Abstract
The 2 related basic helix loop helix genes, LYL1 and TAL-1 are active in hematopoietic and endothelial lineages. While Tal-1 is essential for both hematopoietic and vascular development, the role of Lyl1 appears to be distinct as deficient mice are viable and display modest hematopoietic defects. Here, we reveal a role for Lyl1 as a major regulator of adult neovascularization. Tumors implanted into Lyl1-deficient mice showed higher proliferation and angiogenesis, as evidenced by enlarged lumens, reduced pericyte coverage and increased permeability, compared with wild type littermates. Of note, Lyl1-deficient tumor vessels exhibited an up-regulation of Tal-1, the VE-Cadherin target gene, as well as Angiopoietin-2, 3 major actors in angiogenesis. Hematopoietic reconstitution experiments demonstrated that this sustained tumor angiogenesis was of endothelial origin. Moreover, the angiogenic phenotype observed in the absence of Lyl1 function was not tumor-restricted as microvessels forming in Matrigel or originating from aortic explants were also more numerous and larger than their wild-type counterparts. Finally, LYL1 depletion in human endothelial cells revealed that LYL1 controls the expression of molecules involved in the stabilization of vascular structures. Together, our data show a role for LYL1 in the postnatal maturation of newly formed blood vessels.
Introduction
The vascular system through development and adulthood answers to injury and remodeling and is one of the key systems sustaining normal physiology. Conversely, its deregulation also underlies multiple pathologic processes such as ischemia, inflammation and tumor. Angiogenesis normally occurs as a sequential series of morphogenetic events resulting in a functional network of vessels. Regulation of endothelial cell function in all these processes requires the integration of complex signals by the coordinated action of specific transcription factors.
LYL1 is a member of the large basic helix loop helix (bHLH) family, and is closely related to TAL-1 (also called SCL). Both LYL1 and TAL-1 were identified through their involvement in chromosomal rearrangements in human T-cell leukemia (reviewed in Baer1 ). Tal-1 deficiency causes embryonic lethality due to the absence of hematopoietic cell formation2-5 and several studies have shown that Tal-1 is also involved in the formation of the vascular system.6-10 Unlike Tal-1, Lyl1 is not essential for developmental processes, since homozygous disruption of Lyl1 activity induces only a mild hematopoietic phenotype.11 The viable Lyl1-deficient mice display a reduced number of B cells and impaired long-term hematopoietic reconstitution capacity.11 The highly conserved bHLH motifs between TAL-1 and LYL1 are functionally interchangeable to restore immature hematopoiesis in Tal-1 deficient embryonic stem cells.4,12 Moreover, Tal-1 and Lyl1 display redundant activities to maintain adult hematopoietic stem cell functions.13 However, full-length LYL1 cannot rescue early lethality of embryos lacking Tal-1,4,14 indicating that domains other than the bHLH exert nonredundant functions. We reported that LYL1 but not TAL-1, interacts with p105-NFKappaB1, and that its ectopic expression in human Jurkat T cells reduces NF-KappaB1-dependent transcription.15 Moreover, the N-terminal domain of LYL1, which completely differs from TAL-1, mediates functional interactions with the ubiquitous transcription factors CREB-1 and activates transcription of CREB-1 targets in human K562 erythroid cells.16
A recent study conducted in mouse embryos revealed highly overlapping expression of Lyl1 and Tal-1 not only in hematopoietic ontogeny but also in the developing vasculature and endocardium.17 In adults, TAL-1 expression is undetectable in quiescent endothelium, but is present in newly formed blood vessels,18,19 including vascular proliferations and tumor lymphatic vessels.20,21 We previously reported the requirement of TAL-1 for adult endothelial morphogenesis, where it activates the expression of VE-Cadherin, the major constituent of endothelial adherens junctions.22,23
The expression and the role of LYL1 in adult endothelium are still undefined. We show here that, in contrast to TAL-1, LYL1 is expressed in both angiogenic and mature adult endothelium. Loss of function reveals that Lyl1 is not required for the morphogenetic events leading to endothelial tube formation, but for the maturation and stabilization of newly formed blood vessels in adult mice. At the molecular level, we provide evidence that LYL1 controls the expression of several molecules, involved in the formation and/or the tightening of endothelial adherens junctions. Together, our data suggest a model in which TAL-1 and LYL1 act in a coordinated manner in adult angiogenesis to control lumen formation and vessel maturation, respectively.
Methods
Cell cultures
Primary endothelial cells (ECs) from human umbilical vein ECs (HUVECs), obtained from Clonetics (Lonza) and the immortalized human endothelial cell line hTERT-124 obtained from the United States Centers for Disease Control, were cultured in complete endothelial cell growth medium–2 microvascular (Lonza) with 3% fetal calf serum (FCS; HyClone, Perbio Science). Lewis Lung Carcinoma (LLC) cells or B16-F10 melanoma cells were cultured in Dulbecco Modified Eagle Medium containing 10% FCS.
In vitro quiescence assay
HUVECs (1 × 106) were allowed to grow to confluence onto 60-mm dishes in complete EGM2 medium containing the angiogenic growth factors vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF). After 48 hours, medium was replaced by either EGM2 complete medium, or basal EBM2 medium supplemented with 5% FCS (angiogenic factor starvation). After an additional 24 hours of culture, total RNA was prepared to analyze LYL1 and TAL-1 mRNA expression.
In vitro tubulogenesis assays
Individual HUVECs in basal medium containing 1% FCS were seeded into 1% collagen matrix as described,23 and activated by VEGF and bFGF (20 ng/mL) and Phorbol-12 Myristate 13-acetate (PMA; 80nM) to promote tubulogenesis.
siRNA transfections
Small interfering RNA (siRNA) were transfected in HUVECs as described.23 The sequences of duplex RNAs are presented in supplemental Table 1 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Lentivirus production and endothelial cell transductions are described in supplemental Methods.
RNA preparation, and real-time polymerase chain reaction analysis
Mouse tissues or tumors were lyzed in Trizol (Invitrogen), snap-frozen in liquid nitrogen and kept at −80°C until used. The PureLink Micro-to-Midi Total RNA Purification System (Invitrogen) was used to extract total RNA from cultured cells following the manufacturer's instructions. RNA were primed with oligo(dT) and reverse transcribed with SuperScript II (Invitrogen). Polymerase chain reaction (PCR) primer sequences are shown in supplemental Table 2.
Western blot analysis
Rap1 activity assays
Rap1 activity was measured using the Rap1 activation assay kit (Millipore) following manufacturer's instructions. Briefly, guanosine-5′-triphosphate (GTP)–bound Rap1 was pulled down using RalGDS-Rap binding domain fused to glutathione-S-tranferase (GST) and immobilized to glutathione agarose beads. Precipitated GTP-bound Rap1 and total Rap1 were detected by immunoblotting with a polyclonal anti-Rap1.
Animals
C57BL/6 Lyl1-deficient mice (ΔLyl1/ΔLyl1), previously described,11 were maintained under pathogen-free conditions in our animal facility. All experiments were conducted by authorized personnel (agreements 34-308 for N.P., 34-217 for V.D. and 34-368 for V.P.) and approved by the Institutional Review Board at the Animal Facility of the Institut de Génétique Molécular de Montpellier. Mice genotypes were determined by PCR in the presence of 1M Betain, 1.3% dimethyl sulfoxide and 0.5μM of each primer (supplemental Figure 3).
Tumor implantation
LLC cells (2.5 × 105) kindly given by Philippe Huber (Commissariat à l'Énergie Atomique, Grenoble, France) or B16-F10 melanoma cells (1 × 106) were injected subcutaneously into the flank of 8-week-old mice. Mice were killed before tumor reached 10% of the total weight of the animal (≈2000 mm3 ). Tumors were snap-frozen in Tissue-Tek OCT embedding matrix (CML) and stored at −80°C until they were processed for histologic analysis.
Bone marrow and fetal liver transplantation experiments
For bone marrow (BM) transplantation, C57Bl/6 (Ly 5.1) male mice were used as recipients, whereas BM cells were prepared from C57Bl/6 (Ly 5.2) wild-type (WT) or ΔLyl1/ΔLyl1 donors. BM cells were harvested from both femurs and tibias by repeat flushing of the bone cavity with phosphate buffer saline (PBS) supplemented with 2% fetal bovine serum and passage through a 70-μm nylon cell strainer. For fetal liver (FL) transplantation, C57Bl/6 (Ly 5.2) WT or Lyl1-deficient mice were used as recipients, whereas FL cells were obtained from day 14.5 postcoitus C57Bl/6 (Ly 5.1) embryos by mashing excised livers. Recipients were lethally irradiated with a unique 9.5-Gy dose and underwent transplantation with 2 × 106 BM cells or 4 × 106 FL cells, injected trough the retro-orbital sinus. Hematopoietic reconstitution after BM and FL cell injection was assessed 8 and 6 weeks after transplantation, respectively, through analysis of peripheral blood. To monitor engraftment, peripheral blood mononuclear cells (PBMCs) were double-stained with anti-CD45.2 (Ly5.2) FITC and anti-CD45.1 (Ly5.1) PE before analyzed by flow cytometry.
Immunohistochemistry
Cryostat sections were fixed in acetone/methanol at −20°C for 15 minutes, rehydrated in PBS, and incubated in 3% H2O2 solution for 7 minutes at room temperature. Nonspecific binding of antibody was blocked by incubation with PBS-20% normal horse serum for 30 minutes. Endogenous biotins were blocked using the Avidin-Biotin Blocking kit (Vector Laboratories). Sections were stained for 1 hour with the rat anti–mouse CD31/PECAM-1 mAb (1/250; clone MEC 13.3; BD Biosciences). Sections were incubated 45 minutes with biotinylated anti–rat Immunoglobulin Gs (IgGs; Vector Laboratories), washed, incubated with Extravidin Peroxidase, and revealed with 3-amino-9-ethylcarbazole (Sigma-Aldrich).
Immunofluorescence
Fixed cryostat sections were double-stained with the rat anti–mouse CD31 mAb, a rabbit anti–mouse NG2 polyclonal antibody (1/200; Millipore), or a mouse anti–mouse α-SMA mAb (1/50; R&D Systems). Primary antibodies were detected by a chicken anti–rat IgG Alexa 488, a donkey anti–rabbit IgG Alexa 555, and a chicken anti–mouse IgG Alexa 647, respectively (Invitrogen).
For TAL-1 immunostaining, purified 2TL136 anti–TAL-1 mAb19 was labeled using the Dylight 649 antibody labeling kit (Perbio Science). Frozen sections were fixed with PBS containing 4% formaldehyde, permeabilized with 0.5% Triton, and stained with labeled anti–TAL-1 mAb (5μg/mL). Spleen cryosections were double-stained with a rat anti–mouse CD11b/CD18 mAb (1/200; Mac1, Millipore) and the anti–TAL-1 mAb. Nuclei were stained with 10 μg/mL of 4′,6 diamidino-2-phenylinolole dihydrochloride (Sigma-Aldrich).
VE-Cadherin immunostaining of hTERT1 cells was performed as described23 using the Cadherin-5 mAb (1/125; Transduction Laboratories) and a chicken anti–mouse IgG Alexa 647 (Invitrogen). To visualize the fluorescence, we used a DM6000 Leica microscope, and images were acquired with Metamorph software (Molecular Devices) at room temperature.
Extravasation of albumin-Evans blue within tumor tissues
Mice bearing tumors between 200 and 500 mm3 were injected with 20 mg/kg Evans blue dye (EBD; Sigma-Aldrich) via the tail vein. Thirty minutes after injection, animals were anesthetized by intraperitoneal injection of 2 mg/kg bodyweight xylazine (Rompun 2%; Bayer Pharma) and 50 mg/kg bodyweight ketamin (Imalgène 500; Merial) and the chest was opened. Mice were transcardially perfused with 50 mL of PBS-EDTA 5mM to remove excess of EBD. Tumors were excised, dried, weighed, and placed in formamide at 60°C for 36 hours. EBD in supernatants was quantified by spectrophotometry at 630 nm.
In vivo Matrigel plug assays
WT/ΔLyl1 and ΔLyl1/ΔLyl1 9- to 12-week-old mice were subcutaneously implanted with Matrigel supplemented with bFGF and heparin as described,22 and killed 10 or 11 days after implantation. Matrigel plugs were photographed and treated for histology. Matrigel plug sections (20 μm) were probed with anti-CD31 antibody as described in “Immunochemistry,” revealed with 3-amino-9-ethylcarbazole and counterstained with hematoxylin.
Ex vivo aortic ring cultures
Mouse aortic-ring cultures were essentially prepared as reported.26 The aortic rings were embedded in rat tail collagen gel and cultured at 37°C and 5% CO2 in MCDB131 medium with 25mM NaHCO3 and 2% autologous serum.
Statistical analysis
Differences between experimental groups were analyzed by unpaired Student t test. P less than .05 was considered statistically significant.
Results
Lyl1 is highly expressed in endothelium-rich adult tissues
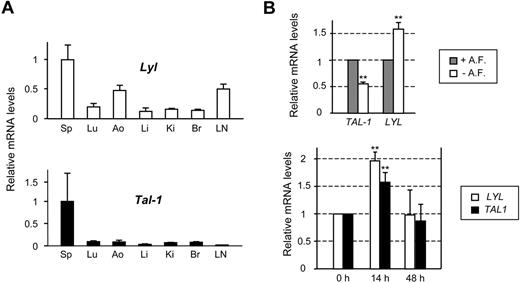
We compared Lyl1 and Tal-1 expression in several tissues derived from C57BL/6 adult mice. Due to the absence of an anti-LYL1 antibody suitable for immunostaining or immunoblotting, this analysis was performed at the mRNA levels by quantitative RT-PCR (qRT-PCR). As expected, strong Lyl1 and Tal-1 expression was detected in the spleen, in keeping with the presence of numerous hematopoietic cells (Figure 1A). Lyl1 expression was observed in all other tissues tested and more particularly in endothelium-rich tissues such as aorta and lymph nodes, whereas Tal-1 mRNAs were barely detected. These data suggested that, unlike Tal-1, Lyl1 is active in mature quiescent endothelium.
LYL1 is expressed in both angiogenic and quiescent adult endothelial cells. (A) Analysis of Lyl1 and Tal-1 mRNA in mouse adult tissues by qRT-PCR. Total RNA was extracted from different tissues derived from 4-week-old C57BL/6 mice (3 animals). cDNAs were amplified in triplicate by specific murine Lyl1 or Tal-1 primers and normalized to beta-Actin; means ± SD are shown. Sp indicates spleen; Lu, lung; Ao, aorta; Li, liver; Ki, kidney; Br, brain; and LN, lymph nodes. mRNA levels in spleen were arbitrarily set at 1. (B top) LYL1 expression in quiescent conditions: confluent HUVECs were maintained in either complete medium containing angiogenic factors (+A. F.) or starved in basal medium supplemented by 5% of FCS (− A. F.) for additional 24 hours. (Bottom) LYL1 expression in angiogenic conditions. Exponentially growing HUVECs were seeded into collagen I matrix and activated by the addition of PMA, VEGF, and bFGF. Total RNAs were extracted at the indicated time points. LYL1 and TAL-1 mRNA levels were assessed by qRT-PCR. cDNAs were amplified in triplicate by specific human LYL1 or TAL-1 primers and normalized to GAPDH. Each bar is the mean ± SD of mRNA levels relative to proliferating cells (set at 1) from 3 independent experiments performed in triplicate. **P < .01
LYL1 is expressed in both angiogenic and quiescent adult endothelial cells. (A) Analysis of Lyl1 and Tal-1 mRNA in mouse adult tissues by qRT-PCR. Total RNA was extracted from different tissues derived from 4-week-old C57BL/6 mice (3 animals). cDNAs were amplified in triplicate by specific murine Lyl1 or Tal-1 primers and normalized to beta-Actin; means ± SD are shown. Sp indicates spleen; Lu, lung; Ao, aorta; Li, liver; Ki, kidney; Br, brain; and LN, lymph nodes. mRNA levels in spleen were arbitrarily set at 1. (B top) LYL1 expression in quiescent conditions: confluent HUVECs were maintained in either complete medium containing angiogenic factors (+A. F.) or starved in basal medium supplemented by 5% of FCS (− A. F.) for additional 24 hours. (Bottom) LYL1 expression in angiogenic conditions. Exponentially growing HUVECs were seeded into collagen I matrix and activated by the addition of PMA, VEGF, and bFGF. Total RNAs were extracted at the indicated time points. LYL1 and TAL-1 mRNA levels were assessed by qRT-PCR. cDNAs were amplified in triplicate by specific human LYL1 or TAL-1 primers and normalized to GAPDH. Each bar is the mean ± SD of mRNA levels relative to proliferating cells (set at 1) from 3 independent experiments performed in triplicate. **P < .01
LYL1 expression occurred in both angiogenic and quiescent endothelial cells
We then assessed whether LYL1 expression was modulated in HUVECs according to culture conditions that recapitulate proliferation, quiescence or angiogenesis. To reproduce the quiescence of endothelium, confluent HUVECs were starved of angiogenic growth factors for 24 hours, in the presence of serum (5%) to avoid apoptosis. In these conditions, ECs tightly contacted each other and stopped to proliferate. As expected, HUVECs undergoing quiescence displayed a 50% decrease in TAL-1 mRNA levels, that we previously associated with a strong reduction in TAL-1 protein levels.22 In contrast, LYL1 mRNA levels were significantly increased (∼ 1.6-fold, Figure 1B top).
The activation of ECs seeded within a 3-dimensional collagen matrix by VEGF, bFGF, and PMA induced angiogenic events leading to tubular structure formation.27 As observed for TAL-1, LYL1 mRNA levels were always present in these angiogenic conditions, with a significant up-regulation within the first 24 hours of tubulogenesis (Figure 1B bottom). Together these data show that LYL1 is always active in endothelial cells, independently of their activated or quiescent phenotype.
Acceleration of tumor growth in Lyl1-deficient mice
Homozygous genetic Lyl1 disruption in mice does not produce developmental abnormalities, but moderate hematopoietic defects.11,13,14 Adult Lyl1-deficient mice are healthy and do not show obvious vascular defects. We took advantage of this mild phenotype to investigate the biologic role of Lyl1 in vivo in adult angiogenesis, using tumors as a model of neovascularization. Syngeneic LLC cells were subcutaneously implanted in WT, heterozygous (WT/ΔLyl1), and Lyl1-deficient (ΔLyl1/ΔLyl1) mice. Tumors grew at a similar rate in WT and WT/ΔLyl1 mice, but faster in ΔLyl1/ΔLyl1 mice resulting in larger tumors at all time points from day 15 to day 21 (Figure 2A). A similar acceleration of tumor growth was observed with B16-F10 melanoma cells implanted in ΔLyl1/ΔLyl1 mice, compared with WT mice.
Accelerated tumor growth and immature tumor blood vessels in Lyl1-deficient mice. (A) Accelerated growth of syngeneic tumors in ΔLyl1/ΔLyl1 mice. (Left) Palpable tumors were measured every day using a caliper. The tumor volumes (in cubic millimeters) were calculated according to the formula: volume = Pi/6 × (width)2 × (length). Shown is the volume of LLC tumors implanted in ΔLyl1/ΔLyl1 and in their WT and WT/ΔLyl1 littermates as a function of time. Error bars represent means ± SEM, WT (n = 7); WT/ΔLyl1 (n = 7); ΔLyl1/ΔLyl1 (n = 8). **P < .01. Data are representative of 4 separate experiments. (Right) Shown is the volume of B16-F10 tumors implanted in ΔLyl1/ΔLyl1 and in their WT littermates as a function of time. Error bars represent means ± SEM, WT (n = 9); ΔLyl1/ΔLyl1 (n = 6). *P < .05. (B) Enlargement of tumor blood vessels in ΔLyl1/ΔLyl1 mice. The entire surfaces of CD31-stained sections of LLC tumor from ΔLyl1/ΔLyl1 and from their WT and WT/ΔLyl1 littermates were visualized with a nanozoomer slide scanner controlled by the NDP.View software. (Left) Representative microscopy images of CD31 immunostaining of tumor sections showing enlargement of tumor blood vessels in Lyl1-deficient mice. Scale bar: 100 μm. (Right) Distribution of tumor blood vessels according to their Ferret diameter in WT, WT/ΔLyl1 and ΔLyl1/ΔLyl1 mice. 80 to 200 open vessels were selected per tumor section stained with anti-CD31 and their Ferret diameter was calculated using ImageJ software (National Institutes of Health). For each section, selected vessels were ranked according to their diameter and the following distribution was established: < 30 μm, 30-70 μm, and > 70 μm. Analyzed tumors: WT (n = 7), WT/ΔLyl1 (n = 6), ΔLyl1/ΔLyl1 (n = 9). **P < .01, using the χ2 test. (C) Reduced pericyte coverage of tumor blood vessels in ΔLyl1/ΔLyl1 mice. (Left) Microscopy images illustrating the severe reduction of pericyte coverage of tumor blood vessels from ΔLyl1/ΔLyl1 mice. LLC tumor sections were double-stained for the endothelial cell marker CD31 (green) and for the pericyte marker NG2 (red). Scale bar: 100 μm. (Right) The extent of vessel coverage by pericytes was determined on 4 to 6 random fields by measuring, with ImageJ software, the proportion of CD31-positive vessels covered by NG2- or α-SMA–immunoreactive cells. NG2 coverage was calculated as the percentage of NG2-positive vessels compared with the number of CD31-positive vessels. The data are presented as the mean ± SEM. Analyzed individual tumors: WT (n = 7), WT/ΔLyl1 (n = 8); ΔLyl1/ΔLyl1 (n = 7). ***P < .001. Tumor sections were double-stained for the endothelial cell marker CD31 and for the pericyte marker α-SMA (see supplemental Figures 2-3). α-SMA coverage was calculated as the percentage of α-SMA–positive vessels compared with the number of CD31-positive vessels. The data are presented as the mean ± SEM. Analyzed individual tumors: WT (n = 4), ΔLyl1/ΔLyl1 (n = 6). ***P < .001. (D) Increased vascular permeability of tumors in ΔLyl1/ΔLyl1 mice ΔLyl1/ΔLyl1 and WT/ΔLyl1 mice with LLC tumor sizes between 200 and 500 mm3 were used to measure tumor vascular permeability by Evans blue extravasation as an index of albumin leakage. Evans blue diffused within tumor was quantified at 630 nm. The amount of EBD (micrograms per gram of dry weight) was calculated from standard curve to determine the concentration of extravasated dye in the tumor. The mean is represented as a black bar on this scatter plot. Each point represents data for a separate mouse: WT/ΔLyl1 (n = 7), ΔLyl1/ΔLyl1 (n = 6). *P < .05.
Accelerated tumor growth and immature tumor blood vessels in Lyl1-deficient mice. (A) Accelerated growth of syngeneic tumors in ΔLyl1/ΔLyl1 mice. (Left) Palpable tumors were measured every day using a caliper. The tumor volumes (in cubic millimeters) were calculated according to the formula: volume = Pi/6 × (width)2 × (length). Shown is the volume of LLC tumors implanted in ΔLyl1/ΔLyl1 and in their WT and WT/ΔLyl1 littermates as a function of time. Error bars represent means ± SEM, WT (n = 7); WT/ΔLyl1 (n = 7); ΔLyl1/ΔLyl1 (n = 8). **P < .01. Data are representative of 4 separate experiments. (Right) Shown is the volume of B16-F10 tumors implanted in ΔLyl1/ΔLyl1 and in their WT littermates as a function of time. Error bars represent means ± SEM, WT (n = 9); ΔLyl1/ΔLyl1 (n = 6). *P < .05. (B) Enlargement of tumor blood vessels in ΔLyl1/ΔLyl1 mice. The entire surfaces of CD31-stained sections of LLC tumor from ΔLyl1/ΔLyl1 and from their WT and WT/ΔLyl1 littermates were visualized with a nanozoomer slide scanner controlled by the NDP.View software. (Left) Representative microscopy images of CD31 immunostaining of tumor sections showing enlargement of tumor blood vessels in Lyl1-deficient mice. Scale bar: 100 μm. (Right) Distribution of tumor blood vessels according to their Ferret diameter in WT, WT/ΔLyl1 and ΔLyl1/ΔLyl1 mice. 80 to 200 open vessels were selected per tumor section stained with anti-CD31 and their Ferret diameter was calculated using ImageJ software (National Institutes of Health). For each section, selected vessels were ranked according to their diameter and the following distribution was established: < 30 μm, 30-70 μm, and > 70 μm. Analyzed tumors: WT (n = 7), WT/ΔLyl1 (n = 6), ΔLyl1/ΔLyl1 (n = 9). **P < .01, using the χ2 test. (C) Reduced pericyte coverage of tumor blood vessels in ΔLyl1/ΔLyl1 mice. (Left) Microscopy images illustrating the severe reduction of pericyte coverage of tumor blood vessels from ΔLyl1/ΔLyl1 mice. LLC tumor sections were double-stained for the endothelial cell marker CD31 (green) and for the pericyte marker NG2 (red). Scale bar: 100 μm. (Right) The extent of vessel coverage by pericytes was determined on 4 to 6 random fields by measuring, with ImageJ software, the proportion of CD31-positive vessels covered by NG2- or α-SMA–immunoreactive cells. NG2 coverage was calculated as the percentage of NG2-positive vessels compared with the number of CD31-positive vessels. The data are presented as the mean ± SEM. Analyzed individual tumors: WT (n = 7), WT/ΔLyl1 (n = 8); ΔLyl1/ΔLyl1 (n = 7). ***P < .001. Tumor sections were double-stained for the endothelial cell marker CD31 and for the pericyte marker α-SMA (see supplemental Figures 2-3). α-SMA coverage was calculated as the percentage of α-SMA–positive vessels compared with the number of CD31-positive vessels. The data are presented as the mean ± SEM. Analyzed individual tumors: WT (n = 4), ΔLyl1/ΔLyl1 (n = 6). ***P < .001. (D) Increased vascular permeability of tumors in ΔLyl1/ΔLyl1 mice ΔLyl1/ΔLyl1 and WT/ΔLyl1 mice with LLC tumor sizes between 200 and 500 mm3 were used to measure tumor vascular permeability by Evans blue extravasation as an index of albumin leakage. Evans blue diffused within tumor was quantified at 630 nm. The amount of EBD (micrograms per gram of dry weight) was calculated from standard curve to determine the concentration of extravasated dye in the tumor. The mean is represented as a black bar on this scatter plot. Each point represents data for a separate mouse: WT/ΔLyl1 (n = 7), ΔLyl1/ΔLyl1 (n = 6). *P < .05.
