In this issue of Blood, Lacombe and colleagues describe the new discovery that Scl/Tal1 plays a role in the quiescence of HSCs.1 This discovery represents an important advance in our understanding of HSC biology.
The mechanisms underlying maintenance of hematopoietic stem cells (HSCs) remain one of the critical mysteries in blood development. Because of advances in whole genome analysis and in reverse genetic animal model systems, there has been accelerating progress in identification of the genes involved in stem cell self-renewal, lineage commitment and differentiation, and cell-cycle regulation. Critical genes include those encoding hematopoietic transcription factors, cell-signaling molecules, epigenetic modifiers of gene expression, and molecular regulators of cell-cycle progression.2-4 Despite much progress, we still lack an integrated understanding of how these complex genetic networks cooperate to maintain the pool of HSCs or methods to manipulate these processes for therapeutic intent.
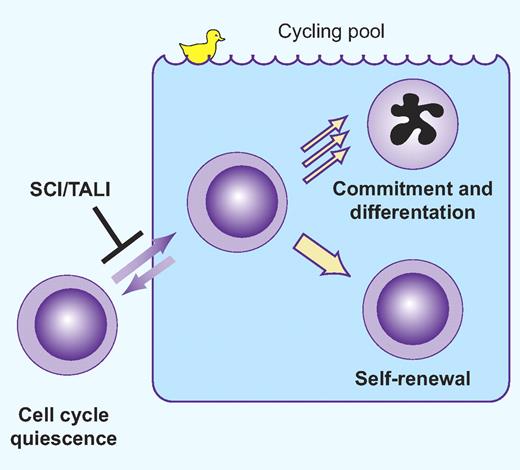
Scl/Tal1 restrains HSC cycling, a duck out of water. Professional illustration by Paulette Dennis.
Scl/Tal1 restrains HSC cycling, a duck out of water. Professional illustration by Paulette Dennis.
One issue of particular importance regards the identification of the mechanisms responsible for stem cell quiescence. At any given point in time, the majority of bone marrow stem cells in mice are in the G0 phase of the cell cycle5 but can be induced to cycle by a variety of stress conditions such as cytotoxic injury6 or hematopoietic stem cell transplantation.7 After hematopoiesis is restored or reconstituted, many of the HSCs return to a quiescent state by mechanisms that are still poorly understood. This regulation of HSC proliferation and pool size is critical for long-term maintenance of hematopoiesis. In this regard, the paper in this issue of Blood by Lacombe et al provides significant new information by demonstrating that Scl/Tal1 is required for maintenance of the quiescent stem cell pool (see figure).
Scl/Tal1 was one of the first hematopoietic transcription factors to be identified and shown to be essential for hematopoiesis through germline deletion experiments in mice.8 However, the role of Scl in adult HSCs is controversial because of potentially conflicting results in conditional Scl deletion models9,10 and because of redundant activities of other related transcription factors.11 The current study shows that relatively high levels of Scl expression specify quiescent HSCs from the adult mouse bone marrow. Functional studies using Scl+/− bone marrow cells in quantitative transplantation experiments demonstrated that haplodeficient HSCs were significantly compromised in their ability to repopulate myeloid and T-cell lineages, and that these defects were magnified in secondary transplant recipients. Similar results were obtained using wild-type cells transduced with a shRNA against Scl. Long-term HSCs from Scl+/− mice showed increased entry into the cell cycle; however, these effects were specific to adult HSCs and not seen in perinatal HSCs up to 4 weeks after birth. The mechanisms for these effects may be explained by the finding that Scl was found to control the expression of several important cell-cycle regulators including Cdkn1a and Id1. Consistent with this finding, Id1 deficiency recapitulates many of the HSC abnormalities found in Scl+/− mice.12,13
These results uncover a new and subtle effect of Scl gene dosage/expression on regulating the cell-cycle transitions required for stress conditions that demand high levels of self-renewal, such as limiting dilution transplantation experiments in serial recipients. Given the importance of maintaining HSC reserve for conditions of proliferative demand, these results provide a significant new insight into the role of Scl/Tal1 in hematopoiesis and illustrate the importance of stress models for teasing out elusive phenotypes. It is also noteworthy that relatively modest changes in Scl gene dosage and expression were sufficient for these effects, an observation that is consistent with other known haplodeficient phenotypes in HSC biology.14,15
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal