Abstract
Recent lineage studies suggest that hematopoietic stem cells (HSCs) may be derived from endothelial cells. However, the genetic hierarchy governing the emergence of HSCs remains elusive. We report here that zebrafish ets1-related protein (etsrp), which is essential for vascular endothelial development, also plays a critical role in the initiation of definitive hematopoiesis by controlling the expression of 2 stem cell leukemia (scl) isoforms (scl-α and scl-β) in angioblasts. In etsrp morphants, which are deficient in endothelial and HSC development, scl-α alone partially rescues angioblast specification, arterial-venous differentiation, and the expression of HSC markers, runx1 and c-myb, whereas scl-β requires angioblast rescue by fli1a to restore runx1 expression. Interestingly, when vascular endothelial growth factor (Vegf) signaling is inhibited, HSC marker expression can still be restored by scl-α in etsrp morphants, whereas the rescue of arterial ephrinb2a expression is blocked. Furthermore, both scl isoforms partially rescue runx1 but not ephrinb2a expression in embryos deficient in Vegf signaling. Our data suggest that downstream of etsrp, scl-α and fli1a specify the angioblasts, whereas scl-β further initiates HSC specification from this angioblast population, and that Vegf signaling acts upstream of scl-β during definitive hematopoiesis.
Introduction
Vertebrate hematopoiesis proceeds in 2 waves: primitive hematopoiesis, which serves as a transient early source of limited blood cell types, and definitive hematopoiesis, which contributes to all adult blood lineages. In zebrafish, the first apparent definitive hematopoietic stem cells (HSCs) or their precursors are marked by the expression of runx1 and c-myb along the ventral wall of the dorsal aorta (DA),1,2 the functionally equivalent structure of the mouse aorta-gonad-mesonephros (AGM) region. These aortic HSCs will later seed the caudal hematopoietic tissue, thymus, and kidney.3-5 Accumulating evidence suggests that the HSCs may be derived from endothelial progenitors through a hemogenic endothelial intermediate.6-8 Although these studies define a lineage relationship during definitive hematopoiesis, the genetic program directing HSC specification remains elusive.
The authors of previous studies9,10 have established the stem cell leukemia (scl) gene as a critical transcription factor controlling vertebrate hematopoiesis. Scl marks both hematopoietic and endothelial precursors during early vertebrate development. When overexpressed, scl expands both angioblasts and erythroblasts in zebrafish embryos9 and partially restores the expression of markers of both lineages in the zebrafish cloche mutants, which are devoid of blood and vessel development.11 In loss-of-function studies, scl deficiency abolishes both primitive and definitive hematopoiesis in mice12,13 and zebrafish.14-16 Two isoforms of scl, the full-length scl-α and the N-terminal truncated scl-β, have been identified in zebrafish, which function redundantly in primitive erythropoiesis and myelopoiesis. However, only scl-β is required for definitive hematopoiesis in vivo, although both isoforms are able to rescue HSC marker expression in scl-splicing morphants where both isoforms are disrupted.16 The authors of a recent report8 in mice further demonstrate that Scl is an indispensable factor for the establishment of the hemogenic endothelium. Thus, identifying the genetic program controlling the dynamic expression of scl during definitive hematopoiesis will contribute to our knowledge of the mechanism underlying HSC emergence and how this process is coordinated with endothelial development.
The vascular endothelial growth factor (Vegf) receptor, fetal liver kinase 1 (flk1), and the transcription factor, ets1-related protein (etsrp), have been indicated as upstream regulators of scl in vertebrates. Flk1−/− mutant mice fail to develop both endothelial and hematopoietic cells,17 which can be rescued by ectopic Scl expression under the Flk1 promoter in vitro.18 Etsrp plays an essential role during angioblast specification and primitive myelopoiesis in zebrafish.19-22 Similarly, knockout of its mammalian homolog, Etv2/Er71, also leads to severe defects in both hematopoietic and endothelial development in mice.23 Epistasis analysis in zebrafish shows that the myelopoietic defect in etsrp morphants can be rescued by scl overexpression.22
In this study, we analyzed the interplay among etsrp, scl, and Vegf signaling in directing the definitive hematopoietic program. Here we show that etsrp is required for HSC initiation and that both scl isoforms function downstream of etsrp in this process. Etsrp controls scl expression in angioblasts of the posterior lateral plate mesoderm (PLPM). We examined the potential of both scl isoforms to rescue the endothelial and HSC defects in etsrp morphants and found that scl-α restores both angioblast and HSC marker expression, whereas scl-β does not. Nonetheless, scl-β partially rescues runx1 expression when angioblasts are first restored by fli1a overexpression. Furthermore, we found that the rescue of arterial and HSC marker expression by scl-α in etsrp morphants can be uncoupled by inhibition of Vegf signaling, wherein overexpression of scl-α can bypass the requirement of arterial identity and Vegf signaling for HSC initiation. This finding suggests that scl plays a role downstream of Vegf signaling in the initiation of definitive hematopoiesis. This idea is further supported by the partial rescue of runx1 expression by both scl isoforms in Vegf signaling-deficient embryos produced by either SU5416 treatment or plcg1 knockdown. Our findings define genetic programs downstream of etsrp during angioblast specification and HSC emergence.