Tumor blood vessels in Lyl1-deficient mice display enlarged diameters
We then investigated the mechanisms responsible for increased tumor growth in mice lacking Lyl1 by carrying out histologic analysis of tumor vascularization. Tumor sections were stained with an anti-CD31/PECAM antibody to reveal blood vessel infiltration. There was no difference in global CD31-staining, when expressed as a percentage of tumor area between WT, WT/ΔLyl1 and ΔLyl1/ΔLyl1 mice (supplemental Figure 1A). However, a more precise examination revealed frequent enlarged CD31-positive structures in ΔLyl1/ΔLyl1 tumors but not in WT and WT/ΔLyl1 (Figure 2B and supplemental Figures 2-3). Quantitative analysis of vessel diameter was performed and showed a significant increase in the number of large vessels (diameter > 70μm) in ΔLyl1/ΔLyl1 mice compared with WT and WT/ΔLyl1 mice (18.6% vs 6.8% and 5.4%, respectively). Conversely, small-size vessels (diameter < 30μm) were more abundant in tumors induced in WT and WT/ΔLyl1 mice than in ΔLyl1/ΔLyl1 mice (67.4%, 66.1%, and 39.7%, respectively). This analysis shows that Lyl1-deficiency leads to a general enlargement of vascular structures in LLC tumors.
Tumor blood vessels in Lyl1-deficient mice are poorly covered and leaky
Newly formed endothelial tubes are initially unstable and subsequently become stabilized through the formation of a perivascular matrix and the association with pericytes.28 To evaluate vessel coverage in the tumors, tumor sections were double-stained with anti-CD31 and an anti-NG2 antibody that recognizes pericytes. As reported,29,30 microvessels of LLC tumors grown in WT mice were nicely covered by mural cells, because a large majority (80% ± 14%) of CD31-structures were also stained with NG2 (Figure 2C and supplemental Figure 2). Tumor vessels from WT/ΔLyl1 mice displayed similar NG2-coverage (72.8% ± 11.2). In contrast, tumor vessels from ΔLyl1/ΔLyl1 mice were significantly less covered (33.9% ± 10.2) by NG2-positive mural cells. We also performed the analysis of α-SMA marker (supplemental Figure 2), because it has been described to reflect a later stage of mural cell recruitment.30 56% CD31-positive vessels in tumors from WT mice were covered with α-SMA-positive mural cells, while only 12% CD31-positive tumor vessels from Lyl1-deficient mice were covered with α-SMA-positive mural cells (Figure 2C). Similarly, vessels of B16-F10 tumors arising in ΔLyl1/ΔLyl1 mice were poorly covered, compared with WT (supplemental Figure 3). Altogether, these studies indicate that Lyl1-deficiency leads to a strong reduction in the coverage of tumor blood vessels by mural cells.
Vascular permeability has been correlated to reduced mural cell recruitment to blood vessels in different pathologies including tumors.31 We therefore addressed whether the reduced recruitment of pericytes to tumor microvessels in mice lacking Lyl1 might affect tumor vascular permeability. As shown in Figure 2D, ΔLyl1/ΔLyl1 LLC tumors displayed a 2.4-fold increase in Evans blue extravasation compared with WT/ΔLyl1 mice (192.8 μg/g vs 80μg/g tumor dry weight, respectively). Altogether, these data strongly suggest that reduced pericyte coverage of tumor vessels might be at the origin of increased vascular tumor leakage observed in ΔLyl1/ΔLyl1 mice.
TAL-1, the VE-cadherin target gene and Angiopoietin-2 are up regulated in tumor vasculature of Lyl1-deficient mice
Given that TAL-1 expression is limited to developing angiogenic vessels,18-20 and given the immature features of tumor vasculature in Lyl1-deficient mice (Figure 2), we investigated Tal-1 expression in endothelial cells of LLC tumors arising in the 3 groups. For each piece of tumor tested, Tal-1 mRNA levels were expressed relative to CD31-PECAM mRNA levels used here as an endothelial-specific gene marker. There was no significant difference in CD31 mRNA expression between the 3 groups in agreement with similar global CD31-immunostaining of the tumors (supplemental Figure 1). In contrast, Tal-1 mRNA expression was significantly up regulated (∼ 2-fold) in ΔLyl1/ ΔLyl1 tumors compared with WT and WT/ ΔLyl1 tumors (Figure 3A). Immunofluorescence analysis confirmed specific TAL-1 protein expression in the nucleus of ECs boarding the blood vessels of ΔLyl1/ ΔLyl1 mice tumors (Figure 3A), but not of WT mice tumors in agreement with a previous study.21 Together, these data demonstrated that Tal-1 expression was sustained in tumor endothelial cells of Lyl1-deficient mice.
Up regulation of TAL-1, its VE-Cadherin target gene and Angiopoietin-2 in tumor vessels of Lyl1 -deficient mice. (A) Endothelial Tal-1 expression in LLC tumors implanted in ΔLyl1/ΔLyl1, WT/ΔLyl1 and WT mice. (Left) Total RNA was extracted from 1 or 2 pieces of tumors from mice of the 3 genotypes. Each point represents data for distinct piece of tumors: ΔLyl1/ΔLyl1 (14 pieces/9 tumors); WT/ΔLyl1 (7 pieces/5 tumors); WT (8 pieces/6 tumors). cDNAs were amplified in triplicate by specific Tal-1. Shown are normalized Tal-1 mRNA levels relative to CD31 mRNA levels (see supplemental Figure 1B) in each piece of tumor. The mean is represented as a black bar on this scatter plot. **P < .01; *P < .05. (Right) Immunofluorescence analysis of TAL-1 protein in LLC tumors from WT and ΔLyl1/ΔLyl1 mice. Representative microscopy images showing TAL-1 expression in the nucleus of endothelial cells boarding a blood vessel in a tumor of ΔLyl1/ΔLyl1 mice (white arrows), but not in tumor of their WT littermates. Tumor sections were double-stained for TAL-1 (red) and for blood vessel marker CD31 (green). Scale bar: 20 μm. (Bottom) TAL-1 expression in immature erythroid cells WT mice used here as positive control for TAL-1 staining. Spleen sections were double-stained for TAL-1 (red) and for the macrophage marker CD11b/CD18 (Mac1, green). Note the specific intense TAL-1 staining in the large nuclei of blast cells, but not in the smaller nuclei of lymphocytes or macrophages. Scale bar: 20 μm. (B) Total RNA was extracted from 1 or 2 pieces of tumors from mice of the 3 genotypes (as in panel A). cDNAs were amplified in triplicate by specific VE-cadherin or Ang-2 primers. VE-cadherin and Ang-2 mRNA levels were normalized to beta-Actin. Each bar is the mean ± SD of mRNA levels relative to mRNA levels of WT mice-derived, set arbitrarily at 1 ***P < .005; **P < .01
Up regulation of TAL-1, its VE-Cadherin target gene and Angiopoietin-2 in tumor vessels of Lyl1 -deficient mice. (A) Endothelial Tal-1 expression in LLC tumors implanted in ΔLyl1/ΔLyl1, WT/ΔLyl1 and WT mice. (Left) Total RNA was extracted from 1 or 2 pieces of tumors from mice of the 3 genotypes. Each point represents data for distinct piece of tumors: ΔLyl1/ΔLyl1 (14 pieces/9 tumors); WT/ΔLyl1 (7 pieces/5 tumors); WT (8 pieces/6 tumors). cDNAs were amplified in triplicate by specific Tal-1. Shown are normalized Tal-1 mRNA levels relative to CD31 mRNA levels (see supplemental Figure 1B) in each piece of tumor. The mean is represented as a black bar on this scatter plot. **P < .01; *P < .05. (Right) Immunofluorescence analysis of TAL-1 protein in LLC tumors from WT and ΔLyl1/ΔLyl1 mice. Representative microscopy images showing TAL-1 expression in the nucleus of endothelial cells boarding a blood vessel in a tumor of ΔLyl1/ΔLyl1 mice (white arrows), but not in tumor of their WT littermates. Tumor sections were double-stained for TAL-1 (red) and for blood vessel marker CD31 (green). Scale bar: 20 μm. (Bottom) TAL-1 expression in immature erythroid cells WT mice used here as positive control for TAL-1 staining. Spleen sections were double-stained for TAL-1 (red) and for the macrophage marker CD11b/CD18 (Mac1, green). Note the specific intense TAL-1 staining in the large nuclei of blast cells, but not in the smaller nuclei of lymphocytes or macrophages. Scale bar: 20 μm. (B) Total RNA was extracted from 1 or 2 pieces of tumors from mice of the 3 genotypes (as in panel A). cDNAs were amplified in triplicate by specific VE-cadherin or Ang-2 primers. VE-cadherin and Ang-2 mRNA levels were normalized to beta-Actin. Each bar is the mean ± SD of mRNA levels relative to mRNA levels of WT mice-derived, set arbitrarily at 1 ***P < .005; **P < .01
We previously reported that TAL-1 activates VE-cadherin expression.23 Moreover, Angiopoietin-2 up-expression is associated with angiogenic vessels. We therefore investigated the expression at the mRNA levels of both genes in LLC tumors grown in the 3 genotypes (Figure 3B). Both VE-cadherin and Ang-2 mRNA levels showed a similar increase (∼ 2-fold) in tumors from ΔLyl1/ΔLyl1 mice compared with WT mice tumors. Hence, in agreement with its angiogenic and immature features, tumor vasculature of Lyl1-deficient mice exhibits a concomitant up-regulation of TAL-1 and its target gene VE-cadherin, as well as Angiopoietin-2.
The hematopoietic defects of Lyl1-deficient mice are not responsible for increased tumor growth and immaturity of tumor blood vessels
LYL1 is active in several hematopoietic cell types, including B cells and immature myeloid cells32 that play important roles in tumor angiogenesis.33 Although the hematopoietic defects of ΔLyl1/ΔLyl1 mice are not severe,11 we could not exclude that they might affect directly tumor growth and angiogenesis. To evaluate this possibility, we carried out reciprocal transplantation of immature hematopoietic progenitors between WT and ΔLyl1/ΔLyl1 mice, and followed the growth of LLC tumors after hematopoietic reconstitution.
First, irradiated WT Ly5.1 recipients were transplanted with BM cells from either Ly5.2 WT or ΔLyl1/ΔLyl1 mice and subcutaneously injected with LLC cells after successful BM engraftment (8 weeks). LLC tumors grew at a similar rate in Ly5.1 mice transplanted with WT or Lyl1-deficient BM cells, and tumor vessels from both Ly5.1 transplanted mice had similar NG2 and α-SMA coverage (Figure 4A). These results show that ΔLyl1/ΔLyl1 BM cells cannot, by themselves, induce increased tumor growth associated with immature angiogenic vessels.
The hematopoietic defects of ΔLyl1/ΔLyl1 mice are not responsible for the accelerated tumor growth and immaturity of tumor blood vessels. (A top) Shown is the volume of LLC tumors implanted in C57Bl/6 Ly5.1 mice transplanted with bone marrow cells from ΔLyl1/ΔLyl1 or WT C57Bl/6 Ly5.2 mice, as a function of time. Eight weeks after transplantation, 86.9% (± 5.5%) and 77.4% (± 8.2%) of PBMCs were Ly5.2-positive in mice transplanted with BM cells from either WT or Lyl1-deficient mice, respectively. Error bars represent means ± SEM, WT (n = 9); ΔLyl1/ΔLyl1 (n = 7). (Bottom) Quantization of mural cell coverage of tumor blood vessels of WT mice transplanted either with WT or ΔLyl1/ΔLyl1 mice BM cells, by immunofluorescence with NG2, or α-SMA antibody. Vessel coverage was calculated as the percentage of NG2-, or α-SMA, positive vessels compared with the number of CD31-positive vessels. The data are presented as the mean ± SEM. Analyzed individual tumors: WT (n = 5); ΔLyl1/ΔLyl1 (n = 6); NG2 coverage: 70% (± 9.2%) and 72.2% (± 13.8%) for WT mice transplanted with WT and Lyl1-deficient BM cells, respectively; α-SMA coverage: 34.8% (± 5.8%) and 46.5% (± 9.5%) for WT mice transplanted with WT and Lyl1-deficient BM cells, respectively. (B top) Shown is the volume of LLC tumors implanted in C57Bl/6 Ly5.2 WT and ΔLyl1/ΔLyl1 mice transplanted with fetal liver cells from C57Bl/6 Ly5.1 WT mice, as a function of time. Six weeks after transplantation, 77.6% (± 9.1%) and 92.8% (± 1.9%) of PBMCs were Ly5.1-positive in Ly5.2 WT or Lyl1-deficient transplanted mice, respectively. Error bars represent means ± SEM, WT (n = 6); ΔLyl1/ΔLyl1 (n = 9). *P < .05. (Bottom) Quantization of mural cell coverage of tumor blood vessels of either WT or ΔLyl1/ΔLyl1 mice transplanted with WT fetal liver cells by immunofluorescence (as in panel A). The data are presented as the mean ± SEM. Analyzed individual tumors: WT (n = 6); ΔLyl1/ΔLyl (n = 6); NG2 coverage: 59.6% (± 7.8%) and 10.5% (± 4.3%) for WT and ΔLyl1/ΔLyl1 mice transplanted with WT FL cells, respectively. For α-SMA coverage: 32.4% (± 11.7%) and 5.6% (± 2.5%) for WT and ΔLyl1/ΔLyl1 mice transplanted with WT FL cells, respectively.
The hematopoietic defects of ΔLyl1/ΔLyl1 mice are not responsible for the accelerated tumor growth and immaturity of tumor blood vessels. (A top) Shown is the volume of LLC tumors implanted in C57Bl/6 Ly5.1 mice transplanted with bone marrow cells from ΔLyl1/ΔLyl1 or WT C57Bl/6 Ly5.2 mice, as a function of time. Eight weeks after transplantation, 86.9% (± 5.5%) and 77.4% (± 8.2%) of PBMCs were Ly5.2-positive in mice transplanted with BM cells from either WT or Lyl1-deficient mice, respectively. Error bars represent means ± SEM, WT (n = 9); ΔLyl1/ΔLyl1 (n = 7). (Bottom) Quantization of mural cell coverage of tumor blood vessels of WT mice transplanted either with WT or ΔLyl1/ΔLyl1 mice BM cells, by immunofluorescence with NG2, or α-SMA antibody. Vessel coverage was calculated as the percentage of NG2-, or α-SMA, positive vessels compared with the number of CD31-positive vessels. The data are presented as the mean ± SEM. Analyzed individual tumors: WT (n = 5); ΔLyl1/ΔLyl1 (n = 6); NG2 coverage: 70% (± 9.2%) and 72.2% (± 13.8%) for WT mice transplanted with WT and Lyl1-deficient BM cells, respectively; α-SMA coverage: 34.8% (± 5.8%) and 46.5% (± 9.5%) for WT mice transplanted with WT and Lyl1-deficient BM cells, respectively. (B top) Shown is the volume of LLC tumors implanted in C57Bl/6 Ly5.2 WT and ΔLyl1/ΔLyl1 mice transplanted with fetal liver cells from C57Bl/6 Ly5.1 WT mice, as a function of time. Six weeks after transplantation, 77.6% (± 9.1%) and 92.8% (± 1.9%) of PBMCs were Ly5.1-positive in Ly5.2 WT or Lyl1-deficient transplanted mice, respectively. Error bars represent means ± SEM, WT (n = 6); ΔLyl1/ΔLyl1 (n = 9). *P < .05. (Bottom) Quantization of mural cell coverage of tumor blood vessels of either WT or ΔLyl1/ΔLyl1 mice transplanted with WT fetal liver cells by immunofluorescence (as in panel A). The data are presented as the mean ± SEM. Analyzed individual tumors: WT (n = 6); ΔLyl1/ΔLyl (n = 6); NG2 coverage: 59.6% (± 7.8%) and 10.5% (± 4.3%) for WT and ΔLyl1/ΔLyl1 mice transplanted with WT FL cells, respectively. For α-SMA coverage: 32.4% (± 11.7%) and 5.6% (± 2.5%) for WT and ΔLyl1/ΔLyl1 mice transplanted with WT FL cells, respectively.
In a second set of experiments, irradiated Ly5.2 WT or Lyl1-deficient recipients were transplanted with day-14.5 FL cells from Ly5.1 WT mice and subcutaneously injected with LLC cells after successful peripheral hematopoietic reconstitution (6 weeks). Compared with WT mice, at days 15 and 16 after injection, ΔLyl1/ΔLyl1 mice transplanted with WT FL stem cells exhibited larger tumors, which were associated with a significant reduction of vessel coverage (Figure 4B, and supplemental Figure 4). Together, these results demonstrate that the increased tumor growth and angiogenesis observed in ΔLyl1/ΔLyl1 mice do not have a hematopoietic origin.
Increased angiogenesis and enlargement of blood vessels in Matrigel implanted in Lyl1-deficient mice
We then evaluated Lyl1 function in the well-characterized neovascularization model, Matrigel plug assays. At day 11, implants were photographed and processed for histologic studies. Macroscopic analysis showed that the majority of ΔLyl1/ΔLyl1 mice plugs displayed a strong angiogenic response compared with WT/ΔLyl1 plugs (see supplemental Figure 5). Histology of heterozygous mice implants showed the development of small caliber capillary structures, and the presence of red blood cells indicated that the neovessels were functionally perfused (Figure 5A). Although some ΔLyl1/ ΔLyl1 implants seemed to be similar at the macroscopic levels to WT/ ΔLyl1 implants, histology revealed great differences in the morphology of vascular structures. Blood vessels from ΔLyl1/ ΔLyl1 implants were highly dilated and in many cases were organized into large vascular cavities engorged with blood and often surrounded with red blood cells, indicative of vascular leakiness (supplemental Figure 6A). Of note, the features of these vascular structures were reminiscent to those developed in TAL-1 overexpressing Matrigel (supplemental Figure 6B) as we previously described.22
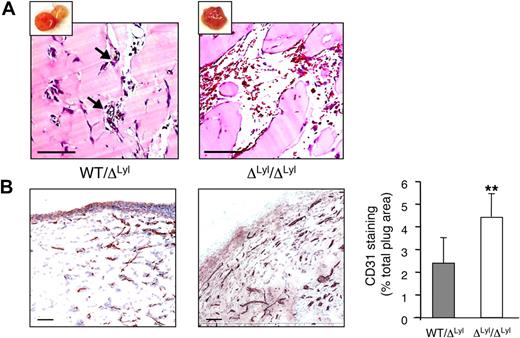
Increased angiogenesis in Matrigel plugs implanted in Lyl1-deficient mice. bFGF-containing Matrigel was subcutaneously implanted in both Lyl1-deficient and heterozygous mice (a total of 11 animals in each group in 2 experiments). Plugs were harvested at day 11, photographed (see supplemental Figure 4), and processed for histology. (A) Hematoxilin/Eosin staining of plug sections was visualized with a Nanozoomer slide scanner (Hamamatsu), and scanned images were analyzed with NDP.view software. Small insets show photograph of the corresponding Matrigel plug. Arrows indicate perfused small blood vessels in WT/ΔLyl1 plug section. Scale bars: 100 μm. (B) CD31 infiltration of Matrigel plugs: The entire surface of CD31-stained plug sections from ΔLyl1/ΔLyl1 and from their WT/ΔLyl1 littermates was visualized with a nanozoomer slide scanner controlled by the NDP.view software. (Left) Representative microscopy images of CD31 staining of plug sections from both genotypes. Scale bars: 100 μm. (Right) Entire plug sections were scanned and analyzed with ImageScope software (Aperio Technologies) using the “Positive Pixel count v9” algorithm. Shown is the quantification of CD31-staining expressed as a percentage of total plug surface. The data are presented as the mean ± SEM. Individual plugs from 2 independent experiments WT/ΔLyl1 (n = 7), ΔLyl1/ΔLyl1(n = 6), **P < .01.
Increased angiogenesis in Matrigel plugs implanted in Lyl1-deficient mice. bFGF-containing Matrigel was subcutaneously implanted in both Lyl1-deficient and heterozygous mice (a total of 11 animals in each group in 2 experiments). Plugs were harvested at day 11, photographed (see supplemental Figure 4), and processed for histology. (A) Hematoxilin/Eosin staining of plug sections was visualized with a Nanozoomer slide scanner (Hamamatsu), and scanned images were analyzed with NDP.view software. Small insets show photograph of the corresponding Matrigel plug. Arrows indicate perfused small blood vessels in WT/ΔLyl1 plug section. Scale bars: 100 μm. (B) CD31 infiltration of Matrigel plugs: The entire surface of CD31-stained plug sections from ΔLyl1/ΔLyl1 and from their WT/ΔLyl1 littermates was visualized with a nanozoomer slide scanner controlled by the NDP.view software. (Left) Representative microscopy images of CD31 staining of plug sections from both genotypes. Scale bars: 100 μm. (Right) Entire plug sections were scanned and analyzed with ImageScope software (Aperio Technologies) using the “Positive Pixel count v9” algorithm. Shown is the quantification of CD31-staining expressed as a percentage of total plug surface. The data are presented as the mean ± SEM. Individual plugs from 2 independent experiments WT/ΔLyl1 (n = 7), ΔLyl1/ΔLyl1(n = 6), **P < .01.
Plug sections were stained with the anti-CD31 antibody to reveal blood vessel infiltration (Figure 5B). Plugs that displayed large vascular cavities were excluded, because they were not suitable for immunohistochemistry studies. Matrigel implants from ΔLyl1/ ΔLyl1 mice showed a 2-fold increase in CD31-postive vessel infiltration compared with implants from WT/ ΔLyl1 mice. Thus, Lyl1-deficiency causes an increased angiogenic response in subcutaneous Matrigel implants.
Increased microvessel outgrowth from mice aortic explant of Lyl1-deficient mice
We further explored the role of LYL1 in normal angiogenesis using the ex vivo aortic ring assay, in which we compared the angiogenic potential of aortic fragments derived from WT and Lyl1-deficient mice. WT and ΔLyl1/ΔLyl1 aortic explants displayed similar outgrowth of myofibroblats (scattered cells, Figure 6A), most of them being α-SMA-positive cells (supplemental Figure 7). Both WT and ΔLyl1/ΔLyl1 aortic cultures developed new endothelial tubes that originated mostly from the 2 wounded edges (Figure 6A and rectilinear CD31-positive structures in supplemental Figure 7). Image analysis of individual rings was performed to count the number of branchings and to measure the total surface occupied by new endothelial tubes (Figure 6B). Microvessels developed from ΔLyl1/ΔLyl1 aortic explants were more numerous and larger than those originating from WT aorta. Altogether, these studies indicate that Lyl1-deficiency increases microvessel formation from aortic explants without affecting mural cell outgrowth.
Lyl1-deficiency increases angiogenic potential of mice aortic explant. (A) Photomicrographs show the angiogenic response of a 5-day culture collagen-embedded thoracic aorta ring explants from ΔLyl1/ΔLyl1 and wild-type mice. Note the outgrowth of scattered myelofibroblast cells (α-SMA-positive cells, supplemental Figure 6) and endothelial tubules (CD31-positive cells, supplemental Figure 6) in both genotypes. (B) Images of microvessel outgrowth from aortic explants were captured at day 5 of culture with a Canon Powershot A650 digital camera mounted on an inverted phase contrast Axiovert25 microscope (Zeiss). The angiogenic response was determined for each individual aortic ring explant by quantifying the number of intersections of growing microvessels (left) and by measuring the total area occupied by the newly formed microvessels (right). Each point represents data for distinct fragment of aorta: WT (20 rings/4 mice) ΔLyl1/ΔLyl1 (30 rings/6 mice). The mean is represented as a black bar on this scatter plot. *P < .05.
Lyl1-deficiency increases angiogenic potential of mice aortic explant. (A) Photomicrographs show the angiogenic response of a 5-day culture collagen-embedded thoracic aorta ring explants from ΔLyl1/ΔLyl1 and wild-type mice. Note the outgrowth of scattered myelofibroblast cells (α-SMA-positive cells, supplemental Figure 6) and endothelial tubules (CD31-positive cells, supplemental Figure 6) in both genotypes. (B) Images of microvessel outgrowth from aortic explants were captured at day 5 of culture with a Canon Powershot A650 digital camera mounted on an inverted phase contrast Axiovert25 microscope (Zeiss). The angiogenic response was determined for each individual aortic ring explant by quantifying the number of intersections of growing microvessels (left) and by measuring the total area occupied by the newly formed microvessels (right). Each point represents data for distinct fragment of aorta: WT (20 rings/4 mice) ΔLyl1/ΔLyl1 (30 rings/6 mice). The mean is represented as a black bar on this scatter plot. *P < .05.
LYL1-silencing reduces the expression of several molecules that contribute to maturation of endothelial adherens junctions
Our above results (Figure 4) showed that the immature phenotype of neovasculature in ΔLyl1/ΔLyl1 mice, that is, increased permeability and poor pericyte coverage, is the consequence of defects intrinsic to endothelial cells. To investigate the mechanisms by which LYL1 regulates EC phenotype, we evaluated the consequences of LYL1 depletion in human ECs. LYL1-silencing was mediated either by siRNA transfection (HUVECs) or by transduction of lentiviruses encoding shRNA (hTERT1). As expected from our above results (Figures 2B and 6A), LYL1-silencing did not affect endothelial tubulogenesis in 3-dimensional collagen matrix (not shown), confirming that LYL1 is not required for this process. The assembly and stabilization of endothelial junctions are determinant for the maturation of new vascular structures.34 To address whether LYL1 impacts the organization of adherens junctions, we compared the immunostaining with anti–VE-Cadherin of shCTL- and shLYL1-transduced hTERT1 cells (Figure 7A). Both cell populations showed clustering of VE-Cadherin at intercellular junctions. However, in LYL1-depleted cells, the staining was often discontinuous and many gaps were present between adjacent cells, compared with the continuous VE-Cadherin staining of CTL-hTERT1 cell perimeters.
LYL1 controls the expression of molecules involved in the formation and stabilization of adherens junctions. (A) LYL1-depletion affects the assembly and maturation of adherens junctions. hTERT1 cells were transduced with lentiviruses encoding either control shRNA or LYL1 shRNA, and puromycin-resistant cell populations were established. Confluent monolayers of the indicated population were immunostained with a VE-Cadherin antibody and vizualized with Alexa 647-conjugated secondary antibody. Note the continuous and regular staining on CTL-hTERT1 cell perimeters (arrows) and the presence of gaps between adjacent cells (arrowheads) leading to an irregular staining. (B) LYL1 depletion reduces constitutive activation of Rap1. Whole cell extract prepared from shRNA-transduced hTERT1 were assayed for RAP1 activity as described in “Rapactivity assay.” Scanned autoradiographs were quantified using Image J to determine the ration GTP-bound Rap1/total Rap1 for each extract. Bars show means ± SD of the ratio GTP-bound Rap1/total-Rap1 of 3 independent experiments. shRNA CTL-treated cell ratio was arbitrarily set at 100%. Images shown are representative of the 3 experiments. Vertical lines have been inserted to indicate a repositioned gel lane. (C) HUVECs were treated with LYL1 or control HLA-A siRNA and total RNA were prepared 48 hours after transfection. hTERT1 cells were transduced with lentiviruses encoding either control shRNA or LYL1 shRNA, and puromycin-resistant cell populations were established. mRNA levels of the indicated gene in siRNA-treated HUVEC (black bars) or in shRNA-transduced hTERT1 (gray bars) were assessed by qRT-PCR. cDNAs were amplified by specific primers and normalized to GAPDH. VE-CAD, ITGA2, Rap-GEF1, Rap-GEF2, genes encoding VE-CADHERIN, INTEGRIN-α2, C3G and DOCK4 proteins, respectively. Each bar is the mean ± SD of mRNA levels relative to control HLA-A siRNA-treated HUVECs (3 independent experiments) or to hTERT1 cells transduced with control shRNA-lentivirus, which were set at 100%. ***P < .005; **P < .01; *P < .05 by Student t test. (D) LYL1-silencing causes down-expression of INTEGRIN-α2: HUVECs or hTERT1 cells were transduced with lentiviruses encoding either control shRNA or shRNA targeting LYL1. Whole cell proteins prepared from puromycin-resistant cell populations were analyzed by immunoblotting for INTEGRIN-α2 and TAL-1 protein expression. beta-CATENIN protein expression was used to control protein loading.
LYL1 controls the expression of molecules involved in the formation and stabilization of adherens junctions. (A) LYL1-depletion affects the assembly and maturation of adherens junctions. hTERT1 cells were transduced with lentiviruses encoding either control shRNA or LYL1 shRNA, and puromycin-resistant cell populations were established. Confluent monolayers of the indicated population were immunostained with a VE-Cadherin antibody and vizualized with Alexa 647-conjugated secondary antibody. Note the continuous and regular staining on CTL-hTERT1 cell perimeters (arrows) and the presence of gaps between adjacent cells (arrowheads) leading to an irregular staining. (B) LYL1 depletion reduces constitutive activation of Rap1. Whole cell extract prepared from shRNA-transduced hTERT1 were assayed for RAP1 activity as described in “Rapactivity assay.” Scanned autoradiographs were quantified using Image J to determine the ration GTP-bound Rap1/total Rap1 for each extract. Bars show means ± SD of the ratio GTP-bound Rap1/total-Rap1 of 3 independent experiments. shRNA CTL-treated cell ratio was arbitrarily set at 100%. Images shown are representative of the 3 experiments. Vertical lines have been inserted to indicate a repositioned gel lane. (C) HUVECs were treated with LYL1 or control HLA-A siRNA and total RNA were prepared 48 hours after transfection. hTERT1 cells were transduced with lentiviruses encoding either control shRNA or LYL1 shRNA, and puromycin-resistant cell populations were established. mRNA levels of the indicated gene in siRNA-treated HUVEC (black bars) or in shRNA-transduced hTERT1 (gray bars) were assessed by qRT-PCR. cDNAs were amplified by specific primers and normalized to GAPDH. VE-CAD, ITGA2, Rap-GEF1, Rap-GEF2, genes encoding VE-CADHERIN, INTEGRIN-α2, C3G and DOCK4 proteins, respectively. Each bar is the mean ± SD of mRNA levels relative to control HLA-A siRNA-treated HUVECs (3 independent experiments) or to hTERT1 cells transduced with control shRNA-lentivirus, which were set at 100%. ***P < .005; **P < .01; *P < .05 by Student t test. (D) LYL1-silencing causes down-expression of INTEGRIN-α2: HUVECs or hTERT1 cells were transduced with lentiviruses encoding either control shRNA or shRNA targeting LYL1. Whole cell proteins prepared from puromycin-resistant cell populations were analyzed by immunoblotting for INTEGRIN-α2 and TAL-1 protein expression. beta-CATENIN protein expression was used to control protein loading.
Given the essential role of the small GTPase Rap1 for VE-Cadherin–dependent adhesion35 and for the stabilization of established endothelial junctions,36,37 we investigated whether LYL1 influences Rap1 activity in hTERT1 cells, by a pull-down assay using a GST fusion protein of Rap1-binding domain of RalGDS. Basal levels of activated Rap1 (GTP-bound) were significantly reduced (∼ 40%) in LYL1-depleted cells compared with control hTERT1 cells (Figure 7B). We then assessed whether LYL1 modulates the expression of genes encoding guanine nucleotide exchange factors (GEFs) that activate Rap1. As shown in Figure 7C, LYL1-depletion in both HUVECs and hTERT1 cells caused a significant reduction in Rap-GEF1/C3G and Rap-GEF2/DOCK4 mRNA levels. In agreement with VE-Cadherin staining (Figure 7A), LYL1-knockdown did not change VE-Cadherin mRNA levels in hTERT1, and produced a 1.4-fold stimulation of VE-Cadherin mRNA levels in HUVECs (Figure 7C). LYL1-depletion in HUVECs and in hTERT1 cells also caused a strong decrease in ITGA2 mRNA levels (∼ 60%), together with a reduced INTEGRIN-α2 protein expression (Figure 7D), which has an important role in endothelial cell adhesion and stability.38 Of note, LYL1 depletion did not change TAL-1 protein levels in both endothelial cell types.
Collectively, these data show that LYL1 depletion decreased the expression of several important mediators of AJ assembly and stabilization, without reducing the major constituent of AJs.
Discussion
The objective of this study was to delineate the role of LYL1 in the endothelial lineage in adulthood. Loss-of-function experiments identified that LYL1, dispensable for initiating angiogenesis, is required during the later steps of postnatal remodeling to promote maturation of newly formed blood vessels.
Whereas Lyl1 activity was described in developing vasculature in embryos,17 its expression in adult endothelium was unknown. We described here that Lyl1 is active in all adult mice tissues tested where quiescent stabilized vasculature dominates, in contrast to Tal-1, which is only expressed in developing vessels.18-20 In vitro experiments confirmed that LYL1 expression occurs in quiescent ECs, but also in activated angiogenic ECs. The mild phenotype of Lyl1-deficient mice gave us the possibility to investigate the role of this transcription factor in adult angiogenesis. We found that Lyl1-deficiency causes an increased angiogenic response in 3 different models, ie implanted tumors, Matrigel plug assays and aortic ring assays.
Importantly, syngeneic LLC and B16-F10 tumors grew faster in Lyl1-deficient mice than in their control or heterozygous littermates. Although tumor blood vessel density and areas were comparable, the architecture of tumor blood vessels from ΔLyl1/ΔLyl1 mice exhibited angiogenic features, that is, poor coverage, increased vascular leakage, lumen enlargement and persistent Tal-1 expression. This sustained angiogenesis is likely to promote tumor growth in ΔLyl1/ΔLyl1 mice. Of importance, reciprocal BM or FL transplantations demonstrate that the higher tumor rate and reduced vessel coverage were not due to hematopoietic defects of ΔLyl1/ΔLyl1 mice. Caveolin-1-deficient mice display a similar increased permeability of tumor vasculature that was correlated with larger LLC tumors,31 and treatments of tumor-bearing mice with molecules that reduce tumor vascular leakage also reduce tumor growth.31,39,40 Thus, the permeable vasculature, by allowing macromolecules to extravasate into the tumor interstitium, presumably also contributes to accelerated tumor growth in Lyl1-deficient mice.
Our data suggest that Lyl1 is required to limit the development of vascular lumen. Indeed, developing vessels in tumors as well as in Matrigel plugs and aortic rings from ΔLyl1/ΔLyl1 mice have larger diameters than in WT mice. Noteworthy, Matrigel implanted in ΔLyl1/ΔLyl1 mice display abnormal dilated vascular structures reminiscent to those previously observed in TAL-1 overexpressing plugs.22 We found a significant increase in Tal-1 expression at both mRNA and protein levels in vessels of tumors developed in Lyl1-deficient-mice. It is unlikely that LYL1 directly influences Tal-1 expression in maturing vessels, because LYL1 silencing in human ECs does not change significantly TAL-1 protein levels (Figure 7). Therefore, Tal-1 up regulation in tumors may rather reflect vessel immaturity caused by sustained angiogenesis.
In keeping with the recent identification of VE-cadherin as a direct TAL-1 target,23 ΔLyl1/ΔLyl1 mice tumors exhibit a concomitant up-expression of Tal-1 and VE-cad, and LYL1 depletion in HUVECs slightly up regulates VE cad mRNA levels. Hence, both the absence of Lyl1 and simultaneous Tal-1 up regulation in tumor ECs may produce excessive VE-Cadherin. A recent study reveals that the aortic lumen develops extracellularly between adjacent ECs through the initial recruitment of CD34-sialomucins to the cell-cell contact, which is mediated by VE-Cadherin.41 Consequently, excessive VE-cadherin expression at the endothelial surface of vessels forming in ΔLyl1/ΔLyl1 mice might contribute to an exaggerated lumen development.
Tumors in ΔLyl1/ΔLyl1 mice also exhibit increased expression of the gene encoding the proangiogenic factor ANG2. The angiopoietin/Tie2 axis plays a central role in vessel maturation and stabilization.42 ANG1 acts as a Tie2 agonist to promote and maintain mature blood vessels, while ANG2 acts a Tie2-antagonist, and its increased production by angiogenic ECs destabilizes vessel coverage by pericytes.43 One possibility is that a local excess of VE-Cadherin and ANG2, that both regulate endothelial responses to VEGF,34 may inhibit pericyte coverage and, as a consequence, vessel maturation.44
Our data strongly suggest that LYL1 serves as a stabilizing signal for developing vessels. Indeed, we identified several downstream LYL1 targets in ECs that operate in different pathways promoting assembly and maturation of endothelial junctions: (1) INTEGRIN-α2, which stabilizes nascent tubular structures through mediating cell adhesion to the extracellular matrix (reviewed in Avraamides et al38 ); (2) Rap1-GEFs, C3G, and Dock4 that function, through activating Rap1, at different levels in network signaling cell junction maturation (reviewed in Kooistra et al36 and Pannekoek et al37 ). Therefore, to explain the immature and highly angiogenic phenotype of the vessels neo-formed in ΔLyl1/ΔLyl1 mice, we propose that a concomitant reduction of these molecules in Lyl1-lacking ECs contributes to impaired maturation of endothelial junctions.
Our data establish that Lyl1 expression occurs in both angiogenic and mature vessels, suggesting that LYL1 participates to the continued maintenance of the quiescent vessels. Lyl1 is dispensable for vascular development, indicating that its loss is compensated by other functionally similar factors that contribute to angiogenesis, namely Tal-1 that is coexpressed with Lyl1 in the developing vasculature.17 However, further studies are required to investigate whether Lyl1-deficiency generates minor vascular anomalies in adult tissues where Tal-1 is supposed to be inactive.
Finally, our findings suggest possible medical implications. Transient normalization of tumor vasculature is emerging as a therapeutic option to improve drug delivery in anticancer treatment.45 A significant increase in vascular permeability is a hallmark of inflammatory diseases such as acute lung injury, sepsis, or epilepsy.46,47 Hence, strategies designed to increase LYL1 activity should be considered to promote or improve endothelial barrier in these pathologic situations.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We are grateful to Jean-Marie Blanchard for constant support, and to Luisa Dandolo for critical reading of the manuscript. We are indebted to the Montpellier-RIO imaging platform (Montpellier, France) and to the Transgenese et Techniques associées platform core facilities for animal experiments. We thank Patricia Cavalier for her expert assistance with histology studies, and Philippe Clair for his help in high throughput qPCR experiments (IFR22, Montpellier). We are grateful to Chamroeun Sar for his help in imaging analyses. N.P. was supported by successive grants from the French Minister of Education and the Ligue Nationale Contre le Cancer (France), R.E.-H. was supported by grants from the Ligue Régionale Contre le Cancer (Hérault, France) and the Conseil National de Recherche Scientifique (Liban), and is now a recipient of the Association pour la Recherche sur le Cancer (ARC; France). D.M. and V.P. are supported by Inserm.
This work was funded by grants from ARC and Institut National du Cancer (France).
Authorship
Contribution: All authors analyzed data; N.P., V.D., and V.P. performed animal studies; R.E.-H.,V.D., C.D., and P.C. performed molecular experiments; F.S. provided Lyl1-deficient mice; and D.M. and V.P. conceived and designed the study and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for N.P. is Institut de Recherche en Cancérologie de Montpellier, Montpellier, France.
Correspondence: Danièle Mathieu or Valérie Pinet, Institut de Génétique Moléculaire de Montpellier, UMR 5535, CNRS, 1919 route de Mende, 34293 Montpellier cedex 5, France; e-mail: daniele.mathieu@igmm.cnrs.fr or valerie.pinet@igmm.cnrs.fr.
References
Author notes
D.M. and V.P. share senior authorship (alphabetical order).






This feature is available to Subscribers Only
Sign In or Create an Account Close Modal