5 mg/m2/12 hours intravenously for 15 days).
Definitions and study end points
Remission induction response was assessed according to the recently revised criteria by Cheson et al8 For morphologic assessment of leukemia resistance, it was required that sufficient time had passed to allow for full terminal differentiation of the malignant promyelocytes (up to 40-50 days). Molecular remission was defined as the disappearance on an ethidium bromide gel of the PML/RARA-specific band visualized at diagnosis with the use of reverse-transcription polymerase chain reaction (RT-PCR) assays with a sensitivity level of one leukemic cell in 10−4 cells. Molecular persistence was defined as PCR positivity in 2 consecutive bone marrow samples collected at the end of consolidation therapy. Molecular relapse was defined as previously reported.3 Diagnosis of the DS was made according to the presence of the following signs or symptoms described by Frankel et al9 : unexplained fever, dyspnea, pleural and/or pericardial effusion, pulmonary infiltrates, renal failure, hypotension, and unexplained weight gain greater than 5 kg. No single sign or symptom was considered sufficient to make a diagnosis of the syndrome. Patients with alternative explanations for the clinical complex, such as pulmonary hemorrhage, septic shock, pneumonia, or cardiac failure, were considered not to have DS. As defined elsewhere,10 patients with 4 or more of the aforementioned signs or symptoms were classified as having severe DS, whereas those with 2 or 3 signs or symptoms were considered to have moderate DS. Relapse-risk groups were defined as reported elsewhere3 as follows: low-risk patients had a WBC count less than 10 × 109/L and a platelet count more than 40 × 109/L; intermediate-risk patients had a WBC count less than 10 × 109/L and a platelet count less than 40 × 109/L; and high-risk patients had a WBC count equal to or more than 10 × 109/L. Hematologic toxicity was graded according to the National Cancer Institute Common Toxicity Criteria, version 2.
Statistical analysis
The χ2 test, with Yates correction if necessary, was used to analyze differences in the distribution of categorical variables between patient subsets. The t test and Mann-Whitney U test were used to detect differences in the distribution of continuous parametric and nonparametric variables, respectively. Results were analyzed on an intention-to-treat basis. Unadjusted time-to-event analyses were performed by use of the Kaplan-Meier estimate11 and, for comparisons, log-rank tests.12 The probability of relapse was also estimated by the cumulative incidence method (for marginal probability).13,14 Overall survival (OS) was calculated from the start date of induction therapy, whereas cumulative incidence of relapse (CIR) and disease-free survival (DFS) were calculated from the date of CR. In the analysis of DFS, relapse, development of secondary myelodysplastic syndrome or acute leukemia, and death in CR were considered uncensored events, whichever occurred first. For cumulative incidence analysis, death in CR and development of secondary myelodysplastic syndrome or acute leukemia were considered as a competing cause of failure. For all estimates in which the event “relapse” was considered as an end point, hematologic and molecular relapse, as well as molecular persistence at the end of consolidation, were each considered as uncensored events. Patient follow-up was updated on October 15, 2009. All P values reported are 2-sided. Multivariate analyses were performed by use of the Cox model for DFS and OS,15 and Fine and Gray model for CIR.16 Computations were performed by use of the 3D, 4F, 1L, and 2L programs from the BMDP statistical library (BMDP Statistical Software), and R 2.9.2 software package for CIR and Fine and Gray model.
Results
Accrual and patient characteristics
Between July 2005 and April 2009, 437 consecutive patients with genetic diagnosis of APL were registered from 81 institutions from Spain, Poland, the Netherlands, Argentina, Uruguay, and the Czech Republic (see the supplemental Appendix, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). A total of 33 patients (7.6%) were considered not eligible for the treatment because of severe clinical condition contraindicating the administration of chemotherapy. Thus, 404 patients met the previously defined entry criteria and were enrolled in the LPA2005 trial. Two additional patients were not evaluated because of protocol violations during induction therapy (addition of cytarabine in one patient and use of daunorubicin instead of idarubicin in another). The main clinical and biologic characteristics of the 402 patients evaluable for induction are shown in Table 1. For comparison, data of 561 patients treated with the previously reported LPA99 trial2 also are included in Table 1. Information on enrollment, eligible patients, lost at follow-up, and exclusion from analysis is shown in a flow diagram (Figure 2). Patients in both trials were comparable for all baseline characteristics except for age and ECOG performance status. The proportion of patients younger than 18 years in the LPA2005 was lower than in the LPA99 trial (P = .01), whereas the proportion of those with ECOG 0 to 1 was greater (P = .02).
Demographic and baseline characteristics of the study population according to PETHEMA/HOVON trial
| Characteristic . | LPA99 . | LPA2005 . | P . | ||
|---|---|---|---|---|---|
| Median (range) . | N (%) . | Median (range) . | N (%) . | ||
| Overall | 561 (100) | 402 (100) | |||
| Age, y | 40 (2-83) | 42 (3-83) | |||
| Younger than 18 | 65 (12) | 26 (6) | .01* | ||
| 18-40 | 223 (40) | 168 (42) | |||
| 41-60 | 171 (30) | 150 (37) | |||
| 61-70 | 68 (12) | 36 (9) | |||
| Older than 70 | 34 (6) | 22 (6) | |||
| Sex | |||||
| Male | 270 (48) | 209 (52) | .22 | ||
| Female | 291 (52) | 193 (48) | |||
| ECOG | 1 (0-3) | 1 (0-3) | |||
| 0-1 | 378 (73) | 267 (80) | .02 | ||
| 2 | 102 (20) | 41 (12) | |||
| 3 | 36 (7) | 26 (8) | |||
| Fever | |||||
| No | 334 (60) | 238 (63) | .33 | ||
| Yes | 220 (40) | 137 (37) | |||
| WBC count, ×109/L | 2.2 (0.2-460) | 3.0 (0.3-126) | |||
| 5 or less | 373 (67) | 248 (62) | .13† | ||
| 5-10 | 47 (8) | 36 (9) | |||
| 10-50 | 100 (18) | 96 (24) | |||
| More than 50 | 40 (7) | 22 (5) | |||
| Platelet count, ×109/L | 22 (1-207) | 23 (1-235) | |||
| 40 or less | 432 (77) | 298 (74) | .24 | ||
| More than 40 | 128 (23) | 104 (26) | |||
| Relapse-risk group | |||||
| Low | 107 (19) | 84 (21) | .14 | ||
| Intermediate | 313 (56) | 200 (50) | |||
| High | 140 (25) | 118 (29) | |||
| Hemoglobin, g/dL | 9.2 (3-16.9) | 9.3 (2.4-14.5) | |||
| 10 or less | 361 (65) | 255 (63) | .74 | ||
| More than 10 | 199 (35) | 147 (37) | |||
| Creatinine, mg/dL | 0.9 (0.2-2.4) | 0.8 (0.3-3.1) | |||
| 1.4 or less | 526 (98) | 365 (97) | .48 | ||
| More than 1.4 | 13 (2) | 12 (3) | |||
| Uric acid, mg/dL | 3.9 (0.29-12.7) | 4.1 (1.0-10.5) | |||
| 7 or less | 445 (95) | 327 (96) | .63 | ||
| More than 7 | 24 (5) | 15 (4) | |||
| Fibrinogen, mg/dL | 158 (0-862) | 179 (37-777) | |||
| 170 or less | 280 (54) | 176 (48) | .07 | ||
| More than 170 | 240 (46) | 193 (52) | |||
| Albumin, g/dL | 4.0 (1.7-6.7) | 4.1 (2.0-6.0) | |||
| 3.5 or less | 107 (24) | 66 (20) | .15 | ||
| More than 3.5 | 335 (76) | 267 (80) | |||
| Morphologic subtype | |||||
| Hypergranular | 452 (82) | 296 (80) | .44 | ||
| Microgranular | 99 (18) | 74 (20) | |||
| PML/RARα isoform | |||||
| BCR1/BCR2 | 300 (60) | 146 (55) | .29 | ||
| BCR3 | 204 (40) | 117 (45) | |||
| Characteristic . | LPA99 . | LPA2005 . | P . | ||
|---|---|---|---|---|---|
| Median (range) . | N (%) . | Median (range) . | N (%) . | ||
| Overall | 561 (100) | 402 (100) | |||
| Age, y | 40 (2-83) | 42 (3-83) | |||
| Younger than 18 | 65 (12) | 26 (6) | .01* | ||
| 18-40 | 223 (40) | 168 (42) | |||
| 41-60 | 171 (30) | 150 (37) | |||
| 61-70 | 68 (12) | 36 (9) | |||
| Older than 70 | 34 (6) | 22 (6) | |||
| Sex | |||||
| Male | 270 (48) | 209 (52) | .22 | ||
| Female | 291 (52) | 193 (48) | |||
| ECOG | 1 (0-3) | 1 (0-3) | |||
| 0-1 | 378 (73) | 267 (80) | .02 | ||
| 2 | 102 (20) | 41 (12) | |||
| 3 | 36 (7) | 26 (8) | |||
| Fever | |||||
| No | 334 (60) | 238 (63) | .33 | ||
| Yes | 220 (40) | 137 (37) | |||
| WBC count, ×109/L | 2.2 (0.2-460) | 3.0 (0.3-126) | |||
| 5 or less | 373 (67) | 248 (62) | .13† | ||
| 5-10 | 47 (8) | 36 (9) | |||
| 10-50 | 100 (18) | 96 (24) | |||
| More than 50 | 40 (7) | 22 (5) | |||
| Platelet count, ×109/L | 22 (1-207) | 23 (1-235) | |||
| 40 or less | 432 (77) | 298 (74) | .24 | ||
| More than 40 | 128 (23) | 104 (26) | |||
| Relapse-risk group | |||||
| Low | 107 (19) | 84 (21) | .14 | ||
| Intermediate | 313 (56) | 200 (50) | |||
| High | 140 (25) | 118 (29) | |||
| Hemoglobin, g/dL | 9.2 (3-16.9) | 9.3 (2.4-14.5) | |||
| 10 or less | 361 (65) | 255 (63) | .74 | ||
| More than 10 | 199 (35) | 147 (37) | |||
| Creatinine, mg/dL | 0.9 (0.2-2.4) | 0.8 (0.3-3.1) | |||
| 1.4 or less | 526 (98) | 365 (97) | .48 | ||
| More than 1.4 | 13 (2) | 12 (3) | |||
| Uric acid, mg/dL | 3.9 (0.29-12.7) | 4.1 (1.0-10.5) | |||
| 7 or less | 445 (95) | 327 (96) | .63 | ||
| More than 7 | 24 (5) | 15 (4) | |||
| Fibrinogen, mg/dL | 158 (0-862) | 179 (37-777) | |||
| 170 or less | 280 (54) | 176 (48) | .07 | ||
| More than 170 | 240 (46) | 193 (52) | |||
| Albumin, g/dL | 4.0 (1.7-6.7) | 4.1 (2.0-6.0) | |||
| 3.5 or less | 107 (24) | 66 (20) | .15 | ||
| More than 3.5 | 335 (76) | 267 (80) | |||
| Morphologic subtype | |||||
| Hypergranular | 452 (82) | 296 (80) | .44 | ||
| Microgranular | 99 (18) | 74 (20) | |||
| PML/RARα isoform | |||||
| BCR1/BCR2 | 300 (60) | 146 (55) | .29 | ||
| BCR3 | 204 (40) | 117 (45) | |||
BCR indicates breakpoint cluster region; ECOG, Eastern Cooperative Oncology Group; PML, promyelocytic leukemia; RAR, retinoic acid receptor; and WBC, white blood cell.
P compares < 18 versus ≥ 18 years old.
P compares ECOG 0 to 1 versus ECOG 2 to 3.
Consolidated Standards of Reporting Trials (CONSORT) diagram for the subsequent LPA99 and LPA2005 PETHEMA trials.
Consolidated Standards of Reporting Trials (CONSORT) diagram for the subsequent LPA99 and LPA2005 PETHEMA trials.
Induction therapy
Response and induction mortality.
Three hundred seventy-two of the 402 evaluable patients achieved morphologic CR (92.5%; 95% confidence interval 90.6%-94.4%). The median time interval to CR was 39 days (range, 18-81 days). The median time to reach neutrophil counts greater than 1 × 109/L, and platelet counts greater than 50 × 109/L was 24 days (range, 6-72 days) and 19 days (range, 4-80 days), respectively. All the 30 induction failures were the result of death during induction.
Hemorrhage and infection accounted for most of the deaths during induction therapy (15 and 6 patients, respectively). Deaths caused by hemorrhage were caused by intracranial (12 patients, 80%), pulmonary (2 patients, 13%), and gastrointestinal hemorrhages (1 patient, 7%). DS and acute myocardial infarction were contributing causes of death in 4 and 2 patients, respectively. The remaining 3 deaths were attributable to massive suprahepatic thrombosis, myocarditis, and cardiac failure in 1 patient each. The response rates and causes of induction death were quite similar in both the LPA2005 and LPA99 studies (Table 2).
Induction outcome and differentiation syndrome of APL patients in the PETHEMA LPA99 and LPA2005 trials
| Characteristic . | LPA99 . | LPA2005 . | P . | ||
|---|---|---|---|---|---|
| Median (range) . | N (%) . | Median (range) . | N (%) . | ||
| Overall | 561 (100) | 402 (100) | |||
| Morphologic CR | 511 (91.1) | 372 (92.5) | .42 | ||
| Days to CR | 37 (21-77) | 39 (18-81) | .38 | ||
| Days to PMN > 1 × 109/L | 23 (0-60) | 24 (6-72) | .36 | ||
| Days to platelets > 50 × 109/L | 19 (0-50) | 19 (4-80) | .08 | ||
| Cause of induction death | |||||
| Hemorrhage | 28 (5.0) | 15 (3.7) | .44 | ||
| Infection | 12 (2.1) | 6 (1.5) | .62 | ||
| Differentiation syndrome | 8 (1.4) | 4 (1.0) | .55 | ||
| Other | 2 (0.4) | 5 (1.2) | .22 | ||
| Differentiation syndrome | |||||
| Severe | 66 (12) | 45 (12) | .12 | ||
| Moderate | 66 (12) | 61 (16) | |||
| Absent | 429 (76) | 266 (72) | |||
| Characteristic . | LPA99 . | LPA2005 . | P . | ||
|---|---|---|---|---|---|
| Median (range) . | N (%) . | Median (range) . | N (%) . | ||
| Overall | 561 (100) | 402 (100) | |||
| Morphologic CR | 511 (91.1) | 372 (92.5) | .42 | ||
| Days to CR | 37 (21-77) | 39 (18-81) | .38 | ||
| Days to PMN > 1 × 109/L | 23 (0-60) | 24 (6-72) | .36 | ||
| Days to platelets > 50 × 109/L | 19 (0-50) | 19 (4-80) | .08 | ||
| Cause of induction death | |||||
| Hemorrhage | 28 (5.0) | 15 (3.7) | .44 | ||
| Infection | 12 (2.1) | 6 (1.5) | .62 | ||
| Differentiation syndrome | 8 (1.4) | 4 (1.0) | .55 | ||
| Other | 2 (0.4) | 5 (1.2) | .22 | ||
| Differentiation syndrome | |||||
| Severe | 66 (12) | 45 (12) | .12 | ||
| Moderate | 66 (12) | 61 (16) | |||
| Absent | 429 (76) | 266 (72) | |||
CR indicates complete response; and PMN, polymorphonuclear leukocytes.
Patients with WBC counts greater than 10 and 50 × 109/L had a poorer response rate (83% and 73%, respectively) compared with those with low- and intermediate-risk patients (99% and 96%, respectively; P < .001). Patients older than 60 years had also a lower CR rate (88%) than younger patients (93%), but this difference was not statistically significant (P = .15),
Differentiation syndrome.
One hundred six of the 372 patients (28.5%) who were evaluable for this complication developed DS. Severe DS was diagnosed in 45 patients (12.1%), 4 of whom died from it, whereas moderate DS was reported in 61 (16.3%; Table 2). In 9 additional patients (2.5%) with possible DS, an unambiguous diagnosis of DS could not be made. This was attributable to the presence of concurrent medical problems that could explain the clinical manifestations. These problems were pulmonary hemorrhage in 5 patients, pneumonia in 2, renal failure in 1, and septic shock in another. ATRA was temporarily discontinued in 78 patients (74%) with DS. Diuretics and intravenous dexamethasone were administered in 95 patients (90%) and 88 patients (83%), respectively, whereas mechanical ventilation and dialysis was needed in 8 patients (8%) and 3 patients (3%), respectively.
Consolidation therapy
Tolerability and treatment feasibility.
Three hundred sixty-eight of the 372 patients who achieved CR proceeded to consolidation therapy as scheduled. Three of the 4 exceptions who developed severe cardiac toxicity during induction received a modified consolidation treatment. It consisted of substituting idarubicin and mitoxantrone for a bioequivalent dose of liposomal daunorubicin in 1 patient and arsenic trioxide in another. The remaining patient with cardiac toxicity and one additional 73-year-old patient who had a perianal abscess during induction proceeded directly to maintenance therapy. Four additional patients died during consolidation therapy (3 high risk; 1 low risk), 3 as the result of infection, and 1 to intracranial bleeding. Three further patients did not receive the third course of consolidation because of toxicity in previous courses. The remaining 361 patients (97%) completed the 3 consolidation courses as scheduled.
Hematologic toxicity.
Details on hematologic toxicity and length of in-hospital stay in each consolidation course are shown in Table 3. Low- and intermediate-risk patients after the second consolidation course of the LPA2005 with reduced dose of mitoxantrone showed a lower rate of prolonged (> 15 days) grade 3 to 4 neutropenia and grade 4 thrombocytopenia compared with the results of the LPA99 trial (P < .001). This reduction of hematologic toxicities was associated with a significantly lower proportion of patients requiring a hospital stay longer than 10 days (P < .001). Overall, the mean length of in-hospital stay during the 3 consolidation courses in low- and intermediate-risk patients was also reduced (ie, 22 days in the LPA99 and 17 days in the LPA2005 trial; P < .001). In contrast, high-risk patients receiving the combination of ATRA, idarubicin, and cytarabine as part of the treatment schedule had a greater rate of prolonged grade 3 to 4 neutropenia and grade 4 thrombocytopenia during the first and third consolidation courses of the LPA2005, compared with the LPA99 trial (P < .001). This increase of hematologic toxicities was associated with a significantly greater proportion of patients requiring hospitalization longer than 10 days (P < .001). The mean length of in-hospital stay during the 3 consolidation courses in this setting was 26 days in the LPA99 and 33 days in the LPA2005 trial (P = .10).
Severity of hematologic toxicity and hospitalization associated with consolidation therapy in the LPA99 and LPA2005 trials
| Toxicity . | Course 1 . | Course 2 . | Course 3 . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| LPA99 N (%) . | LPA2005 N (%) . | P . | LPA99 N (%) . | LPA2005 N (%) . | P . | LPA99 N (%) . | LPA2005 N (%) . | P . | |
| All patients, no. evaluable courses | 472 | 305 | 477 | 292 | 449 | 276 | |||
| Days with grade 3-4 thrombocytopenia, median (range) | 11 (0-52) | 16 (0-52) | 21 (0-91) | 15 (0-117) | 18 (0-90) | 19 (0-85) | |||
| Episodes > 15 d, n (%) | 205 (44) | 159 (52) | .02 | 352 (75) | 140 (48) | < .001 | 257 (53) | 156 (57) | .35 |
| Days with grade 4 neutropenia, median (range) | 17 (0-63) | 18 (0-54) | 22 (0-68) | 18 (0-78) | 20 (0-80) | 19 (0-78) | |||
| Episodes > 15 d, n (%) | 256 (54) | 182 (60) | .12 | 403 (84) | 179 (61) | < .001 | 259 (58) | 165 (60) | .58 |
| Days of hospitalization, median (range) | 4 (0-61) | 4 (0-37) | 10 (0-119) | 0 (0-36) | 0 (0-63) | 0 (0-64) | |||
| Episodes > 10 d, n (%) | 110 (24) | 100 (32) | .02 | 216 (46) | 75 (26) | < .001 | 70 (16) | 73 (26) | .35 |
| Low-risk patients, no. evaluable courses | 93 | 66 | 97 | 68 | 82 | 63 | |||
| Days with grade 3-4 thrombocytopenia, median (range) | 0 (0-37) | 0 (0-28) | 21 (0-36) | 0 (0-57) | 0 (0-67) | 0 (0-31) | |||
| Episodes > 15 d, n (%) | 12 (13) | 16 (24) | .08 | 60 (64) | 27 (40) | < .001 | 13 (16) | 11 (17) | .79 |
| Days with grade 4 neutropenia, median (range) | 0 (0-30) | 0 (0-31) | 21 (0-42) | 17 (0-35) | 0 (0-55) | 0 (0-52) | |||
| Episodes > 15 d, n (%) | 21 (23) | 21 (32) | .19 | 78 (80) | 37 (54) | < .001 | 17 (21) | 12 (19) | .80 |
| Days of hospitalization, median (range) | 0 (0-30) | 4 (0-36) | 8 (0-119) | 0 (0-36) | 0 (0-36) | 0 (0-17) | |||
| Episodes > 10 d, n (%) | 12 (13) | 17 (25) | .06 | 38 (39) | 15 (24) | .02 | 3 (3) | 3 (4) | .99 |
| Intermediate-risk patients, no. evaluable courses | 271 | 161 | 271 | 153 | 264 | 144 | |||
| Days with grade 3-4 thrombocytopenia, median (range) | 17 (0-52) | 16 (0-52) | 22 (0-91) | 0 (0-117) | 24 (0-86) | 20 (0-85) | |||
| Episodes > 15 d, n (%) | 149 (55) | 82 (50) | .34 | 212 (78) | 59 (38) | < .001 | 163 (62) | 84 (59) | .49 |
| Days with grade 4 neutropenia, median (range) | 19 (0-63) | 18 (0-54) | 22 (0-68) | 17 (0-61) | 23 (0-75) | 19 (0-78) | |||
| Episodes > 15 d, n (%) | 171(63) | 96 (60) | .47 | 232 (86) | 88 (57) | < .001 | 174 (66) | 92 (64) | .68 |
| Days of hospitalization, median (range) | 5 (0-61) | 4 (0-34) | 10 (0-49) | 0 (0-33) | 0 (0-63) | 0 (0-64) | |||
| Episodes > 10 d, n (%) | 73 (28) | 42 (26) | .51 | 124 (47) | 32 (21) | < .001 | 45 (18) | 34 (23) | .35 |
| High-risk patients, No. evaluable courses | 107 | 77 | 109 | 70 | 103 | 69 | |||
| Days with grade 3-4 thrombocytopenia, median (range) | 0 (0-34) | 18 (0-33) | 21 (0-64) | 24 (0-102) | 21 (0-90) | 26 (0-56) | |||
| Episodes > 15 d, n (%) | 43 (41) | 61 (81) | < .001 | 80 (77) | 54 (77) | .97 | 61 (61) | 61 (89) | < .001 |
| Days with grade 4 neutropenia, median (range) | 19 (0-46) | 20 (0-43) | 24 (0-64) | 22 (0-78) | 20 (0-80) | 22 (0-49) | |||
| Episodes > 15 d, n (%) | 63 (59) | 65 (84) | < .001 | 93 (85) | 54 (77) | .16 | 68 (66) | 61 (89) | < .001 |
| Days of hospitalization, median (range) | 0 (0-34) | 13 (0-37) | 11 (0-40) | 6 (0-28) | 0 (0-60) | 11 (0-37) | |||
| Episodes > 10 d, n (%) | 78 (23) | 41 (52) | < .001 | 53 (50) | 28 (39) | .13 | 22 (22) | 36 (51) | < .001 |
| Toxicity . | Course 1 . | Course 2 . | Course 3 . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| LPA99 N (%) . | LPA2005 N (%) . | P . | LPA99 N (%) . | LPA2005 N (%) . | P . | LPA99 N (%) . | LPA2005 N (%) . | P . | |
| All patients, no. evaluable courses | 472 | 305 | 477 | 292 | 449 | 276 | |||
| Days with grade 3-4 thrombocytopenia, median (range) | 11 (0-52) | 16 (0-52) | 21 (0-91) | 15 (0-117) | 18 (0-90) | 19 (0-85) | |||
| Episodes > 15 d, n (%) | 205 (44) | 159 (52) | .02 | 352 (75) | 140 (48) | < .001 | 257 (53) | 156 (57) | .35 |
| Days with grade 4 neutropenia, median (range) | 17 (0-63) | 18 (0-54) | 22 (0-68) | 18 (0-78) | 20 (0-80) | 19 (0-78) | |||
| Episodes > 15 d, n (%) | 256 (54) | 182 (60) | .12 | 403 (84) | 179 (61) | < .001 | 259 (58) | 165 (60) | .58 |
| Days of hospitalization, median (range) | 4 (0-61) | 4 (0-37) | 10 (0-119) | 0 (0-36) | 0 (0-63) | 0 (0-64) | |||
| Episodes > 10 d, n (%) | 110 (24) | 100 (32) | .02 | 216 (46) | 75 (26) | < .001 | 70 (16) | 73 (26) | .35 |
| Low-risk patients, no. evaluable courses | 93 | 66 | 97 | 68 | 82 | 63 | |||
| Days with grade 3-4 thrombocytopenia, median (range) | 0 (0-37) | 0 (0-28) | 21 (0-36) | 0 (0-57) | 0 (0-67) | 0 (0-31) | |||
| Episodes > 15 d, n (%) | 12 (13) | 16 (24) | .08 | 60 (64) | 27 (40) | < .001 | 13 (16) | 11 (17) | .79 |
| Days with grade 4 neutropenia, median (range) | 0 (0-30) | 0 (0-31) | 21 (0-42) | 17 (0-35) | 0 (0-55) | 0 (0-52) | |||
| Episodes > 15 d, n (%) | 21 (23) | 21 (32) | .19 | 78 (80) | 37 (54) | < .001 | 17 (21) | 12 (19) | .80 |
| Days of hospitalization, median (range) | 0 (0-30) | 4 (0-36) | 8 (0-119) | 0 (0-36) | 0 (0-36) | 0 (0-17) | |||
| Episodes > 10 d, n (%) | 12 (13) | 17 (25) | .06 | 38 (39) | 15 (24) | .02 | 3 (3) | 3 (4) | .99 |
| Intermediate-risk patients, no. evaluable courses | 271 | 161 | 271 | 153 | 264 | 144 | |||
| Days with grade 3-4 thrombocytopenia, median (range) | 17 (0-52) | 16 (0-52) | 22 (0-91) | 0 (0-117) | 24 (0-86) | 20 (0-85) | |||
| Episodes > 15 d, n (%) | 149 (55) | 82 (50) | .34 | 212 (78) | 59 (38) | < .001 | 163 (62) | 84 (59) | .49 |
| Days with grade 4 neutropenia, median (range) | 19 (0-63) | 18 (0-54) | 22 (0-68) | 17 (0-61) | 23 (0-75) | 19 (0-78) | |||
| Episodes > 15 d, n (%) | 171(63) | 96 (60) | .47 | 232 (86) | 88 (57) | < .001 | 174 (66) | 92 (64) | .68 |
| Days of hospitalization, median (range) | 5 (0-61) | 4 (0-34) | 10 (0-49) | 0 (0-33) | 0 (0-63) | 0 (0-64) | |||
| Episodes > 10 d, n (%) | 73 (28) | 42 (26) | .51 | 124 (47) | 32 (21) | < .001 | 45 (18) | 34 (23) | .35 |
| High-risk patients, No. evaluable courses | 107 | 77 | 109 | 70 | 103 | 69 | |||
| Days with grade 3-4 thrombocytopenia, median (range) | 0 (0-34) | 18 (0-33) | 21 (0-64) | 24 (0-102) | 21 (0-90) | 26 (0-56) | |||
| Episodes > 15 d, n (%) | 43 (41) | 61 (81) | < .001 | 80 (77) | 54 (77) | .97 | 61 (61) | 61 (89) | < .001 |
| Days with grade 4 neutropenia, median (range) | 19 (0-46) | 20 (0-43) | 24 (0-64) | 22 (0-78) | 20 (0-80) | 22 (0-49) | |||
| Episodes > 15 d, n (%) | 63 (59) | 65 (84) | < .001 | 93 (85) | 54 (77) | .16 | 68 (66) | 61 (89) | < .001 |
| Days of hospitalization, median (range) | 0 (0-34) | 13 (0-37) | 11 (0-40) | 6 (0-28) | 0 (0-60) | 11 (0-37) | |||
| Episodes > 10 d, n (%) | 78 (23) | 41 (52) | < .001 | 53 (50) | 28 (39) | .13 | 22 (22) | 36 (51) | < .001 |
Molecular response.
