Abstract
Chronic granulomatous disease (CGD) is associated with significant morbidity and mortality from infection. The first CGD gene therapy trial resulted in only short-term marking of 0.01% to 0.1% of neutrophils. A recent study, using busulfan conditioning and an SFFV retrovirus vector, achieved more than 20% marking in 2 patients with X-linked CGD. However, oxidase correction per marked neutrophil was less than normal and not sustained. Despite this, patients clearly benefited in that severe infections resolved. As such, we initiated a gene therapy trial for X-CGD to treat severe infections unresponsive to conventional therapy. We treated 3 adult patients using busulfan conditioning and an MFGS retroviral vector encoding gp91phox, achieving early marking of 26%, 5%, and 4% of neutrophils, respectively, with sustained long-term marking of 1.1% and 0.03% of neutrophils in 2 of the patients. Gene-marked neutrophils have sustained full correction of oxidase activity for 34 and 11 months, respectively, with full or partial resolution of infection in those 2 patients. Gene marking is polyclonal with no clonal dominance. We conclude that busulfan conditioning together with an MFGS vector is capable of achieving long-term correction of neutrophil oxidase function sufficient to provide benefit in management of severe infection. This study was registered at www.clinicaltrials.gov as #NCT00394316.
Introduction
X-linked chronic granulomatous disease (X-CGD) results from mutations of the CYBB gene encoding gp91phox required by neutrophils to produce microbicidal oxidants. Patients develop recurrent life-threatening bacterial and fungal infections.1 Management involves prophylactic antibiotics, interferon-γ, and aggressive diagnosis and treatment of infection. Allogeneic transplantation (BMT) is curative, but consensus is lacking regarding indications to use what is perceived to be a high-risk modality for a nonfatal disorder. Further, finding a sibling-matched donor in the setting of a genetic disease is a limiting factor. However, outcomes improve when patients are transplanted earlier, before numerous infections and end-organ damage, and when infection free at the time of transplantation.
Gene therapy may be capable of providing protection from or treatment of infection without the risks of BMT.2 Several publications reported protection from infection challenge after gene therapy in gp91 and p47phox-deficient mouse models.3-7 Most studies used myeloablative conditioning to achieve the engraftment levels required for phenotypic correction. Gene-marking studies with myeloablative conditioning in nonhuman primates have achieved levels of long-term engraftment at levels that theoretically would cure patients with CGD.8,9 Full engraftment is unnecessary as demonstrated by X-CGD female carriers who vary in their ratio of oxidase normal versus deficient neutrophils resulting from the stochastic nature of X chromosome inactivation.10 Carriers with more than or equal to 10% oxidase normal neutrophils are generally infection free. However, in the nonhuman primate model, gene marking has been significantly lower with nonmyeloablative conditioning,11,12 particularly where there is no selective advantage conferred by the transgene. Still, an early gene therapy trial for X-CGD in humans resulted in detectable marking even without any conditioning. In that trial, gene-corrected neutrophils occurred at 0.01% to 0.1% of total neutrophils measured at various time points, but none persisted more than a year despite multiple infusions of modified CD34s.13
In disorders where selective advantage is conferred by the corrective transgene, such as X-linked severe combined immunodeficiency (SCID), there has been greater success.14,15 However, even in adenosine deaminase deficiency SCID, the use of low-dose busulfan (4 mg/kg) was required to obtain clinical benefit.16 In 2002, Ott et al17 initiated a clinical trial for X-CGD using a spleen focus forming virus (SFFV)–based vector and busulfan at 8 mg/kg for conditioning. The initial marking was greater than 20% in the 2 patients, although oxidase correction per gene-marked neutrophil was significantly less than normal. The level of marked neutrophils rose because of oligoclonal outgrowth of transduced cells with vector inserted in the EVI1-MDS1 proto-oncogene. Unfortunately, after 2 years, both patients developed myelodysplastic syndromes, with one requiring BMT and the other dying of sepsis. Notably, both patients' infection at the time of gene therapy resolved, despite the use of busulfan.17 Based on their observation of early benefit of gene therapy for infection control, we initiated a gene therapy trial to treat X-CGD patients with a severe infection not responsive to conventional management. We used 10 mg/kg of busulfan followed by autologous CD34s transduced ex vivo with an amphotrophic pseudotyped vector encoding gp91phox. We describe here results in 3 patients treated to date.
Methods
Clinical protocol regulatory review
All procedures and treatments were conducted under National Institutes of Health protocol 07-I-0017 (the current protocol) or under protocol 95-I-0134 (closed to accrual, but results from this protocol are discussed in this paper). Both protocols were approved by the National Institute of Allergy and Infectious Diseases Institutional Review Board, the National Institutes of Health Institutional Biosafety Committee, the Food and Drug Administration, and the members of the Recombinant DNA Advisory Committee of the National Institutes of Health Office of Biotechnology Activities. Patients were eligible for protocol 07-I-0017 if they had an infection not responsive to standard therapy, proven gp91phox-deficient CGD, and no eligible human leukocyte antigen-matched sibling donor. Availability of a human leukocyte antigen-matched unrelated donor was not determined given its more experimental nature and high risk in CGD patients with an ongoing infection. Informed consent was obtained from all patients in accordance with the Declaration of Helsinki.
Vector production
The amphotropic pseudotyped murine Moloney leukemia retrovirus-derived vector encoding gp91phox cDNA (MFGS-gp91phox, supplemental Figure, available on the Blood website; see the Supplemental Materials link at the top of the online article) was produced from a 293-cell producer line under Good Manufacturing Process conditions. Three production lots of the same vector were used, with the first 2 made by Magenta for an earlier gene therapy trial and revalidated for titer and potency. The third lot (Bioreliance-Invitrogen) was made to supplement the current trial.
Autologous CD34+ cells
Patients were enrolled on a separate Institutional Review Board-approved protocol (94-I-0073) for collection of CD34+ hematopoietic stem cells. They underwent standard mobilization and apheresis using filgrastim (Amgen) at 10 to 16 μg/kg daily for 5 to 6 days (Figure 1). On day 5 and/or 6, a 15- to 25-L apheresis collection was performed. CD34+ cells were selected using either the Isolex 300i (Baxter Healthcare; distributed by Miltenyi Biotec) or CliniMACS Cell Selection System (Miltenyi Biotec) cryopreserved and held in the National Institutes of Health, Department of Transfusion Medicine until needed.
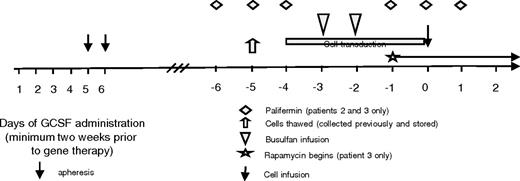
Study design. Patients' cells were collected and stored on a separate protocol. Day 0 indicates the day of cell infusion. Cells were thawed on day −5, and transduction was begun on day −4. The busulfan was infused on days −3 and −2. Patients 2 and 3 received palifermin given on days −6 to −4 and −1 to 1. Patient 3 also started rapamycin on day −1 and continued it for 30 days after transplantation.
Study design. Patients' cells were collected and stored on a separate protocol. Day 0 indicates the day of cell infusion. Cells were thawed on day −5, and transduction was begun on day −4. The busulfan was infused on days −3 and −2. Patients 2 and 3 received palifermin given on days −6 to −4 and −1 to 1. Patient 3 also started rapamycin on day −1 and continued it for 30 days after transplantation.
Transduction
Transduction was performed in the Cell Processing Section of the National Institutes of Health Department of Transfusion Medicine. CD34s were cultured and transduced in X-Vivo 10 (BioWhittaker Media, Cambrex Bio Science) serum-free medium supplemented with 1% human serum albumin and growth factors: interleukin-3 (5 ng/mL), stem cell factor (50 ng/mL), thrombopoietin (50 ng/mL), and Fms-like tyrosine kinase 3 ligand (50 ng/mL; all R&D Systems) in RetroNectin (recombinant fibronectin fragment; Takara Bio)–coated X-FOLD bags (Baxter Healthcare). For each transduction, cells were resuspended in 90% neat vector supernatant at various cell concentrations to maintain a multiplicity of infection of approximately 2. Cells were transduced daily times 4 days for 6 hours/day beginning at 16 to 18 hours of culture, and at 96 hours washed and resuspended in human albumin supplemented phosphate-buffered saline for infusion.
Study design and conditioning
The extent of each patient's infection was assessed before busulfan administration. Using a central venous line, busulfan (Busulfex; Otsuka America Pharmaceutical) 5 mg/kg was infused over 2 hours daily for 2 days and levels measured at 0, 30, 60, 180, 240, 360, and 480 minutes after first infusion (Quest Diagnostics). Area under the curve (AUC) and t1/2 were assessed by Scott Penzak of the National Institutes of Health Clinical Center Pharmacy using analytical software. The cells were given a minimum of 24 to 48 hours after the busulfan was completed (day 0), which, based on the calculated AUC and t1/2, was sufficient to ensure full clearance of drug. Patients 2 and 3 were treated with palifermin (recombinant keratinocyte growth factor; Biovitrum) 60 μg/kg per day, for 3 days before and after the busulfan. Patient 3 also received rapamycin (Wyeth Pharmaceuticals) beginning on day −1, with a loading dose of 5 mg given 3 times a day and then dosed to maintain a level of 10 to 15 mg/dL.
PCR quantification of vector marking
Semiquantitative real-time polymerase chain reaction (PCR) primers were designed to anneal to the 5′ and 3′ long terminal repeats (LTRs) of the MFGS vector.18 Forward primer sequence: CGC AAC CCT GGG AGA CGT CC; reverse primer: CGT CTC CTA CCA GAA CCA CAT ATC C; FAM labeled probe: CCG TTT TTG TGG CCC GAC CTG A. PCR was 40 cycles, melting temperature 95°C 15 seconds, and annealing temperature 60°C 60 seconds (PE Biosystems Realtime PCR 9600).
Detection of gp91phox expression
Intracellular gp91phox expression was quantified by flow cytometry. Briefly, 200 μL whole blood was lysed (ACK buffer), washed with phosphate-buffered saline/bovine serum albumin in 10mM ethylenediaminetetraacetic acid buffer, and permeabilized using a kit (BD Biosciences). Permeabilized leukocytes were resuspended in Solution B along with anti-gp91phox monoclonal antibody (antibody 7D5, a generous gift from Dr Michio Nakamura, Nagasaki, Japan) for 30 minutes, washed, and exposed to rat anti–mouse fluorescein isothiocyanate-conjugated antibody (BD Biosciences) for 30 minutes, and then analyzed using a FACSCaliber (BD Biosciences).
DHR flow cytometry assay of phagocyte respiratory burst
Analysis for dihydrorhodamine (DHR) oxidation was performed and analyzed on a FACSCalibur using forward and side scatter characteristics to define neutrophil and monocyte gates.19
Superoxide measurement
A quantiative ferricytochrome C reduction assay was used to measure superoxide production at 10 and 60 minutes after stimulation.19
LAM PCR analysis of vector insert sites
Linear amplified mediated (LAM) PCR was performed20 on samples from patients on this trial as well as archived samples from the 1998 trial (95-I-0134) using a primer pair set designed for the MFGS vector. Linear PCR product using 5′ biotinylated primer was magnetic selected and purified product mixed with Klenow polymerase and random hexamers. Products were enzymatically cut with TASI (Fermentas; and PUVI in some cases to remove the internal control band) and then ligated to a linker cassette using a Fast-link DNA ligation kit (Epicentre Technologies). Product was denatured to remove single-stranded DNA and beads. Finally, exponential PCR was performed using linker and 5′ end-specific primers (LCI and LTR-R1 and LCIII and LTR-R2) and analyzed on Spreadex gels for visualization. Individual bands were sequenced using primers LCIII and LTR IV and TA cloning. Shotgun cloning was also performed for integration site analysis using pooled LAM PCR products and the TOPO TA cloning kit (primer sequences, supplemental Methods). Clonal tracking was performed by designing primers specific for LAM-detected insertion sites. Samples from various time points were then reanalyzed to assess for presence of the clone with the specific insert using seminested PCR.
Results
Patient characteristics
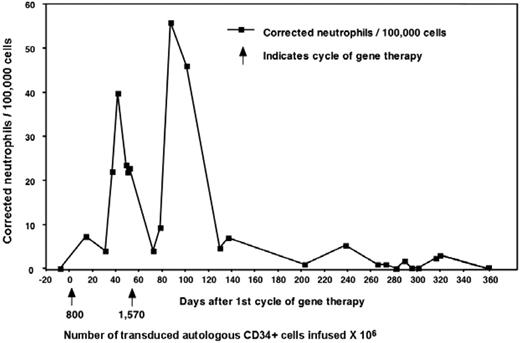
Three adult males with X-CGD (total deficiency of gp91phox protein) were enrolled in this trial (Table 1). In 1995, Malech et al initiated a clinical gene therapy trial for patients with p47phox-deficient CGD without using conditioning.19 This was followed in 1998 by a trial for X-CGD, also without conditioning, the results of which were reported in only preliminary detail.13 There, 6 X-CGD patients were treated with the same MFGS-gp91phox vector as used in the current trial. Those patients were given multiple infusions of genetically modified cells but without conditioning (except for patient 6 where it was used in conjunction with allogeneic transplantation). Patient 1 from the current trial was also the third patient treated in that previous study, allowing comparison of gene transfer in the same person, first without conditioning (Figure 2) and then 7 years later with busulfan, using the same vector lot (results presented in subsequent sections throughout the paper). In the earlier study, he, along with 3 others (4 of 6 patients in that study), produced very low but detectable numbers of oxidase-positive neutrophils, which rose transiently in the circulation after each infusion but did not persist longer than 1 year. However, 2 of 6 patients in that previous study had no detectable marking.
Patient characteristics
| Patient no. . | Age, y . | Indication for gene therapy . | Mutation . | Infection history . | Medical treatment before and during gene therapy . |
|---|---|---|---|---|---|
| 1 | 28 | Staphylococcal liver abscess | 461delA; N154fsX160 (frameshift) | Recurrent infections including previous bacterial abscesses of the liver | Intravenous meropenem, vancomycin, and linezolid |
| 2 | 28 | Paecilomyces chest wall infection | 4 nucleotide deletion that spans splice site 1150_E9(+2)delAA/gt, deletion of Exon 9 in mRNA | Recurrent pneumonias requiring thoracotomies | Intravenous ambisome then intravenous voriconazole, switched to posaconazole and 5 Fluorocytosine, then caspofungin added |
| 3 | 19 | Aspergillus lung infection | Nonsense mutation: C217A; R73X | Prior Aspergillus lung infection, Burkholderia and Bartonella infections, and osteomyelitis | Intravenous ambisome, then treated with voriconazole and micafungin |
| Patient no. . | Age, y . | Indication for gene therapy . | Mutation . | Infection history . | Medical treatment before and during gene therapy . |
|---|---|---|---|---|---|
| 1 | 28 | Staphylococcal liver abscess | 461delA; N154fsX160 (frameshift) | Recurrent infections including previous bacterial abscesses of the liver | Intravenous meropenem, vancomycin, and linezolid |
| 2 | 28 | Paecilomyces chest wall infection | 4 nucleotide deletion that spans splice site 1150_E9(+2)delAA/gt, deletion of Exon 9 in mRNA | Recurrent pneumonias requiring thoracotomies | Intravenous ambisome then intravenous voriconazole, switched to posaconazole and 5 Fluorocytosine, then caspofungin added |
| 3 | 19 | Aspergillus lung infection | Nonsense mutation: C217A; R73X | Prior Aspergillus lung infection, Burkholderia and Bartonella infections, and osteomyelitis | Intravenous ambisome, then treated with voriconazole and micafungin |
Three patients with confirmed X-CGD were enrolled in the study using genetically modified cells to treat an unresolving infection. Patients' infections, specific genetic mutation, and infection history as well as the medical therapy used concurrently are listed.