Methods
Zebrafish husbandry and strains
Construct cloning for mRNAs and probes
Full-length coding sequences of scl-α and scl-β16 were amplified with polymerase chain reaction (PCR) from reverse-transcription product made from zebrafish embryos at 24 hours postfertilization (hpf). Full-length coding sequences of ets1a, fli1a, fli1b, and erg were PCR amplified from cDNA clones purchased from Open Biosystems, Inc (ets1a, BC092935; fli1a, BC066571; fli1b, AY839950; and erg, BC086811). The following primers were used: scl-α: forward, 5′-GGATCCCGCCACCATGGAAAAACTGAAATCCGAG-3′, reverse, 5′-CTCGAGTCAGTGTCTCCAATCAGTCAC-3′; scl-β: forward, 5′-GGATCCCGCCACCATGGTGCAGTTGAGTCCGCCCGCTTTC-3′, reverse, 5′-CTCGAGTCAGTGTCTCCAATCAGTCAC-3′; ets1a: forward, 5′-GAATTCCGCCACCATGACGGCAGCTGTCGATATTAAG-3′, reverse, 5′-CTCGAGTCAGGAGCTCCAACAGGAACTG-3′; fli1a: forward, 5′-GAATTCCGCCACCATGGACGGAACTATTAAGGAG-3′, reverse, 5′-CTCGAGTTAGTAGTAACTACCAAGGTG-3′; fli1b: forward, 5′-GGATCCCGCCACCATGGACTGTACTATTAAGGAAG-3′, reverse, 5′-TCTAGATTAATAATAAGTGTTCAAGTGAG-3′; and erg: forward, 5′-GGATCCCGCCACCATGACGGCGTCTGCAGCCGCTG-3′, reverse, 5′-CTCGAGCTAGTAGTATGAGCCCAGATGTG-3′. These full-length coding sequences were cloned into the pCS2+ vector for mRNA synthesis. To make an scl-3′ probe that recognizes both scl isoforms,16 the coding sequence of scl-β also was cloned into the pCR2.1-TOPO cloning vector (Invitrogen). To make an scl-5′ probe that only recognizes scl-α,16 an scl fragment that covers the most 5′ region of scl-α transcript, including its 5′UTR, was PCR amplified by the use of primers: forward, 5′- ACATGGATGACCCTCCACAAAATG-3′, reverse, 5′- CGCTCCCTCCTCTCCTGGAC-3′, and was cloned into the pCR2.1-TOPO cloning vector.
Microinjection of morpholinos and mRNAs
A mixture of 2 etsrp-specific morpholinos (Gene Tools LLC) was used as described.19 A mixture of 2 tbx16-specific morpholinos (Gene Tools LLC) also was used as described.27 An scl morpholino (Open Biosystems) designed to block the proper splicing of both scl isoforms has been described previously15 and was injected at a dose of 8 ng. A plcg1 morpholino (Open Biosystems) has been described previously28 and was injected at a dose of 15 ng.
The mRNAs of etsrp,19 scl-α, scl-β, ets1a, fli1a, fli1b, and erg were synthesized by the use of mMessage mMachine Kit (Ambion Inc) and injected at the following doses: etsrp (75 pg), scl-α (100 pg), scl-β (200 pg), ets1a (200 pg), fli1a (10 pg), fli1b (10 pg), and erg (10 pg). In rescue experiments, embryos were injected sequentially with morpholinos and mRNAs. All injections were made at the 1-cell stage.
Chemical treatment
SU5416 (Sigma-Aldrich) was dissolved in dimethyl sulfoxide (DMSO) at a stock concentration of 1mM. Embryos were treated with SU5416 at 2μM starting from 12 hpf until fixation at desired stages, whereas control embryos were treated with 0.2% DMSO.
Whole-mount in situ hybridization
For single-color in situ hybridization, antisense riboprobes were labeled with digoxygenin (DIG; Roche). The following probes were used: runx1, c-myb, rag1, scl-3′, scl-5′, lmo2, flk1, gata1, ephrinb2a, and flt4. Whole-mount in situ hybridization was performed as described.29
Double-fluorescent in situ hybridization was performed as described,30 except that biotin-tyramide (PerkinElmer; 1:25 dilution) followed by streptavidin-fluorescein (PerkinElmer; 1:400 dilution) was used for amplification and detection of the DIG signal. To compare the expression of etsrp with that of scl and gata1, etsrp was labeled with DIG, whereas scl and gata1 were labeled with dinitrophenol (Mirus Bio LLC). To compare the expression of runx1 with that of primitive hematopoietic markers in etsrp, morphants overexpressing scl-α, lcp1, mpx, and gata1 were labeled with DIG, whereas runx1 was labeled with dinitrophenol.
Imaging
Images of single-color in situ hybridization and flk1-GFP were taken by the use of Axioskop 2 plus microscope (Zeiss) with 10× magnification and Openlab 4.0 software (Improvision, A PerkinElmer Co). For imaging the flk1-GFP embryos, images of both green fluorescence and bright field were taken and merged with the use of Adobe Photoshop. The brightness and contrast of images was adjusted with Adobe Photoshop. Numbers in the lower right corner of each panel denote the number of embryos with the observed phenotype over the total observed. Images of double fluorescent in situ hybridization were taken with the LSM 510 confocal microscope (Zeiss) with 20× magnification and LSM image software. Unless otherwise noted, images show the lateral view with anterior to left.
Quantitative PCR
For real-time quantitative PCR (qPCR) analysis, RNA was isolated with Trizol (Invitrogen), and equal quantities of cDNA were reverse transcribed between groups with superscript II (Invitrogen). qPCR was performed with the use of the iCycler iQ Real-Time PCR Detection System (BioRad) with iQ SYBR Green Supermix (BioRad). Gene expression levels were analyzed by use of the ΔΔ Ct method, with β-actin serving as the reference gene. Measurements were made in triplicate from 3 independent biologic replicates per condition, and the fold changes reported reflect the comparison of experimental groups relative to uninjected control at the time points stated in the result section. Independent Student t tests were used for statistical analysis. The primers used were as follows: scl-α: forward, 5′-CTGAAATCCGAGCAATTTCC-3′, reverse, 5′-GTTTCCTTGGCAACACCATT-3′; scl-α and -β: forward, 5′-GGAGATGCGGAACAGTATGG-3′, reverse, 5′-GAAGGCACCGTTCACATTCT-3′; and β-actin: forward, 5′-TGTTTTCCCCTCCATTGTTG-3′, reverse, 5′-ACATACATGGCAGGGGTGTT-3′.
Results
Etsrp is required for the initiation of definitive hematopoiesis
In the mouse AGM region, vascular endothelial cadherin+ endothelium instead of its underlying mesenchyme is capable of HSC emergence.6 This finding suggests a close lineage relationship between endothelial cells and HSCs, which is also supported by observations in zebrafish.3,4 Given the critical requirement of etsrp for endothelial development,19-21 we examined whether etsrp is also required for definitive hematopoiesis. Runx1 and c-myb expression in the ventral wall of the DA has been shown to mark the emergence of HSCs or their precursors in zebrafish,1,2 and therefore their expression was analyzed on zebrafish embryos at 30 and 40 hpf, respectively. In etsrp morphants, both markers were missing in the ventral wall of the DA (Figure 1A-D), suggesting that the initiation of definitive hematopoiesis was defective when etsrp was knocked down. This finding was further confirmed in etsrp morphants at 4 days after fertilization by the significantly reduced expression of rag1 (Figure 1E-F), a marker of differentiated thymic T cells that originates from aortic HSCs.3-5 Complementary results were observed in etsrp mutants (y11; data not shown).20
Initiation of definitive hematopoiesis is defective in etsrp morphants. (A-F) runx1, c-myb, and rag1 expression in control (A,C,E) and etsrp morphants (MO; B,D,F). (A-B) runx1 expression in the ventral wall of the DA (arrowhead) at 30 hpf. (C-D) c-myb expression in the ventral wall of the DA (arrowhead) at 40 hpf. (E-F) rag1 expression in the thymus at 4 days after fertilization (arrowheads).
Initiation of definitive hematopoiesis is defective in etsrp morphants. (A-F) runx1, c-myb, and rag1 expression in control (A,C,E) and etsrp morphants (MO; B,D,F). (A-B) runx1 expression in the ventral wall of the DA (arrowhead) at 30 hpf. (C-D) c-myb expression in the ventral wall of the DA (arrowhead) at 40 hpf. (E-F) rag1 expression in the thymus at 4 days after fertilization (arrowheads).
Etsrp colocalizes with and is required for the expression of both scl isoforms in angioblasts of the PLPM
To elucidate the pathway governed by etsrp during HSC initiation, we explored downstream genes that are likely to mediate the diverse functions of etsrp. The dual roles of scl in both hematopoietic and endothelial lineages make it a likely candidate.9,11,14-16 In etsrp morphants, scl is absent from the rostral but not caudal PLPM (Figure 2H).19 Because the rostral PLPM is suggested to be mainly composed of angioblasts,14 we examined whether etsrp controls scl expression in angioblasts of the PLPM.
etsrp colocalizes with and regulates the expression of both scl isoforms in angioblasts of the PLPM. (A-F) Double fluorescent in situ hybridization on wild-type embryos at 14 hpf, flatmount, anterior to left. Arrowheads denote the rostral PLPM (A-D,F), and arrows denote the medial caudal PLPM (A-D,F). (C) Overlay of etsrp (A, green) and scl expression detected by scl-3′ probe (B, red), with coexpression shown in yellow. (F) Overlay of etsrp (D, green) and gata1 (E, red) expression. (G-N) scl expression detected by scl-3′ probe (G-J) and scl-5′ probe (K-N) at 14 hpf in control (G,K), etsrp MO (H,L), tbx16 MO (I,M), and etsrp MO + tbx16 MO (J,N), flatmount, anterior to left. Arrowheads denote the rostral PLPM (G,I,K,M), note that the scl expression in rostral PLPM detected by scl-3′ probe is much stronger than that detected by scl-5′ probe (compare G with K and I with M). Arrows denote the caudal PLPM (G-H,K-L). (O-P) qPCR analysis of scl-α and scl-α and -β transcripts on RNA samples from control or embryos overexpressing etsrp mRNA (etsrp OE) at 10 hpf (O), and from control or etsrp morphants (etsrp MO) at 14 hpf (P). The average fold changes of expression are represented by the bar graphs. Error bars plotted are SEM, and asterisks indicate significant changes at P < .05 on the basis of independent Student t tests.
etsrp colocalizes with and regulates the expression of both scl isoforms in angioblasts of the PLPM. (A-F) Double fluorescent in situ hybridization on wild-type embryos at 14 hpf, flatmount, anterior to left. Arrowheads denote the rostral PLPM (A-D,F), and arrows denote the medial caudal PLPM (A-D,F). (C) Overlay of etsrp (A, green) and scl expression detected by scl-3′ probe (B, red), with coexpression shown in yellow. (F) Overlay of etsrp (D, green) and gata1 (E, red) expression. (G-N) scl expression detected by scl-3′ probe (G-J) and scl-5′ probe (K-N) at 14 hpf in control (G,K), etsrp MO (H,L), tbx16 MO (I,M), and etsrp MO + tbx16 MO (J,N), flatmount, anterior to left. Arrowheads denote the rostral PLPM (G,I,K,M), note that the scl expression in rostral PLPM detected by scl-3′ probe is much stronger than that detected by scl-5′ probe (compare G with K and I with M). Arrows denote the caudal PLPM (G-H,K-L). (O-P) qPCR analysis of scl-α and scl-α and -β transcripts on RNA samples from control or embryos overexpressing etsrp mRNA (etsrp OE) at 10 hpf (O), and from control or etsrp morphants (etsrp MO) at 14 hpf (P). The average fold changes of expression are represented by the bar graphs. Error bars plotted are SEM, and asterisks indicate significant changes at P < .05 on the basis of independent Student t tests.
Two isoforms of scl have been identified in zebrafish, the full-length scl-α and the 5′-truncated scl-β.16 Because both isoforms share the 3′ mRNA sequence, we used an scl-3′ probe that recognizes both isoforms to analyze the expression of scl and compare it with that of etsrp at 14 hpf by double-fluorescent in situ hybridization. We found that etsrp colocalized with scl in the rostral PLPM and in a few cells in the medial caudal PLPM, whereas only scl was expressed in the lateral caudal PLPM (Figure 2A-C). This spatial relationship between etsrp/scl double-positive cells and cells expressing scl alone is reminiscent of that between flk1-positive angioblasts and gata1-positive erythroblasts at the same stage,14 suggesting that etsrp may only colocalize with scl in angioblasts but not in erythroblasts. To confirm this, we compared the expression of etsrp with that of gata1. Indeed, there was no apparent overlap between etsrp and gata1, with gata1 localized adjacent and lateral to etsrp in the caudal PLPM (Figure 2D-F).
To directly test whether etsrp is required for scl expression in angioblasts, we compared the expression of scl in etsrp morphants with that in tbx16 (spadetail) morphants. Tbx16 mutants lack erythroblasts but retain relatively normal levels of angioblasts. Accordingly, in tbx16 mutants, scl expression is absent from erythroblasts but present in angioblasts.31 Embryos were analyzed at 14 hpf with the scl-3′ probe after morpholino knockdowns. Scl was absent from the rostral PLPM in etsrp morphants (Figure 2H), from the caudal PLPM in tbx16 morphants (Figure 2I), and from the entire PLPM in etsrp/tbx16 double morphants (Figure 2J). This finding indicates that scl expression in angioblasts and erythroblasts is controlled separately by etsrp and tbx16, respectively. A similar result was observed when we used the scl-5′ probe that specifically recognizes scl-α (Figure 2K-N), except that the expression in the rostral PLPM detected by the scl-5′ probe (Figure 2K,M) was much weaker than that detected by the scl-3′ probe (Figure 2G,I). This finding may be explained by the lower sensitivity of the shorter scl-5′ probe or the presence of scl-β in angioblasts; however, an scl-β–specific probe cannot be made because the scl-β mRNA overlaps entirely with the 3′ part of the scl-α mRNA. The aforementioned regulatory mechanism is not specific for scl because lmo2, which shares a similar expression pattern with scl and has been suggested to function together with scl in both hematopoietic and endothelial development,31-33 was regulated in a similar manner (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
To further analyze the regulation of both scl isoforms by etsrp, we performed qPCR analysis with 1 pair of primers amplifying an scl-α–specific fragment and another pair amplifying a common fragment to both scl isoforms (scl-α and -β). In 10-hpf embryos overexpressing etsrp mRNA, scl-α was up-regulated by 2-fold (2.11 ± 0.37, P < .05), whereas scl-α and -β together were up-regulated by 10-fold (10.66 ± 3.6, P < .05; Figure 2K). In 14-hpf etsrp morphants, scl-α was slightly decreased (0.73 ± 0.31, P = .22), whereas scl-α and -β together were decreased approximately 70% compared with control (0.31 ± 0.04, P < .001; Figure 2L). This result further supports that scl-β is present in angioblasts and indicates that etsrp is both sufficient and necessary to induce the expression of both scl isoforms in angioblasts.
Scl-α partially rescues both angioblast specification and HSC initiation in etsrp morphants
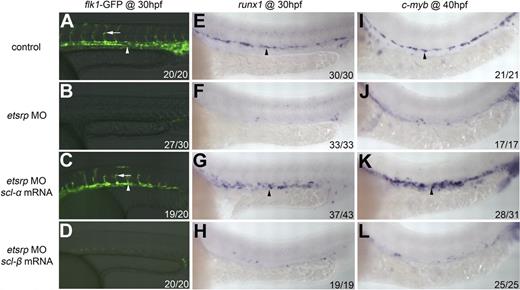
Both trunk endothelial cells and HSCs, which are derived from angioblasts in the PLPM, are deficient in etsrp morphants (Figure 1).19-21 Because etsrp controls the expression of both scl isoforms in angioblasts, we examined the potential of both scl isoforms to rescue the endothelial and HSC defects in etsrp morphants. Flk1-GFP transgenic embryos were used to visualize the vascular phenotype.26 In etsrp morphants injected with scl-α mRNA, the expression of flk1-GFP in axial vessels as well as the sprouting of intersomitic vessels (ISVs) was partially recovered when analyzed at 30 hpf (Figure 3C), whereas injection of scl-β mRNA did not produce significant rescue (Figure 3D). This rescue of flk1-GFP expression was not obvious during the initial hours of endogenous flk1 expression, which is consistent with our previous observation,22 but became evident thereafter. To further evaluate the recovery of the endothelial cell fate, we examined the expression of differentiated arterial marker ephrinb2a and venous marker flt4, which were both absent in etsrp morphants (supplemental Figure 2B,E). In etsrp morphants injected with scl-α mRNA, ephrinb2a was partially restored, which was generally restricted to the anterior trunk region (supplemental Figure 2C), whereas flt4 was restored to a level comparable with that of wild-type embryos (supplemental Figure 2F).
scl-α but not scl-β partially rescues both endothelial and HSC marker expression in etsrp morphants. (A-D) Fluorescent images of flk1-GFP at 30 hpf in control (A), etsrp MO (B), etsrp MO + scl-α mRNA (C), and etsrp MO + scl-β mRNA (D). Arrowheads denote axial vessels, and arrows denote ISVs (A,C). (E-L) runx1 and c-myb expression in control (E,I), etsrp MO (F,J), etsrp MO + scl-α mRNA (G,K), and etsrp MO + scl-β mRNA (H,L). (E-H) runx1 expression in the ventral wall of the DA (arrowheads) at 30 hpf. (I-L) c-myb expression in the ventral wall of the DA (arrowheads) at 40 hpf.
scl-α but not scl-β partially rescues both endothelial and HSC marker expression in etsrp morphants. (A-D) Fluorescent images of flk1-GFP at 30 hpf in control (A), etsrp MO (B), etsrp MO + scl-α mRNA (C), and etsrp MO + scl-β mRNA (D). Arrowheads denote axial vessels, and arrows denote ISVs (A,C). (E-L) runx1 and c-myb expression in control (E,I), etsrp MO (F,J), etsrp MO + scl-α mRNA (G,K), and etsrp MO + scl-β mRNA (H,L). (E-H) runx1 expression in the ventral wall of the DA (arrowheads) at 30 hpf. (I-L) c-myb expression in the ventral wall of the DA (arrowheads) at 40 hpf.
We then extended our analysis to HSC markers, runx1 and c-myb. Again, only scl-α (Figure 3G,K) but not scl-β (Figure 3H,L) partially rescued runx1 and c-myb expression in etsrp morphants. It is known that both runx1 and c-myb are transiently expressed in primitive hematopoietic cells, before their reemergence in the ventral wall of the DA to mark the first emerging HSCs.2,15,34 Also, the etsrp-scl axis has been shown to control primitive myelopoiesis.21,22 To better characterize the HSC marker expression restored by scl-α in etsrp morphants, we performed a series of double-fluorescent in situ hybridization experiments on rescued embryos at 30 hpf and found that the restored runx1 expression did not colocalize with the expression of either primitive myeloid markers, lcp1 (supplemental Figure 3A-D) and mpx (supplemental Figure 3E-H), or the primitive erythroid marker gata1 (supplemental Figure 3I-L). This finding further supports that these runx1-positive cells restored by scl-α are of a definitive hematopoietic nature.
Scl-β partially rescues runx1 expression in etsrp morphants when an angioblast basis is first restored by fli1a overexpression
Scl-β is reported to have lower protein stability than scl-α,16 which may contribute to its inability to rescue the endothelial and HSC defects in etsrp morphants. To examine this possibility, we further compared the functional potentials of the 2 isoforms. It is known that both scl isoforms function redundantly during primitive erythropoiesis,16 and we were able to rescue gata1 expression in scl-splicing morphants with mRNA injections for either scl-α or scl-β (supplemental Figure 4), which validates that the synthetic mRNA encoding scl-β is functional. Scl-α is known to expand both angioblasts and erythroblasts when overexpressed.9 Here we tested whether scl-β is as effective. Overexpression of both scl-α and scl-β ectopically expanded erythroblasts at 14 hpf, as indicated by gata1 expression (Figure 4D-F). However, overexpression of only scl-α but not scl-β expanded the domain of trunk angioblasts at the same stage, as indicated by flk1 expression (Figure 4A-C). This finding indicates that there is an intrinsic functional difference between the 2 scl isoforms besides protein stability, and that although both scl isoforms function equivalently in hematopoiesis,16 only scl-α is able to promote the specification of trunk angioblast.
Overexpression of scl-β expands erythroblasts but not trunk angioblasts and rescues runx1 expression in etsrp morphants when angioblasts are restored by fli1a overexpression. (A-F) flk1 and gata1 expression in the PLPM at 14 hpf, flatmount, anterior to left. (A-C) flk1 expression in trunk angioblasts (arrowheads) is broader in embryos injected with the mRNA of scl-α (B) but not scl-β (C). (D-F) gata1 expression in erythroblasts (arrowheads) is broader in embryos injected with the mRNA of either scl-α (E) or scl-β (F). (G-I) Fluorescent images of flk1-GFP at 30 hpf in control (G), etsrp MO (H), and etsrp MO + fli1a mRNA (I). Arrowheads denote axial vessels, and arrows denote ISVs (G,I). (J-M) runx1 expression in the ventral wall of the DA (arrowheads) at 30 hpf in control (J), etsrp MO (K), etsrp MO + fli1a mRNA (L), and etsrp MO + scl-β and fli1a mRNA (M).
Overexpression of scl-β expands erythroblasts but not trunk angioblasts and rescues runx1 expression in etsrp morphants when angioblasts are restored by fli1a overexpression. (A-F) flk1 and gata1 expression in the PLPM at 14 hpf, flatmount, anterior to left. (A-C) flk1 expression in trunk angioblasts (arrowheads) is broader in embryos injected with the mRNA of scl-α (B) but not scl-β (C). (D-F) gata1 expression in erythroblasts (arrowheads) is broader in embryos injected with the mRNA of either scl-α (E) or scl-β (F). (G-I) Fluorescent images of flk1-GFP at 30 hpf in control (G), etsrp MO (H), and etsrp MO + fli1a mRNA (I). Arrowheads denote axial vessels, and arrows denote ISVs (G,I). (J-M) runx1 expression in the ventral wall of the DA (arrowheads) at 30 hpf in control (J), etsrp MO (K), etsrp MO + fli1a mRNA (L), and etsrp MO + scl-β and fli1a mRNA (M).
Both scl-α and scl-β rescue HSC marker expression in scl-splicing morphants.16 However, here we found that only scl-α was able to do so in etsrp morphants (Figure 3E-L). This finding is surprising because scl-β is required for definitive hematopoiesis in vivo.16 The initial angioblast specification is abolished in etsrp morphants19-21 but remains relatively normal in scl-splicing morphants.14 In light of this, we reasoned that the function of scl-β in promoting definitive hematopoiesis may need to be established on an angioblast basis that was not restored by scl-β in etsrp morphants (Figure 3D). To explore this possibility, we searched for other candidate genes that can rescue the endothelial defect in etsrp morphants. We focused on 4 Ets genes, ets1a, fli1a, fli1b, and erg, because they are also expressed in the endothelial progenitors, and have been implied as downstream targets of etsrp.20,21 On the basis of the recovery of flk1-GFP expression, only fli1a was able to restore angioblasts in etsrp morphants when overexpressed at nontoxic doses (Figure 4G-I). However, runx1 expression was not rescued by fli1a in etsrp morphants (Figure 4J-L), suggesting that additional factors downstream of etsrp are required to initiate HSC specification besides those specifying the angioblasts. We went on to test whether scl-β can act together with fli1a to rescue runx1 expression in etsrp morphants. As shown here, etsrp morphants coinjected with the mRNAs of both scl-β and fli1a displayed restored runx1 expression (Figure 4M). This finding indicates that the ability of scl-β to restore runx1 expression in etsrp morphants requires an angioblast rescue that can be provided by fli1a overexpression, although possible synergistic effects between scl-β and fli1a during angioblast specification or HSC initiation cannot be ruled out.
HSC initiation can proceed without arterial differentiation
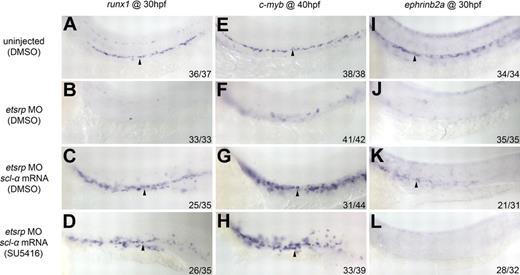
It is known that proper angioblast development is characterized by the expression of Vegf receptors and that both arterial-venous differentiation and angiogenesis is supported by Vegf signaling. Besides the shared role in definitive hematopoiesis,15,16,35,36 both scl and Vegf signaling are also required for arterial specification.15,37 Because scl-α restores the expression of the Vegf receptor flk1 in etsrp morphants, we explored whether the rescue of arterial and HSC marker expression by scl-α in etsrp morphants is a consequence of the reestablishment of Vegf signaling. We treated scl-α mRNA-injected etsrp morphants with a global Vegf receptor tyrosine kinase inhibitor, SU5416,38 to evaluate the effect of inhibiting Vegf signaling on the rescue process. SU5416 treatment did not significantly affect the ability of scl-α to rescue runx1 (Figure 5A-D) and c-myb (Figure 5E-H) expression but completely abolished the recovery of ephrinb2a expression (Figure 5I-L). This finding indicates that the rescue of HSC initiation by scl-α in etsrp morphants can be uncoupled from that of arterial specification and that the arterial fate may not be a prerequisite for HSC induction, at least when scl-α is overexpressed.
The rescue of ephrinb2a but not runx1 or c-myb expression by scl-α in etsrp morphants is abolished by SU5416 treatment. (A-L) runx1, c-myb, and ephrinb2a expression in control (0.2% DMSO; A,E,I), etsrp MO (0.2% DMSO; B,F,J), etsrp MO + scl-α mRNA (0.2% DMSO; C,G,K), and etsrp MO + scl-α mRNA (2μM SU5416; D,H,L). (A-D) runx1 expression in the ventral wall of the DA (arrowheads) at 30 hpf. (E-H) c-myb expression in the ventral wall of the DA (arrowheads) at 40 hpf. (I-L) ephrinb2a expression in the DA (arrowheads) at 30 hpf.
The rescue of ephrinb2a but not runx1 or c-myb expression by scl-α in etsrp morphants is abolished by SU5416 treatment. (A-L) runx1, c-myb, and ephrinb2a expression in control (0.2% DMSO; A,E,I), etsrp MO (0.2% DMSO; B,F,J), etsrp MO + scl-α mRNA (0.2% DMSO; C,G,K), and etsrp MO + scl-α mRNA (2μM SU5416; D,H,L). (A-D) runx1 expression in the ventral wall of the DA (arrowheads) at 30 hpf. (E-H) c-myb expression in the ventral wall of the DA (arrowheads) at 40 hpf. (I-L) ephrinb2a expression in the DA (arrowheads) at 30 hpf.
The Vegf signaling-independent rescue of HSC initiation by scl-α in etsrp morphants suggests a downstream role of scl relative to Vegf signaling during definitive hematopoiesis. To further explore this possibility, we overexpressed either scl isoform in SU5416-treated embryos that were devoid of both ephrinb2a (Figure 6F) and runx1 (Figure 6B) expression. Scl-α partially rescued runx1 expression in SU5416-treated embryos (Figure 6C). Interestingly, scl-β, which was unable to rescue runx1 expression in etsrp morphants, also partially rescued runx1 expression in SU5416-treated embryos (Figure 6D), although to a lesser extent than scl-α did (Figure 6C). This finding is likely attributable to the fact that scl-α but not scl-β is able to expand the angioblast population,9,14,32 which serves as the progenitor pool that gives rise to HSCs.
Both scl isoforms partially rescue runx1 but not ephrinb2a expression in SU5416-treated embryos. (A-H) runx1 and ephrinb2a expression in control (0.2% DMSO; A,E), control (2μM SU5416; B,F), scl-α mRNA (2μM SU5416; C,G) and scl-β mRNA (2μM SU5416; D,H). (A-D) runx1 expression in the ventral wall of the DA (arrowheads) at 30 hpf. (E-H) ephrinb2a expression in the DA (arrowheads) at 30 hpf.
Both scl isoforms partially rescue runx1 but not ephrinb2a expression in SU5416-treated embryos. (A-H) runx1 and ephrinb2a expression in control (0.2% DMSO; A,E), control (2μM SU5416; B,F), scl-α mRNA (2μM SU5416; C,G) and scl-β mRNA (2μM SU5416; D,H). (A-D) runx1 expression in the ventral wall of the DA (arrowheads) at 30 hpf. (E-H) ephrinb2a expression in the DA (arrowheads) at 30 hpf.
However, neither scl isoform was able to rescue ephrinb2a expression in SU5416-treated embryos (Figure 6G-H). To verify the results from SU5416 treatment, we inhibited Vegf signaling alternatively with a morpholino targeting plcg1, an effector of Vegf signaling.28,39 Similarly, runx1 expression in the ventral wall of the DA was absent in plcg1 morphants (supplemental Figure 5B)36 and can be partially rescued by the overexpression of either scl-α (supplemental Figure 5C) or scl-β (supplemental Figure 5D). Thus, scl acts downstream of Vegf signaling during HSC initiation but not arterial specification. Because scl expression initiates before that of Vegf receptors16,35 and its expression in the PLPM during early somitogenesis did not seem to be affected by SU5416 treatment (data not shown), Vegf signaling may be required for the maintenance but not initiation of scl. Scl-β expression in the endothelial lineage becomes restricted to the ventral wall of the DA by 26 hpf,16 which correlates with the emergence of HSCs. However, circulation is blocked in Vegf signaling-deficient embryos and scl-positive primitive erythrocytes remain arrested at their site of formation in trunk vessels, preventing us from analyzing whether Vegf signaling maintains the expression of scl-β at later stages.
Discussion
The anatomical proximity of blood and endothelial cells during vertebrate development led to the hemangioblast hypothesis.40 A recent lineage tracing study in zebrafish also supports the existence of these bipotential progenitors at the late blastula and gastrula stages.41 Scl has been regarded as a molecular marker for the hypothetical hemangioblasts because of its early expression and functions in both hematopoietic and endothelial lineages.9 However, here we show that the expression of both scl isoforms in the PLPM is under distinct control in zebrafish, with their expression in angioblasts and erythroblasts regulated separately by etsrp and tbx16, respectively (Figure 7).31 The dual regulatory programs for scl expression suggest that mesodermal progenitor cells in the PLPM may have bifurcated into distinct erythroid and endothelial lineages before the onset of scl expression. Therefore, if the hemangioblasts for endothelial and primitive hematopoietic cells do exist, they are likely present before the initiation of scl expression, perhaps representing a group of cells with the potential to generate more cell fates than hematopoietic and endothelial cells. This opinion is also supported by the previous study of the kugelig mutant.42
A model of the regulation of scl expression during primitive and definitive hematopoiesis. By the time scl starts to express, the PLPM has already bifurcated into angioblasts and erythroblasts. In erythroblasts, tbx16 is required for the expression of both scl-α and scl-β, which function redundantly during erythropoiesis. While in angioblasts, both scl isoforms are turned on separately by etsrp. Scl-α and fli1a act downstream of etsrp to specify angioblasts. At least some of these angioblasts have the potential to become hemogenic and acquire the competency for definitive hematopoiesis through an scl-β–dependent process. Vegf signaling, which is required for arterial induction, acts upstream of scl-β during definitive hematopoiesis.
A model of the regulation of scl expression during primitive and definitive hematopoiesis. By the time scl starts to express, the PLPM has already bifurcated into angioblasts and erythroblasts. In erythroblasts, tbx16 is required for the expression of both scl-α and scl-β, which function redundantly during erythropoiesis. While in angioblasts, both scl isoforms are turned on separately by etsrp. Scl-α and fli1a act downstream of etsrp to specify angioblasts. At least some of these angioblasts have the potential to become hemogenic and acquire the competency for definitive hematopoiesis through an scl-β–dependent process. Vegf signaling, which is required for arterial induction, acts upstream of scl-β during definitive hematopoiesis.
Lineage tracing study in mice suggests that the HSCs are derived from vacscular endothelial cadherin+ endothelial cells in the AGM.6 The emergence of hematopoietic cells from committed endothelium through hemogenic endothelium is also observed at the single-cell level.7 Scl is shown to be indispensable for the establishment of the hemogenic endothelium.8 By analyzing the temporal requirement of Scl for definitive hematopoiesis, Endoh et al43 propose that the competency of endothelial cells to become HSCs is acquired through an Scl-dependent process at the mesodermal stage and prior to their actual divergence. Studies in zebrafish also indicate that HSCs may be derived from the endothelial cells localized in the ventral wall of the DA.3,4 Herein we find that although scl-β is highly induced by etsrp, it is dispensable for endothelial development16 and unable to rescue the defect in angioblast specification in etsrp morphants. However, scl-β is essential for HSC specification.16 We therefore speculate that this early etsrp-dependent expression of scl-β in angioblasts may act to establish the competency of endothelial cells for definitive hematopoiesis that happens later in development. Thus, besides the role in promoting angioblast specification,19-21 etsrp also makes a direct contribution to definitive hematopoiesis by inducing scl-β (Figure 7).
However, we failed to rescue runx1 expression in etsrp morphants by overexpressing scl-β alone. Nonetheless, scl-β is sufficient to induce HSC marker expression under 3 conditions: scl-splicing morphants,16 etsrp morphants with fli1a overexpression, and Vegf signaling-deficient embryos produced by either SU5416 treatment or plcg1 knockdown. In all 3 circumstances, angioblast specification occurs properly,14,37 which suggests that scl-β requires an endothelial basis to promote definitive hematopoiesis.
In this study, we show that scl-α alone partially rescues the expression of both endothelial and HSC markers in etsrp morphants. Although scl-α is not required for definitive hematopoiesis in vivo,16 given that it has the same hematopoietic potential as scl-β does16 and is able to restore angioblasts in etsrp morphants, it is not surprising that forced and prolonged expression of scl-α also restores runx1 expression in etsrp morphants. Our data suggest that scl acts in 2 waves downstream of etsrp during definitive hematopoiesis. In the first wave, scl-α promotes angioblast specification, and in the second wave, scl-β further promotes HSC emergence on this angioblast basis (Figure 7).
A comprehensive study of the transcriptional program governing endothelial gene expression in mice identifies a FOX:ETS composite motif present in many endothelial enhancers, including an enhancer of Scl. The mouse ortholog of Etsrp, ETV2, which displays the strongest binding to this motif, directly binds to the Scl enhancer in chromatin immunoprecipitation analysis and activates Scl transcription in reporter assays.44 These data demonstrate that Scl is a direct target of ETV2 in mice and suggest that our finding from zebrafish likely represents a conserved transcriptional hierarchy for angioblast and HSC development in vertebrates.
In mice, the role of Vegf signaling during hematopoiesis has been controversial. Although the Vegf receptor Flk1 has been shown to be cell-autonomously required for both primitive and definitive hematopoiesis,45 further analysis of Flk1−/− embryos detects essentially normal numbers of hematopoietic progenitors in a brief developmental window before yolk sac hematopoiesis, which disappear thereafter.46 Besides, Flk1−/− ES cells are able to differentiate into hematopoietic cells, albeit at a lower frequency, in in vitro culture conditions that bypass the requirement of migration to correct hematopoietic microenvironments.46,47 Therefore, Flk1 is dispensable for the initial specification of hematopoietic progenitors but is required for their subsequent migration to proper sites that support further hematopoiesis.46 In support of this, forced expression of Scl under the Flk1 promoter in Flk1-null mice (Flk1Scl/−) produces limited rescue of primitive hematopoiesis in vivo but increases the hematopoietic potential of Flk1-null ES cells to a level equivalent with that of Flk1+/− heterozygotes in vitro.18 A similar migration issue is also present in definitive hematopoiesis of mice, and therefore precludes further study of the role of Vegf signaling and its relationship with Scl during HSC specification in vivo.
However, in zebrafish, not only the initial specification but also subsequent medial migration of hematopoietic and endothelial progenitors appears to be independent of Vegf signaling,35,37 which enables a more direct study of the influence of Vegf signaling on hematopoiesis. When Vegf signaling is inhibited in zebrafish, although primitive erythropoiesis proceeds normally,35,48 definitive hematopoiesis is abolished,35,36 which suggests an intrinsic role of Vegf signaling in HSC specification. In this study, we find that the rescue of runx1 and c-myb expression by scl-α in etsrp morphants is independent of Vegf signaling, indicating a downstream role of scl relative to Vegf signaling during definitive hematopoiesis. This idea is further supported by the partial rescue of runx1 expression by either scl isoform in embryos with Vegf signaling inhibited by either SU5416 treatment or plcg1 knockdown. Given the requirement of scl-β but not scl-α for definitive hematopoiesis in vivo and the scl-β–specific expression in the ventral wall of the DA, we propose that it is scl-β that functions endogenously downstream of Vegf signaling to promote definitive hematopoiesis.
As we showed previously, proper endothelial development is required for definitive hematopoiesis. One of the hallmarks of endothelial development is arterial-venous differentiation. Signaling pathways that play a role in arterial differentiation, such as sonic hedgehog, Vegf, and notch, usually have a concomitant effect on HSC specification.1,35,49 However, it is unknown whether proper arterial differentiation is a prerequisite for HSC specification, or the 2 events proceed in parallel but share the requirement of some signaling pathways because of their close apposition. The authors of a recent report50 show that mice deficient in the Notch ligand Jagged1 have impaired definitive hematopoiesis but normal arterial development. Accordingly, heat-induced notch activation expands HSCs without significantly increasing arterial ephrinb2a expression in zebrafish.1 These findings indicate that notch signaling has a role in promoting definitive hematopoiesis independent of that for arterial specification. Here we show that in etsrp morphants with Vegf signaling inhibited, HSC initiation can be rescued by scl-α overexpression whereas arterial differentiation remains blocked, suggesting that the initiation of HSCs can be uncoupled from arterial specification. Our data indicate that different downstream branches of Vegf signaling are used for arterial specification and HSC initiation, and the function of Vegf signaling on HSC development is mediated by scl-β (Figure 7).
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank M. Veldman, Y. Zhao, and Z. Tehrani for critical reading of the manuscript; D. Yelon (University of California, San Diego) for sharing the protocol for double fluorescent in situ hybridization; H. Jiang for assistance with double fluorescent in situ hybridization; and A. Liu and Y. Dong for zebrafish husbandry.
This work was supported by a research grant from the National Institutes of Health to S.L. (DK054508-13) and by research grants from NSFC (No. 30721064 and 30730056) and 973 Program from MOST of China (2005CB522504, 2006CB943801 and 2007CB914502, 2009CB941203) to B.Z. and S.L. X.R. was supported in part by China Scholarship Council, Ministry of Education. G.A.G. was supported by a predoctoral fellowship grant from the National Institutes of Health (5F31HL091713-02).
National Institutes of Health
Authorship
Contribution: X.R. designed and performed research, analyzed data, and wrote the paper; G.A.G. performed research, analyzed data, and wrote the paper; B.Z. wrote the paper; and S.L. designed experiments, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Shuo Lin, Department of Molecular, Cell and Developmental Biology, University of California, Los Angeles, 621 Charles E. Young Dr South, Rm LS4325, Los Angeles, CA 90095; e-mail: shuolin@ucla.edu.






This feature is available to Subscribers Only
Sign In or Create an Account Close Modal