RT-PCR tests for PML/RARA at the end of consolidation therapy were available in 320 patients (89%). No patient showed evidence of molecular persistence, whereas 3 patients were positive at this point in time in the LPA99 trial (P = .37). Among the 41 patients who were not tested for RT-PCR at the end of consolidation therapy, one bone marrow relapse occurred at 6 months.
Maintenance therapy
All of the 361 patients alive after completing consolidation therapy proceeded to maintenance therapy. Cytopenias, especially neutropenia, and slight liver function test abnormalities were commonly observed in this phase, often requiring dose reduction or temporary discontinuation of chemotherapy. No death in remission was reported during maintenance.
Outcome
Median follow-up of patients in the LPA99 and LPA2005 trials were 82 months (range, 17-120 months) and 28 months (range, 2-51 months) from diagnosis, respectively. In addition to the 4 cases of death in CR, 2 patients developed colorectal adenocarcinoma at 25 and 31 months from APL diagnosis. Deaths in remission occurred in patients aged 23, 38, 45, and 52 years as the result of infection (3 patients) and cerebral hemorrhage (1 patient) belonging to high- and low-risk groups (3 and 1 patients, respectively). Twenty-one relapses occurred among the 361 patients who achieved CR (16 overt morphologic and 5 molecular relapses). Three were extramedullary relapses. These relapses occurred in the central nervous system at 17 and 20 months from diagnosis in 2 intermediate-risk patients and the skin at 6 months in 1 high-risk patient with WBC count 55 × 109/L at presentation.
Relapse rate.
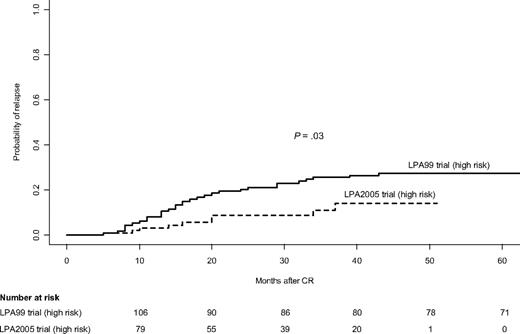
For patients in the LPA2005 study, the 3-year CIR rate was 7%, whereas for patients in the LPA99 study it was 9% (P = .39). For patients in the LPA2005 study, the CIR rates for low- and intermediate-risk patients were 6% and 6%, respectively, whereas in the LPA99 study, they were 4% and 5% (P = .58 and P = .69, respectively; Table 4). Among high-risk patients, the 3-year CIR rate was significantly lower in those treated with the LPA2005 protocol compared with those treated with the LPA99 protocol (11% vs 26%, P = .03; Figure 3). Multivariate analysis showed that the PETHEMA protocol was the sole independent risk factor for relapse in this setting (P = .02).
Postremission outcome of APL patients in the PETHEMA LPA99 and LPA2005 trials
| Characteristic . | No. of patients . | CIR . | DFS . | OS . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Overall . | CR, n (%) . | % at 3 y . | % at 4 y . | P . | % at 3 y . | % at 4 y . | P . | % at 3 y . | % at 4 y . | P . | |
| Overall | 963 | 883 (92) | 8 | 11 | 88 | 85 | 87 | 85 | |||
| All patients | |||||||||||
| LPA99 | 561 | 511 (91) | 9 | 11 | .39 | 87 | 84 | .06 | 85 | 83 | .08 |
| LPA2005 | 402 | 372 (92) | 7 | 9 | 92 | 90 | 89 | 88 | |||
| Low-risk | |||||||||||
| LPA99 | 107 | 103 (96) | 4 | 4 | .58 | 90 | 89 | .49 | 91 | 89 | .14 |
| LPA2005 | 84 | 83 (99) | 6 | 6 | 93 | 93 | 96 | 96 | |||
| Intermediate-risk | |||||||||||
| LPA99 | 313 | 294 (94) | 5 | 7 | .69 | 91 | 87 | .21 | 89 | 88 | .18 |
| LPA2005 | 200 | 191 (95) | 6 | 8 | 94 | 92 | 93 | 91 | |||
| High-risk | |||||||||||
| LPA99 | 140 | 113 (81) | 26 | 27 | .03 | 73 | 71 | .11 | 71 | 68 | .34 |
| LPA2005 | 118 | 98 (83) | 11 | 14 | 82 | 82 | 79 | 79 | |||
| Characteristic . | No. of patients . | CIR . | DFS . | OS . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Overall . | CR, n (%) . | % at 3 y . | % at 4 y . | P . | % at 3 y . | % at 4 y . | P . | % at 3 y . | % at 4 y . | P . | |
| Overall | 963 | 883 (92) | 8 | 11 | 88 | 85 | 87 | 85 | |||
| All patients | |||||||||||
| LPA99 | 561 | 511 (91) | 9 | 11 | .39 | 87 | 84 | .06 | 85 | 83 | .08 |
| LPA2005 | 402 | 372 (92) | 7 | 9 | 92 | 90 | 89 | 88 | |||
| Low-risk | |||||||||||
| LPA99 | 107 | 103 (96) | 4 | 4 | .58 | 90 | 89 | .49 | 91 | 89 | .14 |
| LPA2005 | 84 | 83 (99) | 6 | 6 | 93 | 93 | 96 | 96 | |||
| Intermediate-risk | |||||||||||
| LPA99 | 313 | 294 (94) | 5 | 7 | .69 | 91 | 87 | .21 | 89 | 88 | .18 |
| LPA2005 | 200 | 191 (95) | 6 | 8 | 94 | 92 | 93 | 91 | |||
| High-risk | |||||||||||
| LPA99 | 140 | 113 (81) | 26 | 27 | .03 | 73 | 71 | .11 | 71 | 68 | .34 |
| LPA2005 | 118 | 98 (83) | 11 | 14 | 82 | 82 | 79 | 79 | |||
CIR indicates cumulative incidence of relapse; CR, complete response, DFS, disease-free survival; and OS, overall survival.
Cumulative incidence of relapse in high-risk patients according to PETHEMA trial.
Cumulative incidence of relapse in high-risk patients according to PETHEMA trial.
DFS and OS.
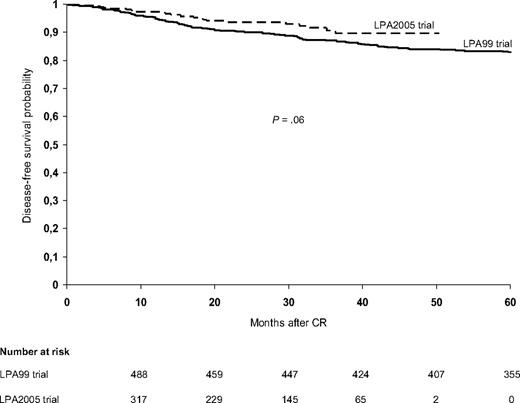
The 3-year DFS rates were 92% plus or minus 4% in the LPA2005 study and 87% plus or minus 3% in the LPA99 study (P = .06; Figure 4). Multivariate analysis showed that lower relapse-risk group (P < .001), female sex (P = .04), and the LPA2005 protocol (P = .04) were favorable risk factors for DFS. For patients in the LPA2005 and LPA99 study, the 3-year DFS rates for low-risk (93% ± 8% vs 90% ± 6%, P = .49), intermediate-risk (94% ± 4% vs 91% ± 3%, P = .21), and high-risk patients (82% ± 10% vs 73% ± 8%, P = .11) were not statistically different (Table 4). The probability of remaining alive after 3 years was 89% plus or minus 4% in the LPA2005 study and 85% plus or minus 3% in the LPA99 study (P = .08). In addition, for OS there were no significant differences between LPA2005 and LPA99 among low-, intermediate-, and high-risk patients (Table 4).
Discussion
This study shows a significant improvement in the outcome of APL patients treated with a successive risk-adapted PETHEMA/HOVON trial (LPA2005) on the basis of the combination of ATRA with idarubicin for induction plus anthracycline-based chemotherapy for consolidation, followed by ATRA and low-dose methotrexate and mercaptopurine for maintenance therapy. A reduction in the dose of mitoxantrone for low- and intermediate-risk patients in the LPA2005 trial led to a significant lower toxicity during the second consolidation course without decreasing the high antileukemic efficacy achieved in the previous LPA99 trial. Furthermore, the addition of cytarabine to the combination of ATRA and idarubicin in consolidation for high-risk patients resulted in a significantly greater antileukemic activity coupled with an increased but tolerable toxicity. Overall, compared with the previous LPA99, the LPA2005 protocol resulted in an improvement of the outcome in APL patients.
Following standard practice of the PETHEMA Group, the LPA2005 trial was nonrandomized and designed to provide a historical comparison with the previous LPA99 trial with the minimum possibility of bias. Given the sequential nature of the studies, patients in the LPA2005 series had a shorter follow-up than those in the LPA99 study (median, 28 and 82 months, respectively). Notwithstanding, the follow-up of patients in the LPA2005 study falls within the range reported by the major trials published to date. Participating institutions in all PETHEMA trials registered all eligible and noneligible patients with newly diagnosed APL and the eligibility criteria were unchanged throughout the 2 subsequent studies. Regarding therapy, only a single change was made in each relapse risk group to facilitate the interpretation of the potential impact on outcome in comparable series of patients. In this respect, it should be noted that the proportion of non eligible and nonevaluable patients were similar in the LPA99 and LPA2005 trial. Patients in both trials were comparable for all baseline characteristics, except for the somewhat-greater proportion of pediatric patients in the LPA99 trial and those with ECOG grade 0 to 1 in the LPA2005. These findings could explain, at least in part, the slightly lower induction failure rate, although not statistically significant, observed in the LPA2005 trial. Induction results in the LPA2005 study confirmed again a practical absence of leukemia resistance, as well as a similar CR rate and time interval to CR compared with previous trials of our group using the same AIDA regimen.6 Regarding the incidence of moderate and severe DS in the LPA2005 trial compared with the LPA99 trial, only a slight increase of the moderate form was observed (16% vs 12%), but this difference was not statistically significant. The changes made in the LPA2005, in which patients with WBC count lower than 5 × 109/L within the 2 first weeks of ATRA therapy did not received DS prophylaxis, might explain the small increase of moderate DS observed, but this is speculative.
Similar to a previous comparison between the LPA99 and the LPA96 study,1,2 we demonstrate here again a stepwise significant further improvement in outcome in the present LPA2005 study, that appears the consequence of selected modifications in consolidation therapy. We had hypothesized that patients with APL with a comparatively low risk of relapse, such as the low- and intermediate-risk groups, might receive overtreatment. Therefore, a reduction in the dose intensity of mitoxantrone in the second consolidation course was implemented in the LPA2005 trial with the aim of decreasing short- and long-term toxicities, without sacrificing the antileukemic efficacy of the LPA99 trial. This objective was fully achieved in terms of hematologic toxicities, relapse, and survival rates. In patients treated with reduced dose of mitoxantrone, that is, all patients older than 60 years and those younger with low and intermediate relapse risk, which overall represents 86% of the study population, prolonged grade 4 neutropenia, grade 3 to 4 thrombocytopenia, and hospitalization were reported significantly less frequently in the second course of the LPA2005 trial compared with the LPA99. Besides this favorable outcome, the dose reduction of mitoxantrone did not negatively impact on the antileukemic efficacy as is apparent from the similar relapse rates, DFS, and OS rates in the LPA2005 and the previous LPA99 trials.
Concerning the role of cytarabine in APL, the study conducted by the European APL group4 in younger patients randomly assigned to receive a state-of-the-art approach with daunorubicin alone or daunorubicin plus cytarabine, the addition of this agent resulted in a better outcome. However, the authors cautiously confined the interpretation of these results to the boundaries of the type and the cumulative dose of anthracycline given in this study. In the present LPA 2005 trial, the addition of cytarabine to the combination of ATRA and idarubicin in consolidation therapy for the high-risk patients younger than 60 years was mainly conducted to verify the outstanding but preliminary results reported by the GIMEMA group with a similar approach.6 The results of our study also demonstrate improved antileukemic efficacy of the cytarabine enriched schedule in high-risk APL. Also a recently updated analysis of the aforementioned GIMEMA study (Front-line treatment of acute promyelocytic leukemia with AIDA induction followed by risk-adapted consolidation: results of the AIDA-2000 trial of the Italian GIMEMA group, F. Lo-Coco, G. Avvisati, M. Vignetti, et al, manuscript submitted) has meanwhile confirmed the significant improvement of the therapeutic regimen supplemented with cytarabine.
However, the different way in which the GIMEMA and PETHEMA studies have substantiated this improvement deserves further discussion. It should be noted that the GIMEMA study showed a dramatic reduction of relapse rate in high-risk patients when, in the AIDA2000 trial, ATRA was added to the classical cytarabine-containing chemotherapy consolidation given in the.AIDA0493 trial.17 In contrast, the PETHEMA study demonstrated a similar favorable impact on relapse rate when, in the LPA2005 trial, cytarabine was added to the consolidation therapy administered in the LPA99 trial, which was based on the combination of ATRA and anthracycline/anthraquinone monochemotherapy. Taken together, these results allow us to speculate on a possible supra-additive effect of the combination of ATRA plus cytarabine that might support the improvement observed in high-risk patients. Interestingly, in vitro studies with human NB4 promyelocytic leukemia cells have showed an increased sensitivity to cytarabine after treatment with ATRA.18 In these experiments, the combination effect was supra-additive, with a maximum cytotoxicity and potency of cytarabine administration when it was closely followed by ATRA administration. In addition to the improved relapse rate observed in the present study, we also noticed an apparent reduction in the molecular persistence rate at the end of consolidation therapy in the LPA2005 (0 of 320 patients assessed) compared with the LPA99 trial (3 of 444). It should be noted that considering both the LPA99 and LPA2005 trials together, 3 of 764 patients assessed had molecular persistence at the end of consolidation therapy, this finding being significantly lower than in the LPA96 study (5 of 147 patients assessed, P = .002; data not shown). Besides the improvement in the antileukemic efficacy, as expected, the cytarabine enriched schedule increased the hematologic toxicities compared with the LPA99. However, both a high protocol compliance (97%) and a low death rate during consolidation therapy were observed in the LPA2005 trial, even when high-risk patients receiving cytarabine were analyzed separately (3 deaths of 85 patients, data not shown).
In conclusion, following a risk-adapted strategy, the LPA2005 trial provided an overall improvement of results in APL patients by means of specific changes in consolidation therapy. A decrease in dose intensity of mitoxantrone in low- and intermediate-risk patients resulted in a significant reduction of hematologic toxicities and hospital stay without compromising the antileukemic activity. Furthermore, the combination of ATRA, idarubicin, and cytarabine in consolidation therapy for high-risk APL patients demonstrated a striking antileukemic efficacy coupled with a significant but tolerable increase of toxicity. Once the use of arsenic trioxide to reinforce consolidation therapy in standard ATRA plus chemotherapy regimens has been supported by a large randomized study by the US Intergroup,19 future studies should address the role of this agent in front-line therapy. These studies may establish whether arsenic trioxide can permit a reduction of chemotherapy intensity while maintaining cure rates or confirm the improvement on outcomes currently achieved with optimal ATRA and anthracycline-based protocols.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Leticia Llopis for data collection and management.
This study was supported in part by the Fundación para la Investigación Hospital Universitario La Fe-Ayudas Bancaja (grant 2006/0137) and Red Temática de Investigación Cooperativa en Cáncer (RD06/0020/0031).
Authorship
Contribution: M.A.S. and P.M. conceived the study and analyzed and interpreted the data; M.A.S., P.M., and B.L. wrote the paper; P.M. performed the statistical analyses; and C.R., A.H., J.d.l.S., G.M., E.d.L., S.B., V.R., J.M.R., C.R., I.K., J.B., J.G., J.D.-M., R.R., F.M., G.O., and J.D.G. included data of patients treated in their institutions, reviewed the manuscript, and contributed to the final draft.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
For a complete list of PETHEMA and HOVON participants, see the supplemental Appendix.
Correspondence: Dr Miguel A. Sanz, Hospital Universitario La Fe Avenida Campanar 21, Valencia, Spain 46009; e-mail: msanz@uv.es.