Results from patient 1 during his first gene therapy trial in 1998. Patient 1 from our trial was enrolled previously on a gene therapy trial that used the same vector but did not include conditioning. The patients received 2 separate infusions of cells as marked by the arrows and the number of cells given × 104. Marking was detectable early and increased after each infusion but eventually was undetectable after 1 year.
Results from patient 1 during his first gene therapy trial in 1998. Patient 1 from our trial was enrolled previously on a gene therapy trial that used the same vector but did not include conditioning. The patients received 2 separate infusions of cells as marked by the arrows and the number of cells given × 104. Marking was detectable early and increased after each infusion but eventually was undetectable after 1 year.
For the current study, patient 1 presented with fevers caused by Staphylococcus aureus liver abscesses not amenable to surgical resection because of their size, location, and his previous complications from 5 prior liver resections. He was enrolled and treated in November 2006. Patient 2 had a Paecilomyces fungus pneumonia extending into the chest wall and rib with recurrent accumulations of pus and spontaneous drainage despite 2 years of combination antifungal treatment and surgical resections. Patient 3 had Aspergillus fungus lung infection extending to ribs and vertebrae persisting and expanding despite multiagent antifungal therapy for more than 1 year.
Effects of busulfan
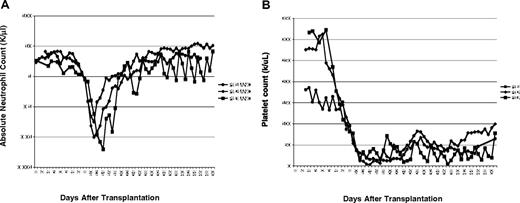
All patients developed neutropenia and thrombocytopenia (Figure 3). Time to neutrophil nadir varied from day 11 to 14, but all patients had more than or equal to 500 000/μL by day 28. Patient 3 required granulocyte colony-stimulating factor for prolonged neutropenia. All patients required platelet transfusions, with patient 3 requiring them to day 48. Patient 1 developed moderate mucositis. With the addition of palifermin, patients 2 and 3 had little mucositis. Overall, the patients tolerated the busulfan well.
Effect of busulfan on neutrophil and platelet counts. (A) The absolute neutrophil count for each patient is plotted beginning before transplant (baseline) to day 50 after transplantation. Cell infusion was day 0 and the busulfan was given days −3 and −2. (B) The platelet count for each patient is plotted beginning before the busulfan and until all patients no longer required any transfusions, day 48.
Effect of busulfan on neutrophil and platelet counts. (A) The absolute neutrophil count for each patient is plotted beginning before transplant (baseline) to day 50 after transplantation. Cell infusion was day 0 and the busulfan was given days −3 and −2. (B) The platelet count for each patient is plotted beginning before the busulfan and until all patients no longer required any transfusions, day 48.
Patient 2 received intravenous nutrition supplementation because of inanition from infection. All patients were maintained on their infection-specific antimicrobials. The protocol was amended to include rapamycin treatment for patient 3 because of early loss of marking in patient 2. This was tapered off after 4 weeks, and there was no evidence of toxicity from rapamycin.
Graft characteristics
The characteristics of the autologous mobilized transduced CD34+ cell graft and busulfan AUC are shown in Table 2. Note that significantly fewer cells were available for transduction for patient 3 compared with patients 1 and 2, as he mobilized poorly.
Graft description
| Patient no. . | CD34/kg dose at start . | CD34/kg dose after transduction . | CD34, percentage . | GP91, percentage . | Vector copy number . | Vector lot number . | Busulfan AUC, μM/min . |
|---|---|---|---|---|---|---|---|
| 1 | 14 | 43 | 85 | 73 | 4.05 | 1 | 11 324 |
| 2 | 13.7 | 71 | > 90 | 41 | .8 | 2 | 7638 |
| 3 | 4.1 | 18.9 | 70 | 25 | .005 | 3 | 7560 |
| Patient no. . | CD34/kg dose at start . | CD34/kg dose after transduction . | CD34, percentage . | GP91, percentage . | Vector copy number . | Vector lot number . | Busulfan AUC, μM/min . |
|---|---|---|---|---|---|---|---|
| 1 | 14 | 43 | 85 | 73 | 4.05 | 1 | 11 324 |
| 2 | 13.7 | 71 | > 90 | 41 | .8 | 2 | 7638 |
| 3 | 4.1 | 18.9 | 70 | 25 | .005 | 3 | 7560 |
The CD34 doses as well as the marking of the graft as measured by either flow or GP91 expression or PCR for copy number, along with the busulfan levels for each patient are given.
Functional correction of oxidase activity in circulating neutrophils and monocytes
At 2 to 3 weeks after infusion, DHR analysis of peripheral blood circulating neutrophils and monocytes from patients 1, 2, and 3 demonstrated that 26%, 5%, and 4%, respectively, had become oxidase normal. Figure 4 shows the results for neutrophils in each patient over time, but monocytes had similar percentage correction at each time point. In patient 1, the percentage of oxidase normal cells declined slowly, reaching a plateau of 1% at 7 months; was still 1.1% at 2 years after gene therapy (Figure 5); and remains stable at 1.1% at 34 months (not shown). After initially producing 5% oxidase normal neutrophils, patient 2 had no DHR-positive neutrophils and no PCR detectable inserts at 4 weeks or longer. Like patient 1, patient 3 has persistence of oxidase normal neutrophils in the circulation, declining from a high of 4% to a steady-state level of 0.03% (Figure 4) that has persisted at this level now with 11 months of follow-up (not shown).
Percentage of DHR+ cells after cell infusion (day 0). Flow cytometric analysis was performed at various time points for each patient to assess for oxidase-positive cells in the peripheral blood. Each patient had undetectable DHR before beginning the therapy. The percentage is plotted on a logarithmic scale with each patient having a different range starting at 0.1 for patient 1, 0.001 for patient 2, and 0.01 for patient 3.
Percentage of DHR+ cells after cell infusion (day 0). Flow cytometric analysis was performed at various time points for each patient to assess for oxidase-positive cells in the peripheral blood. Each patient had undetectable DHR before beginning the therapy. The percentage is plotted on a logarithmic scale with each patient having a different range starting at 0.1 for patient 1, 0.001 for patient 2, and 0.01 for patient 3.
Flow cytometry panel showing DHR analysis for patient 1 at 2 years after gene therapy. Patient 1 who had the best results continues to have approximately 1% DHR-positive cells in the peripheral blood. The MFI for this patient's cells is consistently in the same range as the normal control run concurrently, indicating a close to normal level of oxidase production on a per cell basis.
Flow cytometry panel showing DHR analysis for patient 1 at 2 years after gene therapy. Patient 1 who had the best results continues to have approximately 1% DHR-positive cells in the peripheral blood. The MFI for this patient's cells is consistently in the same range as the normal control run concurrently, indicating a close to normal level of oxidase production on a per cell basis.
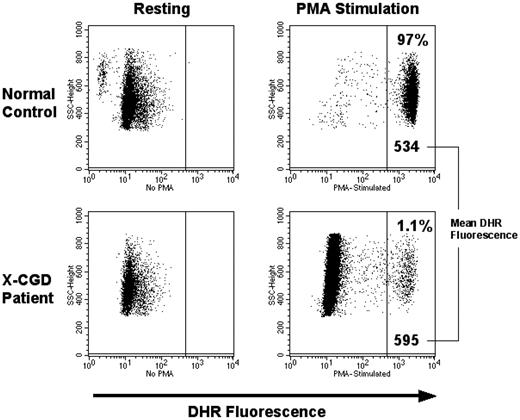
Superoxide production
Before gene therapy, DHR assays of oxidase function in phorbol myristate acetate (PMA)–stimulated neutrophils from all 3 patients were uniformly negative with low mean fluorescence intensity (MFI) similar to unstimulated cells. After gene therapy, at all time points at which marking was detectable, the MFI of all 3 patients' PMA stimulated neutrophils was not merely increased, but at each assay was identical to the MFI of the PMA stimulated normal neutrophils from a healthy volunteer (example of this comparison shown in Figure 5). The ferricytochrome C reduction assay was performed on patient neutrophils to provide precise quantitative measurement of superoxide production. These assays confirmed that the amount of superoxide produced by patient gene-corrected neutrophils was the same as that from healthy volunteer neutrophils, when the data are corrected for the number of DHR-positive neutrophils detected in the same sample (data not shown). This demonstrates that the MFGS-gp91phox vector achieves sufficient transgene protein expression in the cell to fully reconstitute phagocyte oxidase activity.
Vector copy number and clonal tracking
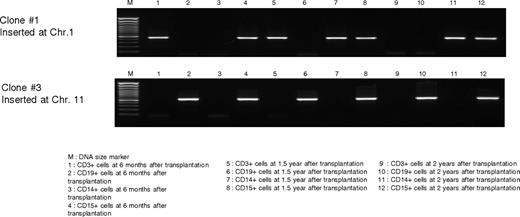
PCR analysis of patient 1 total neutrophils (CD15 cells) at the earliest time point, when 26% of neutrophils were DHR-positive, showed a vector insert copy number of 1.335 per transduced cell. The overall marking has declined to and stabilized at approximately 0.7%, consistent with the decline in the percentage of DHR-positive cells. Marking was also detectable in the B-cell fraction of patient 1 (0.6% currently). As expected, peripheral T-cell marking in patient 1 was lower, starting at 0.057 and now persisting at 0.002%. In addition, clonal tracking in this patient shown in Figure 6 demonstrates the presence of the same clones at both early (6 months) and late (2 years) time points in different lineages, suggesting transduction and engraftment of long-term repopulating progenitors (Figure 4). Patient 2 had early loss of any marking detectable by PCR, correlating with the DHR analysis. Although PCR detectable marking in patient 3 has persisted, correlating with a low level persistence of DHR-positive cells, the very low marking precludes accurate PCR quantification or clonal tracking.
Patient 1 clonal tracking PCR. Primers were designed based on inserts identified from the LAM PCR from patient 1, and a seminested PCR was performed on samples obtained at various time points to determine their presence in the various lineages.
Patient 1 clonal tracking PCR. Primers were designed based on inserts identified from the LAM PCR from patient 1, and a seminested PCR was performed on samples obtained at various time points to determine their presence in the various lineages.
Infection course
As shown in Figure 7, patient 1 demonstrated resolution of his liver abscesses after gene therapy. Near the end of his prolonged course of antibiotic therapy for liver abscess (8 months after gene therapy), the patient developed an infection of the central venous catheter that resolved quickly after removal of the catheter plus a short course of antibiotics. For the next 2 years (now 34 months after gene therapy), this patient has been free of any infection and remains well on standard CGD antimicrobial prophylaxis.
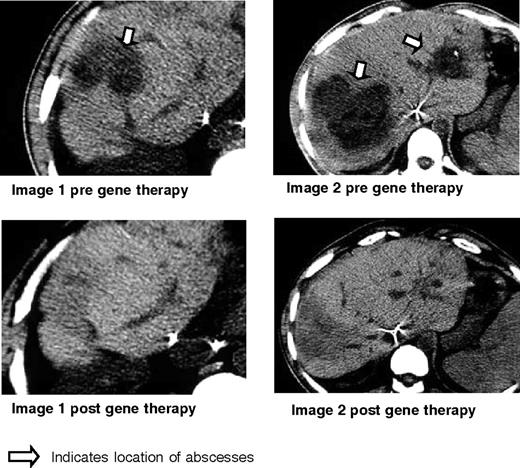
Clinical results for patient 1. Shown here are the CT scans obtained before gene therapy and 6 months after gene therapy for patient 1 who had biopsy-proven Staphylococcal aureus liver abscesses as indicated by the arrow. These disappeared, leaving only some scarring and regenerating liver as shown in the corresponding post films.
Clinical results for patient 1. Shown here are the CT scans obtained before gene therapy and 6 months after gene therapy for patient 1 who had biopsy-proven Staphylococcal aureus liver abscesses as indicated by the arrow. These disappeared, leaving only some scarring and regenerating liver as shown in the corresponding post films.
Patient 2, who had early gene marking that quickly disappeared, died of his invasive Paecilomyces fungus infection, dying almost 6 months after his gene therapy despite continuous intensive antifungal therapy and allogeneic irradiated granulocyte transfusions. A donor search was initiated, but the best possible matches were only 5 of 6 cord blood products. Unfortunately, the patient's infection continued to worsen, precluding even the possibility of a double-cord transplantation.
Patient 3 demonstrated significant radiologic evidence of regression of his Aspergillus infection followed by stabilization at 6 months after gene therapy with residual scarring and chronic pulmonary changes. Given the extent of disease and the development of neutropenic fevers, this patient was treated with allogeneic irradiated granulocyte infusions until recovery of an absolute neutrophil count more than 500 cells/μL, complicating interpretation of the role of gene therapy in resolution of his fungus infection.
Patients were not treated with granulocytes before the gene therapy to avoid the development of alloimmunization. However, as in both patients 2 and 3, they were used once gene therapy had been performed. For patient 3, they were given for only the first few weeks, a time that overlapped in part with the period of rapamycin administration, and alloimmunization did not occur.
Insertional mutagenesis monitoring
All patients were closely monitored for evidence of clonality of gene-marked cells. At no point has there been any evidence of oligoclonality or clonal dominance. In particular, for patient 1 who is now almost 3 years from treatment, the marking levels have remained steady and monitoring by LAM PCR shows a diverse polyclonal population with multiple bands seen in all marked lineages. A bone marrow biopsy performed at 1 year and repeated at approximately 3 years after gene therapy shows normal marrow histology and karyotype. Of note, we have also performed an analysis on archived samples from our 1998 X-CGD gene therapy trial. Despite the very low marking, we cloned and characterized 42 unique sites from the first trial, and none involved EV11/MDS, PRDM16 or Set BP1 nor were any sites found within 100 kb of those genes. In addition, none of the original participants has developed any evidence of MDS with follow-up more than 10 years.
Discussion
Gene therapy has achieved lasting clinical benefit and restoration of immunity for some primary immune deficiencies.14,16 However, in disorders such as CGD where there is no selective advantage provided by the corrective therapeutic gene, conditioning appears necessary for long-term persistence of marked cells. In our 1998 gene transfer trial for X-CGD without conditioning, 4 of the 6 patients achieved only very low levels of gene marking, and that marking did not persist. Busulfan conditioning in this current protocol greatly improved initial marking and resulted in persistence of marking in 2 of the 3 patients. Patient 1 had early marking peaking at 26% and has continued stable production of oxidase normal cells composing more than or equal to 1% of circulating neutrophils and monocytes from 7 months to almost 3 years at last follow-up. Because this patient was also treated on the previous gene therapy study without conditioning but using the same vector lot, it is noteworthy that marking in the same patient in the previous study never exceeded 0.055% and did not have any evidence of genetically marked cells 9 months after the last infusion. Although there are some differences in the transduction methods used in these 2 studies, the initial presence of marked cells during the first trial with their subsequent disappearance versus continued presence of marked cells after busulfan supports the need for bone marrow suppression to promote long-term engraftment.
Clinical benefit from adenosine deaminase (ADA)–SCID gene therapy required some busulfan conditioning (4 mg/kg) despite selective growth advantage conferred on lymphocytes. In that setting, marrow engraftment of gene-marked CD34+ cell compartment was 5.1%, versus the 88% seen for the peripheral blood T cells.21,22 Because no selective growth advance was anticipated for correction of X-CGD, we used a higher but still not ablative dose of busulfan (10 mg/kg). The source of CD34s, that is, bone marrow versus mobilized peripheral blood, may also play a role; however, cell dose, which is also a factor, is usually larger with peripheral blood grafts. Ott et al chose busulfan at 8 mg/kg for their trial as they also assumed a greater degree of conditioning was necessary for long-term engraftment in the CGD setting.17 We chose 10 mg/kg based on our own experience of using this dose in an allogeneic transplantation for CGD. The difference between 8 and 10 mg/kg is unknown, although the increased effect may be more than linear.22,23 Patient 1, who had the highest AUC perhaps because of altered metabolism from his liver abscesses, had the highest degree of marking. Despite the elevated AUC and his underlying abscesses, he had no evidence of liver toxicity. Moreover, his duration of neutropenia, thrombocytopenia, and other side effect profile were similar to the other 2 patients.
Our second patient had initial marking of 5% but lost that marking extremely rapidly. Gene silencing occurs often in murine gene transfer studies; it can also occur, although much less often in human cells and human clinical trials24,25 ; and there was evidence for silencing of transgene protein production in the Ott et al CGD gene therapy trial.17 New vectors may incorporate transcriptional insulators to reduce silencing.26,27 However, vector marking by PCR analysis also became undetectable in patient 2 blood in parallel with loss of oxidase activity, confirming that loss of graft rather than gene silencing had occurred. We looked for development of an immune response as an etiology but did not find evidence for this.
Nonetheless, development of an immune response to the transgene, vector components, or other immunogens has clearly been shown to occur in a large animal model of gene therapy.18 Thus, despite our inability to detect an immune cause of the rapid loss of graft in patient 2, we could not ignore this possibility as we planned the treatment of our third patient in this study. Because the purpose of this gene therapy treatment trial is to provide salvage therapy for an infection not controlled by standard therapy, there was concern that we not interpret our failure to detect immune-mediated graft loss as definitive evidence that immune elimination of graft did not occur. We therefore sought to incorporate a way of preventing immunity to the graft and enhancing tolerance without significantly increasing risk to the patient. A consensus settled on short-term treatment with rapamycin, a well-tolerated and widely used immune suppressant with few side effects, a short half-life, and a proven track record of inducing tolerance. For this reason, patient 3 was treated with rapamycin starting 2 days before gene therapy until 30 days after the gene therapy. The graft that patient 3 received had much fewer cells at lower transduction than patient 2, although initial marking of patient 3 was similar to that of patient 2, 4.1% versus 5.0%, respectively. However, 0.03% of circulating neutrophils and monocytes remain oxidase normal in patient 3 at last assessment at 13 months. Although we cannot be conclusive about any benefits from rapamycin, it was well tolerated and a persistence of corrected neutrophils was achieved that did not occur in patient 2.
Regardless of the several mechanisms that may lead to cell clearance, it is clear from our results that both the early and late in vivo marking is directly affected by the ex vivo transduction efficiency and total number of transduced cells infused. This effect of transduction efficiency and cell dose has also been noted in the NOD/SCID mouse xenograft model and in nonhuman primate gene marking studies.28-30 Although profound effects on outcome may also occur from vector insert effects such as observed in the study using the SFFV vector, where gene marking of neutrophils was very high in vivo despite only a 30% ex vivo transduction efficiency, there were undesired side effects from this outgrowth. We did not observe any evidence of insertional mutagenesis or clonal expansion in our patients but do not think that vector insertion driven expansion is a desirable way to enhance gene marking. Although the degree of conditioning we achieved with 10 mg/kg of busulfan significantly improved both the short-term and long-term marking, it is doubtful that higher doses will achieve significantly higher levels of marking without increasing the toxicity beyond acceptable levels. To achieve clinically beneficial levels of marking, we think that maneuvers enhancing ex vivo gene targeting of true hematopoietic stem cells, such as the use of lentivirus vectors, may be one means of reaching this goal.31
The MFGS-gp91phox vector mediates sufficient production of gp91phox transgene to fully correct oxidase function in an X-CGD neutrophil. This was clearly demonstrated in our study by the DHR and supporting quantitative assays of superoxide production. The SFFV vector used in the Ott et al study17 mediated oxidase function correction on a per neutrophil basis that was estimated by the authors as only 15% to 30% of the amount produced by a normal neutrophil. The level of oxidase production on a per neutrophil basis may be as crucial for infection management as overall transduction efficiency because microbial killing occurs within a person cell's phagosome. Although there are safety and other advantages to lentiviral vectors that continue to drive an interest in their development, our experience with production of gp91phox proteins from lentivectors is that production levels to date have been significantly much lower than what we can achieve with the MFGS vector. Even if fewer cells are transduced, cells with normal function can act synergistically with residing abnormal cells to improve infection response.
Patient 1 had complete resolution of his massive liver infection. This is a setting where experience has demonstrated32 that antibiotics alone, without the major surgery that was contraindicated in this patient, would not have cured this infection. Although this does not constitute proof that gene therapy cured his infection, a very strong case can be made for significant clinical benefit to this patient from the gene therapy. Furthermore, this patient has been infection free for almost 23 months, the longest infection free period he has experienced in the last 10 years despite being only partially compliant with his maintenance antimicrobial prophylaxis.
Despite significantly less efficient gene correction than in patient 1, patient 3 may also have obtained clinical benefit from the gene therapy in that the progression of his Aspergillus infection that was occurring before gene therapy stopped, significant resolution of infection was documented, and the infection may be cured. Patient 3 did receive a short course of allogeneic granulocyte transfusions during the neutropenic period after busulfan conditioning, complicating any firm conclusion regarding the exclusive role of the gene therapy in helping to control and resolve his fungus infection.
The 2 X-SCID trials33-36 and the Ott et al trial17 demonstrate that retroviral vectors pose a risk of insertional mutagenesis. There does appear to be a predisposition of the X-SCID phenotype to oncogenesis with gene therapy and possibly a cooperation of the common γ chain therapeutic gene itself to tumorigenesis.37,38 By contrast, there has been no leukemia or myelodysplasia seen in any ADA-SCID gene therapy patients. The CGD trial by Ott et al17 demonstrated a predisposition to outgrowth of clones with vector insertions seen in EVI1, SetBP1, and PRDM16, but this may be in part an effect of the strong myeloid enhancing element of the SFFV vector LTR. Interestingly, these insertions were not seen in an ADA-SCID patient treated with an SFFV-based vector.39 EVI1 has been shown to be a target in other large animal studies using non-SFFV gammaretrovirus vectors,40 although there was no progression to clonal hematopoiesis or leukemia.41 Still, an ex vivo mouse marrow myeloid immortalization study demonstrated that SFFV-transduced clones had a higher rate of transformation, compared with murine stem cell virus-transduced cells (C. Baum, oral communication, June 2008), possibly explaining the difference seen in our study compared with the CGD trial using the SFFV.17 In our current study, we saw no evidence of any clonal outgrowth in our 2 patients who we followed to date to 34 and 11 months of follow-up. Analysis of more than 60 insertions characterized from patient 1 to date did find 1 in EVI1 from 2 separate time points but not in all analyses, nor does it predominate, and the overall pattern of inserts remains polyclonal with no evidence of clonal outgrowth and with continued evidence for normal hematopoiesis (supplemental Table).
A very recent brief report appearing in electronic print described the outcome of gene therapy in an 8.5-year-old X-CGD child who had severe Aspergillus pneumonia.42 The patient received busulfan conditioning of 8.8 mg/kg, and CD34+ cells gene-marked ex vivo at 31% to 34% (using the Ott et al vector17 ) were administered intravenously and intraosseously. The level of early gene marking of neutrophils reported at 26% to 29% was similar to that seen with our patient 1, and as with him, their patient appeared to have significant clinical benefit with control of his infection. Although their report focused on neutrophil extracellular DNA nets as a possible mechanism for benefit, less than 3 months of follow-up from gene therapy is provided in the report, and level of oxidase function per neutrophil cannot be determined from the paper. However, their report supports the notion that gene therapy for X-CGD can help control a life-threatening infection.
We show here the results of 3 patients treated with busulfan and ex vivo genetically modified cells. Patient 1 had unequivocal clinical benefit with continued production of 1% oxidase normal cells in his peripheral blood now almost 3 years after treatment. This represents the highest level of sustained marking with production of functional gene product in neutrophils seen in any patient to date beyond 1 year of follow-up with any disease that does not have a selective advantage conferred by the corrective gene or as a result of unintended clonal outgrowth. Patient 3 may also have benefited in the control of his infection despite the substantially lower level of long-term gene marking. All patients tolerated the busulfan, which appears to be a critical element to achieve engraftment of gene-corrected long-term repopulating cells. Additional progress in the field will require higher rates of gene transfer into pluripotent stem cells. Work to achieve this goal has focused on new vectors, such as lentivectors,43 or novel pseudotyping, such as RD114.43-45 Although gene therapy has not yet cured patients with CGD, the field has made significant progress with the demonstration that gene therapy can provide clinical benefit to patients.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the clinical staff who helped to care for the patients as well as the Cell Processing Section of the Department of Transfusion Medicine at National Institutes of Health for help with the collection, processing, and transduction of the cell products.
National Institutes of Health
Authorship
Contribution: E.M.K. designed and implemented the study and wrote the paper; U.C. performed analyses, created figures, helped with vector design and production, and reviewed the paper; N.T., G.L., D.A.L.P., and D.K. performed the majority of the assays in the study; and H.L.M. helped design and support the study as well as write the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Elizabeth M. Kang, Laboratory of Host Defenses, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bldg 10-CRC, 6W Rm 6-3752, 9000 Rockville Pike, Bethesda, MD 20892; e-mail: ekang@niaid.nih.gov.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal