Abstract
The lymphatic vasculature is important for the regulation of tissue fluid homeostasis, immune response, and lipid absorption, and the development of in vitro models should allow for a better understanding of the mechanisms regulating lymphatic vascular growth, repair, and function. Here we report isolation and characterization of lymphatic endothelial cells from human intestine and show that intestinal lymphatic endothelial cells have a related but distinct gene expression profile from human dermal lymphatic endothelial cells. Furthermore, we identify liprin β1, a member of the family of LAR transmembrane tyrosine phosphatase-interacting proteins, as highly expressed in intestinal lymphatic endothelial cells in vitro and lymphatic vasculature in vivo, and show that it plays an important role in the maintenance of lymphatic vessel integrity in Xenopus tadpoles.
Introduction
The main function of the lymphatic system is to drain extravasated interstitial fluid and to carry antigens to the lymph nodes, where immune responses are provoked by activated lymphocytes. Furthermore, lacteals, lymphatic capillaries in the intestinal villi, are responsible for the uptake of dietary fat and fat-soluble vitamins. Thus, lymphatic vessels from different organs can be functionally and morphologically divergent and consequently display diverse molecular expression patterns.1 Moreover, studies of Vegfc+/− mice show that postnatal internal and skin lymphatic vessels have differential growth requirements.2 However, no studies have been undertaken to investigate the organ-specific differences of lymphatic endothelial cells on a genome-wide level.
Here, we have compared dermal and intestinal lymphatic endothelial cells (LECs), and we show that, although the 2 cell populations display similar gene expression profiles and functional responses, significant differences still exist. Based on our data, we identify liprin β1, previously only reported as a binding partner of calcium-binding protein S100A4,3 as a novel mediator of lymphatic vessel integrity.
Methods
Isolation and analysis of LECs
Intestinal microvascular cells were isolated essentially as described.4,5 After enzymatic digestion of the mucosa from jejunum, cells were seeded on fibronectin-coated plates in Endothelial Cell Growth Medium MV (PromoCell) and subjected to negative selection using α-CD44 antibodies (Clone F10-44-2; Serotec) and paramagnetic beads (Dynal). Dermal lymphatic endothelial cells (dLECs) were isolated from human dermal microvascular endothelial cells (HDMECs; PromoCell) as described.6 All microarray data have been uploaded to ArrayExpress under accession number E-MEXP-2452.
Morpholino knockdown in Xenopus laevis
Xenopus laevis frogs were from Nasco Biology. All animal studies were approved by the ethical committee for animal experimentation of the Katholieke Universiteit Leuven. The generation of the transgenic Tg(Flk1:eGFP) frogs expressing green fluorescent protein (GFP) in the vasculature will be reported elsewhere. Fertilized Xenopus eggs were injected with 50 to 87.5 ng of morpholino (Gene Tools) into the 1-cell stage. The sequence of the ATG-targeted antisense morpholino was designed based on NM_001089047 X laevis sequence of liprin β1 after DNA sequence confirmation using cDNA amplified from Xenopus embryos. Primer and morpholino sequences as well as details of the analysis are provided in the supplemental Methods (available on the Blood website; see the Supplemental Materials link at the top of the online article).
Results and discussion
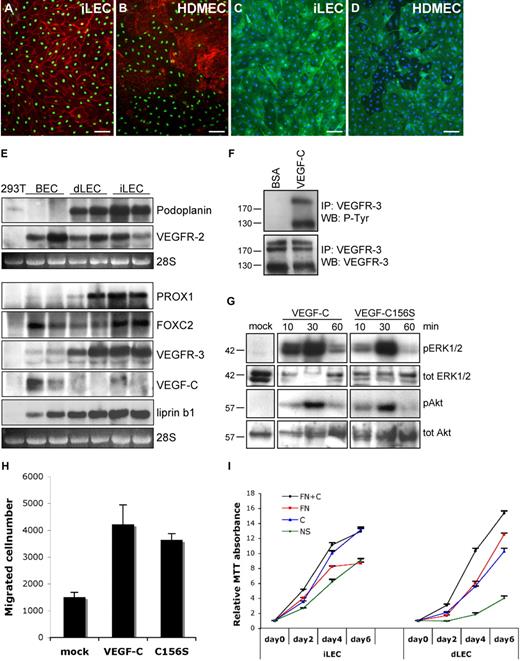
dLECs, isolated as a subpopulation of dermal microvascular endothelial cells, represent a distinct endothelial cell lineage with a characteristic gene expression profile.6-8 To characterize lymphatic endothelial cells from a different vascular bed, we purified human intestinal microvascular endothelial cells.4,5 Surprisingly, the analysis of the resulting endothelial cell fraction revealed that it was composed mainly of lymphatic endothelial cells (iLECs). Indeed, iLECs expressed high levels of LEC markers podoplanin, PROX1 and VEGFR-3 (Figure 1A-E) and, similar to dLECs, produced very low levels of lymphangiogenic growth factor VEGF-C, whereas the latter was produced by dermal blood vascular endothelial cells (BECs; Figure 1E).9 VEGFR-2, a major transducer of VEGF-mediated signaling, was present in all 3 cell populations (Figure 1E). Interestingly, iLECs expressed higher levels of forkhead transcription factor FOXC2, shown to control lymphatic vascular maturation.10,11
Characterization of iLECs. (A-D) Immunofluorescent staining of iLECs (A,C) and human dermal microvascular endothelial cells (HDMECs; B,D) for PROX1 (green) and β-catenin (red; A-B) or podoplanin (green) and 4,6-diamidino-2-phenylindole (DAPI; blue; C-D). HDMECs are mixed dermal endothelial cell populations containing both LECs and BECs. BECs are PROX1-negative and express higher levels of β-catenin.9 Bars represent 50 μm. (E) Northern blotting of mRNA from 293T cells, and BECs, dLECs, and iLECs from 2 different persons hybridized with probes for the indicated transcripts. The 28S ribosomal RNA is shown as loading control. (F) Increased VEGFR-3 phosphorylation on stimulation of iLECs with VEGF-C. (G) Activation of ERK1/2 and Akt phosphorylation after stimulation of iLECs with VEGF-C or VEGF-C156S. Total ERK1/2 and Akt levels are shown as control. (H) VEGF-C and VEGF-C156S induce iLEC migration. The results are shown as mean ± SEM. (I) VEGF-C and fibronectin (FN) enhance survival and proliferation of iLECs and dLECs. The results are shown as mean, relative to day 0, ± SEM.
Characterization of iLECs. (A-D) Immunofluorescent staining of iLECs (A,C) and human dermal microvascular endothelial cells (HDMECs; B,D) for PROX1 (green) and β-catenin (red; A-B) or podoplanin (green) and 4,6-diamidino-2-phenylindole (DAPI; blue; C-D). HDMECs are mixed dermal endothelial cell populations containing both LECs and BECs. BECs are PROX1-negative and express higher levels of β-catenin.9 Bars represent 50 μm. (E) Northern blotting of mRNA from 293T cells, and BECs, dLECs, and iLECs from 2 different persons hybridized with probes for the indicated transcripts. The 28S ribosomal RNA is shown as loading control. (F) Increased VEGFR-3 phosphorylation on stimulation of iLECs with VEGF-C. (G) Activation of ERK1/2 and Akt phosphorylation after stimulation of iLECs with VEGF-C or VEGF-C156S. Total ERK1/2 and Akt levels are shown as control. (H) VEGF-C and VEGF-C156S induce iLEC migration. The results are shown as mean ± SEM. (I) VEGF-C and fibronectin (FN) enhance survival and proliferation of iLECs and dLECs. The results are shown as mean, relative to day 0, ± SEM.
Stimulation of iLECs with VEGF-C, which activates VEGFR-3 and VEGFR-2, or with its mutant form VEGF-C156S, which only activates VEGFR-3, increased cell proliferation, migration, and phosphorylation of ERK1/2 and Akt (Figure 1F-I). Thus, similar to dLECs, iLECs respond to lymphangiogenic stimuli provided by VEGF-C/VEGFR-3. Interestingly, however, whereas dLECs required the presence of exogenous VEGF-C or extracellular matrix protein fibronectin for efficient survival and proliferation,12 iLECs were able to grow even in their absence (Figure 1I).
To get a better understanding of differences between intestinal and dermal LECs, we compared expression profiles of 5 dLEC and 6 iLEC samples from different persons. Overall expression profiles of dLECs and iLECs were similar, and none of pan-endothelial or lymphatic endothelial specific markers was differentially expressed between the 2 cell populations, confirming the purity of our iLEC preparations (supplemental Table 1). However, the expression levels of 206 genes were found to be statistically significantly different between dLECs and iLECs, of which 29 genes were differentially expressed with log2 more than or equal to 1 (supplemental Table 2, functional annotation).
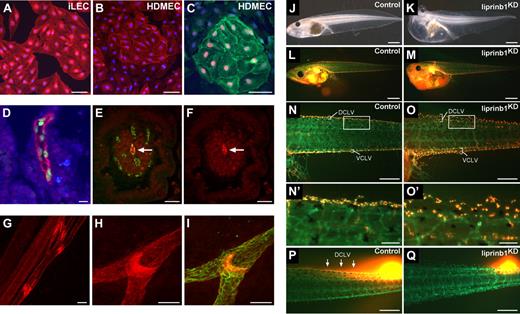
The microarray analysis revealed high levels of the LAR protein-tyrosine phosphatase-interacting protein liprin β1 (PTPRF interacting protein, binding protein 1 [PPFIBP1]) in iLECs (supplemental Table 1). The liprin family is composed of 2 subfamilies: liprin α1-4 and liprin β1-2.13 α-Liprins are important for synaptic development and axon guidance,14-18 but the physiologic role of β-liprins has not been studied.3,13 In line with the microarray data, we found that liprin β1 is highly expressed in iLECs, whereas lower levels were found in cultured dLECs and BECs (Figures 1E, 2A-C). Importantly, liprin β1 was expressed in vivo in PROX1-positive lacteals in humans, whereas blood capillaries were negative (Figure 2D-F). In mouse tissues, liprin β1 expression was observed in lacteals, skin, and mesenteric collecting lymphatic vessels and their valves (Figure 2G-I; and data not shown), whereas the skin lymphatic capillaries were liprin β1 negative (data not shown).
Liprin β1 is expressed in lymphatic endothelial cells and mediates lymphatic vessel integrity. (A-B) Immunofluorescent staining of iLECs (A) and HDMECs (B) for liprin β1 (red) and DAPI (blue). HDMECs are mixed dermal endothelial cell populations containing both LECs and BECs. Bars represent 50 μm. (C) Immunofluorescent staining of HDMECs for liprin β1 (green), PROX1 (red), and DAPI (blue). HDMECs are mixed dermal endothelial cell populations containing both LECs and BECs. Bar represents 50 μm. (D) Liprin β1 is expressed in human small intestinal lacteals. Liprin β1 (red), PROX1 (green), and DAPI (blue). Bar represents 20 μm. (E-F) Blood capillaries in small intestinal villi do not express liprin β1. Immunofluorescent staining of liprin β1 (red) and VE-cadherin (green). Arrow indicates lacteal. Bars represent 50 μm. (G-I) Whole-mount immunofluorescent staining of the mouse mesentery at P5 for liprin β1 (red) and VEGFR-3 (green). Note the high liprin β1 expression in lymphatic valves. Bars represent 100 μm. (J-K) Liprin β1 knockdown in Xenopus tadpoles results in edema formation. Tadpoles are from stage 45. Bar represents 1000 μm. (L-O′) Impaired assembly of DCLVs and ventral caudal lymph vessels (VCLVs) in stage 46 liprin β1-morphants of the Tg(Flk1:eGFP) transgenic line. Lymphatic endothelial cells are identified by the uptake of TRITC-dextran after intracardial injection and extravasation from blood vessels, whereas blood vessels express only GFP. Note grossly normal blood vascular assembly. (N′-O′) Close-up view of DCLVs from panels N-O. Bars represent the following: L-M, 1000 μm; N-O, 500 μm; N′-O′, 100 μm. (P-Q) Impaired drainage of locally injected fluorescent TRITC-dextran dye after liprin β1 knockdown. Arrows indicate normal dye drainage in control tadpoles. Tadpoles are at stage 46. Bar represents 500 μm.
Liprin β1 is expressed in lymphatic endothelial cells and mediates lymphatic vessel integrity. (A-B) Immunofluorescent staining of iLECs (A) and HDMECs (B) for liprin β1 (red) and DAPI (blue). HDMECs are mixed dermal endothelial cell populations containing both LECs and BECs. Bars represent 50 μm. (C) Immunofluorescent staining of HDMECs for liprin β1 (green), PROX1 (red), and DAPI (blue). HDMECs are mixed dermal endothelial cell populations containing both LECs and BECs. Bar represents 50 μm. (D) Liprin β1 is expressed in human small intestinal lacteals. Liprin β1 (red), PROX1 (green), and DAPI (blue). Bar represents 20 μm. (E-F) Blood capillaries in small intestinal villi do not express liprin β1. Immunofluorescent staining of liprin β1 (red) and VE-cadherin (green). Arrow indicates lacteal. Bars represent 50 μm. (G-I) Whole-mount immunofluorescent staining of the mouse mesentery at P5 for liprin β1 (red) and VEGFR-3 (green). Note the high liprin β1 expression in lymphatic valves. Bars represent 100 μm. (J-K) Liprin β1 knockdown in Xenopus tadpoles results in edema formation. Tadpoles are from stage 45. Bar represents 1000 μm. (L-O′) Impaired assembly of DCLVs and ventral caudal lymph vessels (VCLVs) in stage 46 liprin β1-morphants of the Tg(Flk1:eGFP) transgenic line. Lymphatic endothelial cells are identified by the uptake of TRITC-dextran after intracardial injection and extravasation from blood vessels, whereas blood vessels express only GFP. Note grossly normal blood vascular assembly. (N′-O′) Close-up view of DCLVs from panels N-O. Bars represent the following: L-M, 1000 μm; N-O, 500 μm; N′-O′, 100 μm. (P-Q) Impaired drainage of locally injected fluorescent TRITC-dextran dye after liprin β1 knockdown. Arrows indicate normal dye drainage in control tadpoles. Tadpoles are at stage 46. Bar represents 500 μm.
The high level of sequence identity between human and X laevis proteins suggested an evolutionary conserved role for liprin β1. Furthermore, liprin β1 is expressed in X laevis lymphatic endothelial cells (supplemental Figure 1). We down-regulated liprin β1 in X laevis tadpoles, a recently established model for studies of lymphangiogenesis, using a morpholino-mediated knockdown approach.19 Silencing of liprin β1 resulted in edema, suggesting defects in lymphatic vascular development (Figure 2J-K). We next analyzed the Tg(Flk1:eGFP) line, in which lymphatic vessels are identified by their uptake of tetramethylrhodamine isothiocyanate (TRITC)–dextran after intracardial injection and dye extravasation. Notably, whereas the GFP+ blood vessels appeared normal in liprin β1 morphants, GFP+;TRITC+ lymphatic endothelial cells displayed dispersed appearance, demonstrating failure of dorsal caudal lymph vessels (DCLVs) and ventral caudal lymph vessels (VCLVs) to coalesce into a single compact vessel (Figure 2L-O′). Functional analysis by fluorescent lymphangiography moreover demonstrated impaired dye drainage by the DCLVs (Figure 2P-Q). Taken together with the expression of liprin β1 in lacteals and collecting lymphatic vessels in higher vertebrates, ie, anatomic locations where lymphatic vessels are expected to be highly stable, these results suggest a previously unsuspected role of liprin β1 in the regulation of lymphatic vessel integrity.
In conclusion, we have isolated and characterized lymphatic endothelial cells from human intestine and compared them with dermal lymphatic endothelial cells. The 2 lymphatic endothelial cell types display comparable expression of known lymphatic endothelial cell markers and responses to lymphangiogenic stimuli; however, they are also characterized by distinct expression profiles and growth requirements. Furthermore, we identify liprin β1 as a potential novel regulator of lymphatic vessel integrity. These results provide a basis for further research to study the mechanisms of formation of functional lymphatic vasculature in different organs.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank A. Parsons, T. Tainola, S. Wallin, K. Makkonen, S. Lampi, P. Hyvärinen, and M. Helanterä for technical support; S. Vinkx, P. Vandervoort, and A. Luttun for help with Xenopus experiments; and the Biomedicum Helsinki Molecular Imaging Unit for assistance on imaging. Microarray analysis was carried out at Biomedicum Biochip Center.
This work was supported by the Academy of Sciences of Finland, the Sigrid Juselius Foundation, the Louis-Jeantet Foundation, European Union (Lymphangiogenomics LSHG-CT-2004-503573 and grant EU7 TuMIC-HEALTH-F2-2008-201662), Magnus Erhnrooth Foundation, Swiss National Science Foundation (PPP0033-114898), and the National Institutes of Health (R01 HL 75183-01).
National Institutes of Health
Authorship
Contribution: C.N. designed the research, performed experiments, and wrote the paper; W.V., A.N., and P.S., performed experiments; M.G. analyzed Affymetrix data; G.H. provided iLECs at initial stages and discussed results; P.P. provided intestinal samples; E.L. produced liprin β1 antibodies and discussed results; M.D. designed the research, performed experiments, and wrote the paper; K.A. designed the research and wrote the paper; and T.V.P. designed the research, performed experiments, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current address of C.N. is Institute of Cell Biology, ETH Zürich, CH-8093 Zurich, Switzerland. The current address of T.V.P. is Division of Experimental Oncology, Multidisciplinary Oncology Center, University of Lausanne and CHUV, Epalinges, Switzerland.
Correspondence: Kari Alitalo, Molecular and Cancer Biology Program, Biomedicum Helsinki, POB 63 (Haartmaninkatu 8), 00014 University of Helsinki, Helsinki, Finland; e-mail: kari.alitalo@helsinki.fi; and Tatiana V. Petrova, Division of Experimental Oncology, Multidisciplinary Oncology Center, University of Lausanne and CHUV, Ch Des Boveresses 155, CH-1066 Epalinges, Switzerland; e-mail: tatiana.petrova@unil.ch.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal