Abstract
HIV up-regulates cell-surface expression of specific ligands for the activating NKG2D receptor, including ULBP-1, -2, and -3, but not MICA or MICB, in infected cells both in vitro and in vivo. However, the viral factor(s) involved in NKG2D ligand expression still remains undefined. HIV-1 Vpr activates the DNA damage/stress-sensing ATR kinase and promotes G2 cell-cycle arrest, conditions known to up-regulate NKG2D ligands. We report here that HIV-1 selectively induces cell-surface expression of ULBP-2 in primary CD4+ T lymphocytes by a process that is Vpr dependent. Importantly, Vpr enhanced the susceptibility of HIV-1–infected cells to NK cell–mediated killing. Strikingly, Vpr alone was sufficient to up-regulate expression of all NKG2D ligands and thus promoted efficient NKG2D-dependent NK cell–mediated killing. Delivery of virion-associated Vpr via defective HIV-1 particles induced analogous biologic effects in noninfected target cells, suggesting that Vpr may act similarly beyond infected cells. All these activities relied on Vpr ability to activate the ATR-mediated DNA damage/stress checkpoint. Overall, these results indicate that Vpr is a key determinant responsible for HIV-1–induced up-regulation of NKG2D ligands and further suggest an immunomodulatory role for Vpr that may not only contribute to HIV-1–induced CD4+ T-lymphocyte depletion but may also take part in HIV-1–induced NK-cell dysfunction.
Introduction
HIV-1 has evolved multiple strategies to induce a persistent infection in hosts. Several of these strategies rely on an array of virally encoded accessory proteins, including Vif, Vpr, Vpu, and Nef, which collectively appear to manipulate host cell biology as a means to ensure a favorable cellular state for viral replication, transmission, dissemination, and immune evasion.1 Vpr (Viral protein R), one of these accessory proteins, is a 96-amino acid protein that is expressed at the late stage of the virus life cycle but is present during the early steps of infection because it is packaged into viral particles via an interaction with the p6 domain of Gag.2,3 The protein also exists in an extracellular form because it can be detected in the serum and cerebrospinal fluid of HIV-1–infected persons.4 One of the main biologic activities of Vpr is the induction of a G2 cell-cycle arrest.5-7 Interestingly, soluble and virion-associated Vpr molecules also display cytostatic activities,8-10 raising the possibility that Vpr may exert this biologic activity beyond infected cells. The observations that Vpr-mediated G2 cell-cycle arrest is well conserved among the primate lentiviruses11,12 and that HIV-1–infected persons display an abnormal number of cells accumulating in the G2 phase13 suggest that this activity probably plays an important role in HIV-1 pathogenesis. Although its functional significance is still not well understood, its mechanism has recently been in part elucidated. Vpr appears to induce a G2 cell-cycle arrest by mimicking a DNA stress/damage checkpoint arrest initiated by the DNA damage-sensing protein kinase ATR (ataxia telangiectasia-mutated and Rad3-related).14 The proximal events that trigger G2 cell-cycle arrest were recently found to rely on the engagement by Vpr of a cullin-RING E3 ubiquitin (Ub) ligase complex, DDB1-CUL4A (VprBP). The recruitment of the complex would lead to polyubiquitination and proteasomal degradation of a yet unknown cellular protein(s) resulting ultimately in activation of ATR signaling pathway.15-21
DNA stress/damage checkpoint pathways initiated by ATM (ataxia telangiectasia-mutated) or ATR protein kinases are essential to the maintenance of genomic integrity and stability, but interestingly, recent findings suggest that they are also involved in innate immune surveillance. Indeed, it has recently been demonstrated that genotoxic agents up-regulate expression of ligands of the activating natural killer group 2, member D (NKG2D) receptor through the activation of ATM and ATR and enhance destruction of treated cells by natural killer (NK) cells.22 NKG2D is a potent activating receptor expressed not only on NK cells, but also on γδ T cells, CD8+ T cells and a small subset of CD4+ T cells.23,24 Human NKG2D ligands consist of 2 classes of MHC-I-like molecules: MHC-1-related chains (MIC) and human cytomegalovirus UL16 binding proteins (ULBP), which are generally poorly expressed by normal cells and up-regulated on virus-infected, tumor, and stressed cells.23,24 Activating signals delivered through the NKG2D receptor expressed by NK and T cells induce the killing of pathogen-infected cells as well as cancer cells both in vitro and in vivo.23,24
Because HIV-1 infection of primary CD4+ T lymphocytes was recently reported to increase cell-surface expression of specific NKG2D ligands both in vitro and in vivo,25-27 we investigated in the present study whether the HIV-1 Vpr accessory protein could regulate the expression of ligands for the activating NKG2D receptor and modulate NK-cell cytotoxic responses. Herein, we provide evidence that Vpr, in the absence of any other viral gene products, is sufficient to up-regulate cell-surface expression of MICA, MICB, and ULBP-1, -2, and -3, with the strongest effect observed with ULBP-2. We also show that Vpr-mediated up-regulation of ULBP-2 is dependent on the ability of Vpr to engage the DDB1-CUL4A (VprBP) E3 Ub ligase and to activate the ATR-mediated DNA damage/stress checkpoint. Importantly, we show that up-regulation of ULBP-2 can be induced by intracellular as well as virion-associated Vpr leading to an enhanced susceptibility of cells to NK cell–mediated killing. These data indicate that Vpr is a key determinant responsible for HIV-1–induced up-regulation of cell-surface NKG2D ligands and further suggest an immunomodulatory role for Vpr.
Methods
Antibodies and reagents
Mouse antihuman ULBP-1,-2, -3, MIC-A or -B monoclonal antibodies (mAbs), soluble NKG2D-IgG Fc fusion proteins and matched IgG Fc fusion molecules, interfering Abs to NKG2D and matched-IgG control Abs were obtained from R&D Systems and the fluorochrome-conjugated secondary Abs from Invitrogen. The anti-p24 (HB-9725) and anti-HA (12CA5) mAbs were isolated from supernatants of cultured hybridoma cells obtained from ATCC. The anti-Vpr mouse mAb, 8D1, was a kind gift of Dr Y. Ishizaka (Research Institute, International Medical Center of Japan, Tokyo, Japan).4 The Chk2-phospho(Thr68) Abs were obtained from Cell Signaling, and the H2AX-phospho(Ser139) Abs were from Upstate Biotechnology. Phytohemagglutinin-L, aphidicolin (APC), caffeine, and indinavir were purchased from Sigma-Aldrich, KU55933 from Calbiochem, and the human recombinant interleukin-2 (rIL-2) from the National Institutes of Health AIDS Research and Reference Reagent Program.28
Cell lines and isolation of primary cells
HEK 293T and HeLa TZM cells were cultured as described previously.29 CEM.NKR T cells were cultured in RPMI 1640 complete medium (20% fetal bovine serum, 100 U/mL penicillin, 100 μg/mL streptomycin). Peripheral blood samples were obtained from healthy, HIV-1-seronegative adult donors who gave written informed consent in accordance with the Declaration of Helsinki under research protocols approved by the research ethics review board of the Institut de Recherches Cliniques de Montréal. Peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll-Paque centrifugation as recommended by the manufacturer (GE Healthcare). NK cells and CD4+ T lymphocytes were purified from freshly isolated PBMCs by negative selection using immunomagnetic beads according to the manufacturer's instructions (StemCell Technologies). NK cells were cultured overnight in RPMI 1640 complete medium before use. CD4+ T lymphocytes were activated with phytohemagglutinin-L (5 μg/mL) for 48 hours and then maintained in RPMI 1640 complete medium supplemented with rIL-2 (100 U/mL).
Plasmids and proviral DNA constructs
The plasmids pSVCMV-VprWT, pSVCMV-HA-VprWT, pSVCMV-Vpr-R80A, and pSVCMV-IN-VSVg were described previously.15,30 The plasmids pSVCMV-VprQ65R and pSVCMV-HA-VprQ65R were generated by site-directed mutagenesis. The lentiviral vectors pWPI as well as the packaging plasmid psPAX2 were kindly provided by D. Trono (School of Life Sciences, Swiss Federal Institute of Technology, Lausanne, Switzerland). The lentiviral vector pWPI-VprWT was described previously,15 whereas pWPI-VprR80A and pWPI-VprQ65R were generated using a similar strategy. The Vpr-, reverse transcriptase-, and integrase-defective provirus construct HxBruΔVprΔRTΔIN was described previously.31 The pNL4.3 infectious molecular clone was obtained from the National Institutes of Health AIDS Research and Reference Reagent Program,32 and pNL4.3ΔVpr was generated by insertion of a frameshift mutation in a unique AflII site. The infectious isogenic CCR5-tropic HxBru.ADA.GFP and HxBru(Vpr-).ADA.GFP proviruses coexpress Nef and GFP (green fluorescent protein) from an internal ribosome entry site-containing open reading frame. The Vpr-defective HxB89LF-PS-R- proviral construct that contains point mutations in the P6 domain of Gag, which prevent incorporation of Vpr, was a kind gift from Dr H. Göttlinger (University of Massachussetts Medical School, Worcester, MA).33
Production of lentiviral vectors and HIV-1 viruses
Lentiviral vectors and HIV-1 infectious viruses were produced and titrated as described previously.15,29 Noninfectious HIV-1-defective viruses trans-packaged with VprWT or VprQ65R were produced by transient transfection of 40 μg of provirus construct (NL4.3ΔVpr or HxBruΔVprΔRTΔIN), 30 μg of pSVCMV-VprWT or pSVCMV-VprQ65R, and 12 μg of pSVCMV-IN-VSV-G, in 5 × 106 HEK 293Tcells using the standard calcium phosphate method. To produce defective particles, 100nM of indinavir was added to the medium 24 hours after transfection. Defective viruses were titrated by p24 enzyme-linked immunosorbent assay as recommended by the manufacturer (AIDS and Cancer virus program, National Cancer Institute-Frederick). To evaluate the packaging efficiency of VprWT and VprQ65R, virus particles were produced similarly using NL4.3ΔVpr or HxBR89LF-PS-R- provirus construct and pSVCMV-HA-VprWT or pSVCMV-HA-VprQ65R plasmids.
Lentiviral vector transduction and viral infection
Infection of activated primary CD4+ T lymphocytes was performed as previously described.29 CEM.NKR T cells and primary CD4+ T lymphocytes were transduced with lentiviral vectors by spinoculation (1200g for 2 hours at 25°C with 8μg/mL of polybrene) at a multiplicity of infection (MOI) of 1.0. Exposure of primary CD4+ T lymphocytes to noninfectious HIV-1 particles was also performed by spinoculation, using equivalent amounts of viral particles.
Cytotoxicity assay
Infected primary CD4+ T-cell targets were prepared by sorting infected GFP-expressing primary CD4+ T cells using an Influx cell sorter (BD Biosciences). The whole-cell population was used as targets in case of CEM.NKR T cells transduced with lentiviral vectors or primary CD4+ T cells exposed to noninfectious defective particles. NK cells obtained from the same donors who provided CD4+ T cells were used for cytotoxicity assay. For some experiments, saturating concentrations (10 μg/mL) of NKG2D Abs or matched-IgG control Abs were added to NK cells or soluble NKG2D-IgG Fc fusion proteins (3 μg/mL) and matched IgG Fc fusion molecules were added to target cells for 30 minutes at 4°C before cytotoxic assay and were maintained throughout the assay. Determination of NK-cell killing of target cells was done using a standard 4-hour 51Cr release assay, as described elsewhere.34
Results
HIV-1 up-regulates cell-surface expression of NKG2D ligand ULBP-2 in primary CD4+ T cells in a Vpr-dependent manner
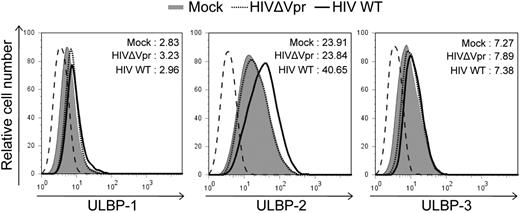
HIV-1 was recently shown to up-regulate the cell-surface expression of NKG2D ligands, ULBP-1, -2, -3, but not MICA or MICB in infected primary CD4+ T cells.25 To determine whether the Vpr accessory protein encoded by HIV-1 was playing a role in this up-regulation, human primary CD4+ T cells were infected with Vpr+ or Vpr-defective isogenic CCR5-tropic GFP-expressing HIV-1 virus and cell-surface expression of the NKG2D ligands were analyzed by flow cytometry 5 days after infection. Basal expression of NKG2D ligands at the cell-surface of mock-infected CD4+ T lymphocytes was at the limit of detection except for ULBP2 (Figure 1; and data not shown for MICA and MICB), even though these ligands could be specifically detected in various transformed cell lines after treatment of cells with APC, an inhibitor of cellular DNA polymerase and a known inducer of NKG2D ligands22 (supplemental Figure 1, available on the Blood website; see the Supplemental Materials link at the top of the online article; and Figure 3). HIV-1 infection consistently induced an increased expression of ULBP-2 on infected cells (mean fluorescence intensity [MFI] = 40.65 vs 23.91 for mock), yet this up-regulation of ULBP-2 was completely abrogated when cells were infected with Vpr-defective viruses (MFI = 23.84; Figure 1). In contrast, infection of primary CD4+ T cells with Vpr+ or Vpr-defective virus did not lead to any significant change in the expression levels of ULBP-1, ULBP-3, MICA, or MICB (Figure 1; and data not shown) compared with mock-infected cells. Thus, HIV-1 selectively induces cell-surface expression of the NKG2D ligand ULBP-2 by a process that is dependent on Vpr.
CD4+ T lymphocytes infected with HIV-1 express ULBP-2 in a Vpr-dependent manner. Human primary CD4+ T lymphocytes were mock-infected or infected with infectious CCR5-tropic HxBru.ADA.GFP or HxBru(Vpr-)ADA.GFP at an MOI of 0.5. After 5 days, mock-infected or GFP-expressing infected CD4+ T lymphocytes were monitored for expression of NKG2D ligands by flow cytometry using specific mAbs directed against ULBP-1, -2, and -3 and appropriate fluorochrome-conjugated secondary reagents. The histogram with the dashed line represents cells stained with the isotype control Abs; the filled histogram represents mock-infected cells, and the histograms with the bold and dotted lines represent, respectively, Vpr+ (HIV WT) and Vpr-defective (HIVΔVpr) HIV-infected cells, as indicated. MFI values were calculated by subtracting the corresponding isotype control values. Results shown are representative of the data obtained from 5 different donors.
CD4+ T lymphocytes infected with HIV-1 express ULBP-2 in a Vpr-dependent manner. Human primary CD4+ T lymphocytes were mock-infected or infected with infectious CCR5-tropic HxBru.ADA.GFP or HxBru(Vpr-)ADA.GFP at an MOI of 0.5. After 5 days, mock-infected or GFP-expressing infected CD4+ T lymphocytes were monitored for expression of NKG2D ligands by flow cytometry using specific mAbs directed against ULBP-1, -2, and -3 and appropriate fluorochrome-conjugated secondary reagents. The histogram with the dashed line represents cells stained with the isotype control Abs; the filled histogram represents mock-infected cells, and the histograms with the bold and dotted lines represent, respectively, Vpr+ (HIV WT) and Vpr-defective (HIVΔVpr) HIV-infected cells, as indicated. MFI values were calculated by subtracting the corresponding isotype control values. Results shown are representative of the data obtained from 5 different donors.
Vpr enhances the susceptibility of HIV-1–infected CD4+ T lymphocytes to NK cell–mediated killing
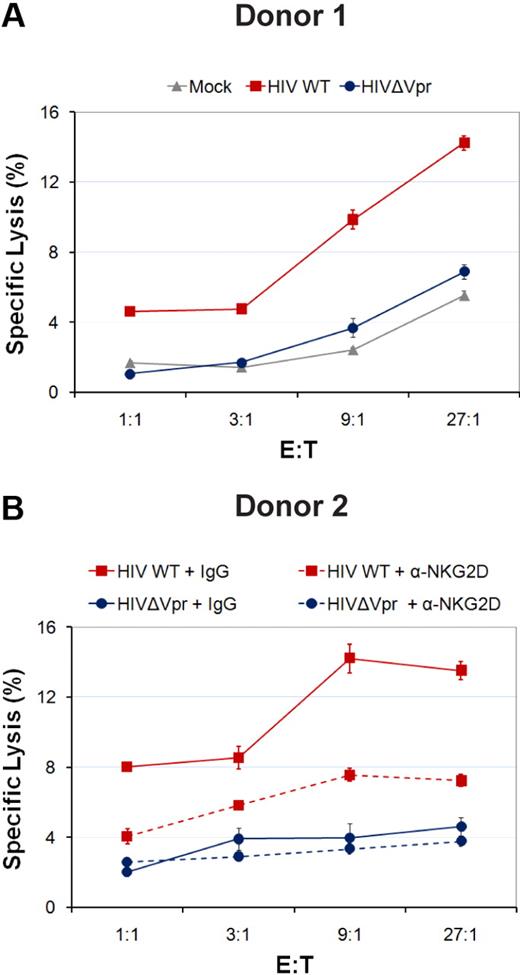
Because HIV-1 up-regulates cell-surface expression of ULBP-2 in a Vpr-dependent manner, we next determined whether the presence of Vpr triggers NK cells to kill infected cells. For this purpose, we evaluated the ability of nonactivated human primary NK cells to kill autologous CD4+ T lymphocytes infected with Vpr+ or Vpr-defective GFP-expressing HIV-1 virus in a 4-hour 51Cr release assay. As expected, cells infected with WT HIV-1 virus displayed a marked increase in their susceptibility to NK-cell killing relative to cells infected with Vpr-defective virus and mock-infected cells (Figure 2A). The extent of killing of Vpr-defective HIV-1–infected cells by NK cells was very similar to that of the mock-infected cells (Figure 2A). Importantly, the increased susceptibility to NK-cell killing was significantly reduced when the ability of the NKG2D receptor to bind its ligands was blocked using NKG2D Abs (Figure 2B), thus indicating that the effect of Vpr in triggering NK-cell killing was mediated in a large part through the NKG2D receptor. Thus, expression of Vpr during HIV-1 infection of primary CD4+ T lymphocytes promotes NK cell–mediated killing at least in part through the NKG2D receptor.
HIV-1 Vpr enhances the killing of HIV-1–infected CD4+ T lymphocytes by autologous NK cells. Human primary CD4+ T lymphocytes were mock-infected or infected with infectious CCR5-tropic HxBru.ADA.GFP or HxBru(Vpr-)ADA.GFP at an MOI of 0.5. After 5 days, mock-infected or GFP-expressing infected primary CD4+ T lymphocytes were sorted and subsequently exposed to autologous primary NK cells in a 4-hour 51Cr release assay in the absence (A) or presence (B) of interfering Abs to NKG2D (α-NKG2D) or matched-IgG control Abs (IgG), as indicated. Error bars represent SEM. Results shown are representative of the data obtained with 3 different donors.
HIV-1 Vpr enhances the killing of HIV-1–infected CD4+ T lymphocytes by autologous NK cells. Human primary CD4+ T lymphocytes were mock-infected or infected with infectious CCR5-tropic HxBru.ADA.GFP or HxBru(Vpr-)ADA.GFP at an MOI of 0.5. After 5 days, mock-infected or GFP-expressing infected primary CD4+ T lymphocytes were sorted and subsequently exposed to autologous primary NK cells in a 4-hour 51Cr release assay in the absence (A) or presence (B) of interfering Abs to NKG2D (α-NKG2D) or matched-IgG control Abs (IgG), as indicated. Error bars represent SEM. Results shown are representative of the data obtained with 3 different donors.
Expression of Vpr alone is sufficient to increase the expression of NKG2D ligands
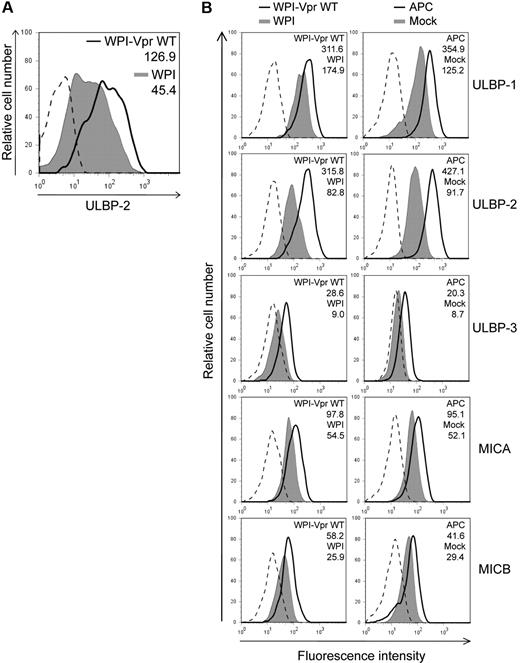
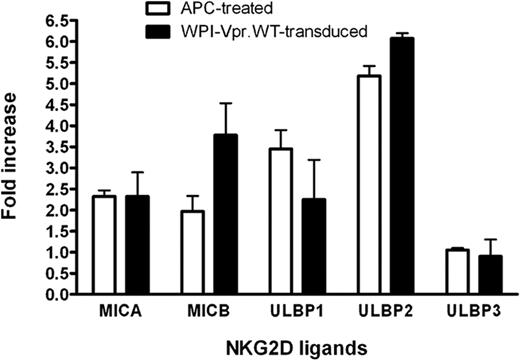
Having shown that HIV-1 up-regulated ULBP-2 expression in a Vpr-dependent manner, we then evaluated whether the expression of Vpr, in the absence of any other HIV-1 gene products, was sufficient to mediate a similar increase in the expression of ULBP-2. To this end, human primary CD4+ T lymphocytes were transduced with lentiviral vectors that coexpressed Vpr and GFP (WPI-VprWT) or GFP alone (WPI) and GFP-expressing cells were analyzed for ULBP-2 expression 48 hours after transduction. Figure 3A reveals that CD4+ T lymphocytes transduced with the Vpr-expressing lentiviral vector displayed an increased expression of ULBP-2 at the cell surface (MFI = 126.9) relative to control vector-transduced cells (MFI = 45.4). To further analyze the effect of Vpr on the expression of NKG2D ligands and on NK-cell cytotoxic responses in a more sensitive system, we took advantage of a natural subclone of a transformed human NK cell–resistant CD4+ T-lymphoblastoid cell line CEM, designated CEM.NKR. Transduction of CEM.NKR T cells with WPI-VprWT led to a detectable increase of all tested NKG2D ligands at the cell surface of GFP+ cells relative to the WPI control (Figure 3B). Consistent with the results obtained with infected primary CD4+ T cells, ULBP-2 showed the strongest up-regulation in the presence of Vpr (MFI = 315.8 vs 82.8 for the control). This effect of Vpr on cell-surface expression of the NKG2D ligands correlated with an up-regulation of the ligands at the mRNA level (Figure 4). Similarly, CEM.NKR T cells treated with APC expressed higher levels of the NKG2D ligands at the cell surface and at the mRNA level (Figures 3B, 4). Overall, these results indicate that Vpr alone is sufficient to up-regulate expression of NKG2D ligands.
Up-regulation of cell-surface NKG2D ligands in cells expressing HIV-1 Vpr. (A) Human primary CD4+ T lymphocytes were transduced with lentiviral vectors expressing GFP alone (WPI) or coexpressing GFP and VprWT (WPI-VprWT). GFP-expressing cells were monitored for ULBP-2 cell-surface expression by flow cytometry 48 hours after transduction using specific mAbs directed against ULBP-2 and appropriate fluorochrome-conjugated secondary reagents. (B) CEM.NKR T cells were transduced with WPI-VprWT or WPI lentiviral vectors or treated with APC (4μM) as indicated. Cell-surface expression of NKG2D ligands was monitored on the GFP-expressing cells 48 hours after transduction or after a 24-hour treatment with APC, using specific mAbs directed against ULBP-1, -2, and -3, MICA, MICB, and appropriate fluorochrome-conjugated secondary reagents. MFI values were calculated by subtracting the corresponding isotype control values (dashed line). Results shown are representative of the data obtained from at least 2 independent experiments.
Up-regulation of cell-surface NKG2D ligands in cells expressing HIV-1 Vpr. (A) Human primary CD4+ T lymphocytes were transduced with lentiviral vectors expressing GFP alone (WPI) or coexpressing GFP and VprWT (WPI-VprWT). GFP-expressing cells were monitored for ULBP-2 cell-surface expression by flow cytometry 48 hours after transduction using specific mAbs directed against ULBP-2 and appropriate fluorochrome-conjugated secondary reagents. (B) CEM.NKR T cells were transduced with WPI-VprWT or WPI lentiviral vectors or treated with APC (4μM) as indicated. Cell-surface expression of NKG2D ligands was monitored on the GFP-expressing cells 48 hours after transduction or after a 24-hour treatment with APC, using specific mAbs directed against ULBP-1, -2, and -3, MICA, MICB, and appropriate fluorochrome-conjugated secondary reagents. MFI values were calculated by subtracting the corresponding isotype control values (dashed line). Results shown are representative of the data obtained from at least 2 independent experiments.
Augmentation of NKG2D ligand mRNA expression in CEM.NKR T cells expressing HIV-1 Vpr. CEM.NKR T cells were transduced with lentiviral vectors expressing GFP alone (WPI) or coexpressing GFP and VprWT (WPI-VprWT). GFP+ cells were sorted for analysis 48 hours after transduction. Alternatively, transduced cells were treated (or not) with 4μM APC for 24 hours. DNase-treated RNA was analyzed for NKG2D ligand expression by real-time reverse-transcriptase polymerase chain reaction. Target gene expression in Vpr-transduced and APC-treated CEM.NKR T cells was normalized for glyceraldehyde 3-phosphate dehydrogenase and hypoxanthine-guanine phosphoribosyltransferase (HPRT) expression, and the data were subsequently expressed as a fold increase relative to WPI-transduced or untreated cells, respectively. Results shown represent a mean fold increase. Error bars represent SEM. Results are representative of the data obtained from 4 independent experiments.
Augmentation of NKG2D ligand mRNA expression in CEM.NKR T cells expressing HIV-1 Vpr. CEM.NKR T cells were transduced with lentiviral vectors expressing GFP alone (WPI) or coexpressing GFP and VprWT (WPI-VprWT). GFP+ cells were sorted for analysis 48 hours after transduction. Alternatively, transduced cells were treated (or not) with 4μM APC for 24 hours. DNase-treated RNA was analyzed for NKG2D ligand expression by real-time reverse-transcriptase polymerase chain reaction. Target gene expression in Vpr-transduced and APC-treated CEM.NKR T cells was normalized for glyceraldehyde 3-phosphate dehydrogenase and hypoxanthine-guanine phosphoribosyltransferase (HPRT) expression, and the data were subsequently expressed as a fold increase relative to WPI-transduced or untreated cells, respectively. Results shown represent a mean fold increase. Error bars represent SEM. Results are representative of the data obtained from 4 independent experiments.
Up-regulation of NKG2D ligands is dependent on HIV-1 Vpr-mediated activation of the ATR DNA damage/stress pathway
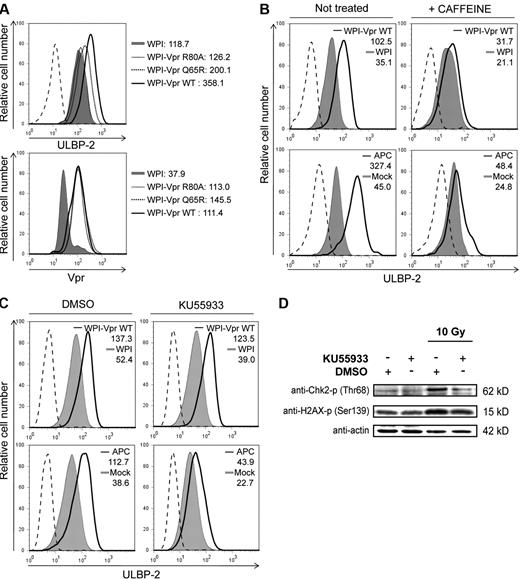
We and others have recently shown that HIV-1 Vpr engages the DDB1-CUL4A (VprBP) E3 Ub ligase through a direct association with the substrate specificity receptor VprBP to induce a cell-cycle arrest in the G2 phase.15-21 In that context, Vpr mutants that lost the ability to interact efficiently with the E3 ligase (VprQ65R) or were presumably unable to associate with putative host-cell substrate(s) (VprR80A) were found to have a reduced capability to induce a G2 cell-cycle arrest.15 To assess whether Vpr-mediated up-regulation of NKG2D ligands depended on the ability of the protein to recruit the DDB1-CUL4A (VprBP) E3 Ub ligase and to promote a G2 cell-cycle arrest, we analyzed the effect of VprR80A and VprQ65R on cell-surface expression of ULBP-2 because the expression of this specific NKG2D ligand was found to be consistently up-regulated by Vpr in all tested cell types. For this purpose, CEM.NKR T cells were transduced with lentiviral vectors expressing GFP alone (WPI) or coexpressing GFP and VprWT (WPI-VprWT) or Vpr mutants (WPI-VprR80A or WPI-VprQ65R). Figure 5A (top panel) reveals that, whereas VprWT induced an up-regulation of ULBP-2 expression compared with the WPI control (MFI = 358.1 vs 118.7), VprR80A and VprQ65R were drastically attenuated in their ability to induce G2 arrest (supplemental Figure 2A) and to increase expression of this ligand (MFI = 126.2 and 200.1, respectively), even though their expression levels were very similar to that of VprWT (Figure 5A bottom panel). Similar results were also obtained with the VprR80A mutant in HeLa cells (supplemental Figure 1).
Vpr-mediated up-regulation of ULBP2 requires the recruitment of the DDB1-CUL4A (VprBP) E3 ligase complex and activation of the DNA damage/stress checkpoint arrest initiated by ATR. (A) CEM.NKR T cells were transduced with lentiviral vectors expressing GFP alone (WPI) or coexpressing GFP and VprWT (WPI-VprWT) or Vpr mutants (WPI-VprR80A or WPI-VprQ65R), as indicated. At 48 hours after transduction, GFP-expressing cells were monitored for ULBP-2 cell-surface expression by flow cytometry using specific mAbs directed against ULBP-2 and appropriate fluorochrome-conjugated secondary reagents (top panel). Expression of VprWT and Vpr mutants (VprR80A and VprQ65R) was evaluated by intracellular staining and flow cytometry using anti-Vpr mAbs and appropriate fluorochrome-conjugated secondary reagents (bottom panel). (B-C) CEM.NKR T cells were transduced with lentiviral vectors WPI or WPI-VprWT or treated with APC (4μM), in the presence or absence of caffeine (2.5mM) (B) and in the presence of DMSO or KU55933 (10μM) (C) as indicated. Cell-surface expression of ULBP-2 was monitored on GFP-expressing cells 48 hours after transduction or on the total cell population after a 24-hour treatment with APC. MFI values were calculated by subtracting the corresponding isotype control values (dashed line). Results shown are representative of the data obtained from at least 2 independent experiments. (D) HeLa cells were irradiated with γ rays (10 Gy from a Cs137 source) in the presence of DMSO or KU55933 (10μM). Cells were then lysed and sonicated 1 hour after irradiation, and phosphorylation of Chk2 and H2AX was monitored by Western blotting using specific Abs.
Vpr-mediated up-regulation of ULBP2 requires the recruitment of the DDB1-CUL4A (VprBP) E3 ligase complex and activation of the DNA damage/stress checkpoint arrest initiated by ATR. (A) CEM.NKR T cells were transduced with lentiviral vectors expressing GFP alone (WPI) or coexpressing GFP and VprWT (WPI-VprWT) or Vpr mutants (WPI-VprR80A or WPI-VprQ65R), as indicated. At 48 hours after transduction, GFP-expressing cells were monitored for ULBP-2 cell-surface expression by flow cytometry using specific mAbs directed against ULBP-2 and appropriate fluorochrome-conjugated secondary reagents (top panel). Expression of VprWT and Vpr mutants (VprR80A and VprQ65R) was evaluated by intracellular staining and flow cytometry using anti-Vpr mAbs and appropriate fluorochrome-conjugated secondary reagents (bottom panel). (B-C) CEM.NKR T cells were transduced with lentiviral vectors WPI or WPI-VprWT or treated with APC (4μM), in the presence or absence of caffeine (2.5mM) (B) and in the presence of DMSO or KU55933 (10μM) (C) as indicated. Cell-surface expression of ULBP-2 was monitored on GFP-expressing cells 48 hours after transduction or on the total cell population after a 24-hour treatment with APC. MFI values were calculated by subtracting the corresponding isotype control values (dashed line). Results shown are representative of the data obtained from at least 2 independent experiments. (D) HeLa cells were irradiated with γ rays (10 Gy from a Cs137 source) in the presence of DMSO or KU55933 (10μM). Cells were then lysed and sonicated 1 hour after irradiation, and phosphorylation of Chk2 and H2AX was monitored by Western blotting using specific Abs.
Given that the up-regulation of NKG2D ligands was found to be dependent on the activation of DNA damage/stress checkpoint pathway initiated by ATM or ATR,22 we next sought to test whether Vpr-mediated up-regulation of NKG2D ligands relied on its ability to activate the ATR DNA damage/stress pathway. To this end, CEM.NKR T cells were transduced with WPI or WPI-VprWT lentiviral vectors in the presence or absence of caffeine, an inhibitor of ATR and ATM previously reported to prevent Vpr-mediated G2 cell-cycle arrest.14 Results from these experiments revealed that caffeine treatment not only inhibited Vpr-mediated G2 cell-cycle arrest (supplemental Figure 2B) but also significantly reduced cell-surface up-regulation of ULBP-2 (Figure 5B). Similarly, induction of ULBP-2 by APC, a known activator of DNA damage checkpoints,22 was drastically diminished in the presence of caffeine (Figure 5B). Equivalent results were obtained in HeLa cells (data not shown). To exclude the involvement of ATM, we evaluated the effect of the specific ATM inhibitor KU5593335 on Vpr-induced up-regulation of ULBP-2. We found that treatment of transduced cells with KU55933 had no effect on Vpr-mediated G2 cell-cycle arrest (supplemental Figure 2C) and up-regulation of ULBP-2 (Figure 5C). Comparable results were also obtained in HeLa cells (data not shown). In contrast, KU55933 affected APC-induced ULBP-2 up-regulation (Figure 5C) and prevented phosphorylation of 2 known targets of ATM, Chk2 and H2AX after γ irradiation (Figure 5D). Taken together, these results suggest that Vpr up-regulates cell-surface expression of NKG2D ligands by a process that depends on the recruitment of the DDB1-CUL4A (VprBP) E3 ligase and on the activation of the ATR-mediated DNA damage/stress checkpoint.
Vpr-mediated up-regulation of NKG2D ligands triggers NK-cell–mediated killing
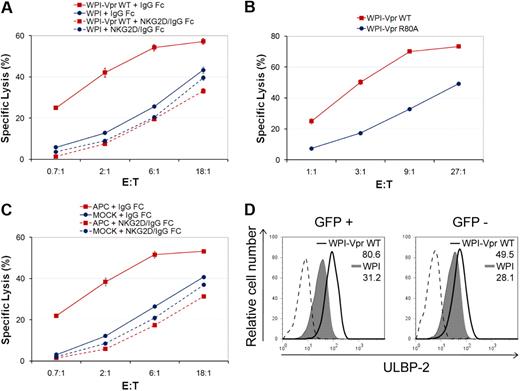
Given that expression of Vpr alone is sufficient to up-regulate expression of NKG2D ligands in CEM.NKR T cells, we next determined whether Vpr-expressing cells are more sensitive to NK cell–mediated killing. To this end, we analyzed the susceptibility of the entire population of lentiviral vector-transduced CEM.NKR T cells to NK cell–mediated killing, 48 hours after transduction, in a 4-hour 51Cr release assay. Figure 6A shows that CEM.NKR T cells transduced with Vpr-expressing lentiviral vectors displayed a remarkable sensitivity to NK cell–mediated killing compared to the control vector-transduced cells. Importantly, the enhanced NK-cell–mediated lysis of Vpr-expressing cells was completely abrogated when binding of NKG2D ligands to the NKG2D receptor was blocked using soluble NKG2D-IgG Fc fusion proteins, providing additional evidence that the NK-cell cytotoxic response triggered by Vpr occurred via expression of NKG2D ligands. Furthermore, as expected, the G2 arrest-defective mutant, VprR80A, which fails to efficiently up-regulate NKG2D ligands, was unable to trigger NK cell–mediated killing at levels comparable to those induced by VprWT (Figure 6B). Interestingly, although we obtained a transduction efficiency of approximately 30% (data not shown), the extent of NK cell–mediated killing induced by Vpr was very similar to that observed with APC treatment when using NK cells from the same donor (compare Figure 6A with Figure 6C). These results raised the possibility that Vpr may be acting beyond transduced cells in this experimental setting. Indeed, an analysis of GFP+ and GFP− cells within the WPI-VprWT-treated cell population revealed that ULBP-2 cell-surface expression was up-regulated in both cell populations relative to the WPI control lentiviral vector (Figure 6D). However, although Vpr-mediated up-regulation of ULBP-2 was stable in GFP+ cells over a 96-hour period after transduction, it occurred in a transient manner in GFP− cells, thus suggesting that the effect in GFP− cells may be mediated by delivery of virion-associated Vpr rather than expression of the transgene (data not shown). Taken together, these results indicate that expression of Vpr is sufficient to trigger NK cell–mediated killing through an increased expression of NKG2D ligands.
Vpr-mediated up-regulation of NKG2D ligands in target cells promotes NK cell–mediated killing. (A) CEM.NKR T cells were transduced with lentiviral vectors WPI or WPI-VprWT and then exposed to primary NK cells in a 4-hour 51Cr release assay 48 hours after transduction, in the presence of soluble NKG2D-IgG Fc fusion proteins or matched-IgG Fc fusion molecules, as indicated. (B) CEM.NKR T cells were transduced with lentiviral vectors WPI-VprWT or WPI-VprR80A and then assessed for cell lysis by primary NK cells in a 51Cr release assay 48 hours after transduction. (C) CEM.NKR T cells were treated or not with APC (4μM) and analyzed, as in panel A, 24 hours after treatment. Primary NK cells used in panels A and C were isolated from the same donor. Error bars represent SEM. (D) Cell-surface expression of ULBP-2 was monitored on GFP+ and GFP− CEM.NKR T cells 48 hours after transduction with lentiviral vectors expressing GFP alone (WPI) or coexpressing GFP and VprWT (WPI-VprWT) as indicated. MFI values were calculated by subtracting the corresponding isotype control values (dashed line). Results shown are representative of the data obtained from at least 2 independent experiments.
Vpr-mediated up-regulation of NKG2D ligands in target cells promotes NK cell–mediated killing. (A) CEM.NKR T cells were transduced with lentiviral vectors WPI or WPI-VprWT and then exposed to primary NK cells in a 4-hour 51Cr release assay 48 hours after transduction, in the presence of soluble NKG2D-IgG Fc fusion proteins or matched-IgG Fc fusion molecules, as indicated. (B) CEM.NKR T cells were transduced with lentiviral vectors WPI-VprWT or WPI-VprR80A and then assessed for cell lysis by primary NK cells in a 51Cr release assay 48 hours after transduction. (C) CEM.NKR T cells were treated or not with APC (4μM) and analyzed, as in panel A, 24 hours after treatment. Primary NK cells used in panels A and C were isolated from the same donor. Error bars represent SEM. (D) Cell-surface expression of ULBP-2 was monitored on GFP+ and GFP− CEM.NKR T cells 48 hours after transduction with lentiviral vectors expressing GFP alone (WPI) or coexpressing GFP and VprWT (WPI-VprWT) as indicated. MFI values were calculated by subtracting the corresponding isotype control values (dashed line). Results shown are representative of the data obtained from at least 2 independent experiments.
Delivery of virion-associated Vpr via defective HIV-1 particles up-regulates ULBP-2 expression and triggers NK cell–mediated killing
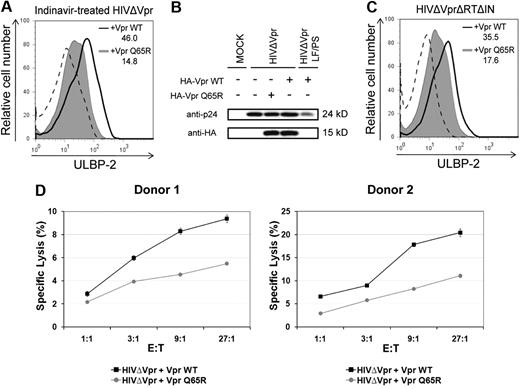
We and others have previously shown that virion-associated Vpr could trigger G2 cell-cycle arrest in transformed or primary T cells by a process that was insensitive to antiretroviral agents.9,10 Therefore, we next examined whether delivery of virion-associated Vpr by defective HIV-1 particles could up-regulate NKG2D ligands. To mimic infection by defective viruses, we infected primary CD4+ T lymphocytes with HIV-1 particles that are unable to express any HIV-1 proteins de novo but contain virion-associated Vpr. First, HIV-1 particles trans-packaged with VprWT or VprQ65R were produced in the presence of the protease inhibitor indinavir to make them defective for infection. In this context, indinavir-treated viruses were found to be noninfectious but still able to deliver virion-associated Vpr.9,10 These indinavir-treated viruses were unable to establish productive infection as evaluated by infection of HeLa TZM (data not shown). Nevertheless, noninfectious HIV-1 particles trans-packaged with VprWT were still capable of inducing an up-regulation of ULBP-2 cell-surface expression in primary CD4+ T lymphocytes compared to isogenic noninfectious virus trans-packaged with VprQ65R (Figure 7A). Notably, the VprQ65R mutant was found to be packaged into viral particles in quantities comparable to VprWT (Figure 7B), thus excluding the possibility that the lack of ULBP-2 up-regulation observed with this mutant was the result of inefficient packaging. As a negative control, we used a proviral construct (LF/PS) containing mutations in the P6 domain of Gag, which prevent the incorporation of Vpr (Figure 7B). Similar results were also obtained with naturally noninfectious viral particles produced from a well-characterized reverse transcriptase and integrase-defective proviral construct (HxBruΔVprΔRTΔIN)31 that was trans-packaged with VprWT or VprQ65R (Figure 7C). Importantly, primary CD4+ T lymphocytes exposed to indinavir-treated VprWT-containing HIV-1–defective particles were more susceptible to autologous NK-cell–mediated killing than cells exposed to HIV-1–defective particles containing the VprQ65R mutant (Figure 7D). Overall, these results suggest that delivery of virion-associated Vpr protein through HIV-1–defective particles can up-regulate cell-surface expression of NKG2D ligands in noninfected cells and as a result can trigger NK-cell cytotoxic responses.
Virion-associated Vpr up-regulates ULBP-2 expression in noninfected target cells and triggers NK cell–mediated killing. (A) Human primary CD4+ T lymphocytes were exposed to indinavir-treated noninfectious viral particles that were trans-packaged with VprWT or the VprQ65R mutant, as indicated, and cell-surface expression of ULBP-2 was monitored 24 hours after exposure using specific mAbs directed against ULBP-2 and appropriate fluorochrome-conjugated secondary reagents. (B) HIV-1ΔVpr and HIV-1ΔVpr LF/PS viruses (P6-mutated Gag-encoding virus that does not incorporate Vpr) trans-packaged with HA-tagged VprWT or VprQ65R were produced as described in “Production of lentiviral vectors and HIV-1 viruses.” Virion-associated HA-tagged VprWT and VprQ65R were detected by Western blotting using anti-HA mAbs. (C) Primary CD4+ T lymphocytes were exposed to reverse transcriptase- and integrase-defective (HIVΔVprΔRTΔIN) viral particles that were trans-packaged with VprWT or the VprQ65R mutant as indicated, and cell-surface expression of ULBP-2 was monitored 24 hours after exposure. MFI values were calculated by subtracting the corresponding isotype-control values (dashed line). (D) Primary CD4+ T lymphocytes exposed to indinavir-treated noninfectious viral particles containing VprWT or VprQ65R were added 24 hours after exposure to autologous primary NK cells in a 4-hour 51Cr release assay, as indicated. Error bars represent SEM. Results shown are representative of the data obtained from 2 independent donors.
Virion-associated Vpr up-regulates ULBP-2 expression in noninfected target cells and triggers NK cell–mediated killing. (A) Human primary CD4+ T lymphocytes were exposed to indinavir-treated noninfectious viral particles that were trans-packaged with VprWT or the VprQ65R mutant, as indicated, and cell-surface expression of ULBP-2 was monitored 24 hours after exposure using specific mAbs directed against ULBP-2 and appropriate fluorochrome-conjugated secondary reagents. (B) HIV-1ΔVpr and HIV-1ΔVpr LF/PS viruses (P6-mutated Gag-encoding virus that does not incorporate Vpr) trans-packaged with HA-tagged VprWT or VprQ65R were produced as described in “Production of lentiviral vectors and HIV-1 viruses.” Virion-associated HA-tagged VprWT and VprQ65R were detected by Western blotting using anti-HA mAbs. (C) Primary CD4+ T lymphocytes were exposed to reverse transcriptase- and integrase-defective (HIVΔVprΔRTΔIN) viral particles that were trans-packaged with VprWT or the VprQ65R mutant as indicated, and cell-surface expression of ULBP-2 was monitored 24 hours after exposure. MFI values were calculated by subtracting the corresponding isotype-control values (dashed line). (D) Primary CD4+ T lymphocytes exposed to indinavir-treated noninfectious viral particles containing VprWT or VprQ65R were added 24 hours after exposure to autologous primary NK cells in a 4-hour 51Cr release assay, as indicated. Error bars represent SEM. Results shown are representative of the data obtained from 2 independent donors.
Discussion
HIV-1 infection of primary CD4+ T cells up-regulates cell-surface expression of specific ligands for the activating NKG2D receptor, including ULBP-1, -2, and -3, but not MICA or MICB.25,26 This up-regulation was also found to occur in vivo because NKG2D ligands were expressed at high levels on endogenously HIV-1–infected CD4+ T cells.27 In particular, ULBP molecules (especially ULBP-2) were clearly expressed on p24+ cells, whereas the levels of MICA and MICB were almost undetectable. However, the identity of the viral factor(s) involved in NKG2D ligands expression has remained undefined. We demonstrated in this study that the HIV-1 Vpr accessory protein is responsible for increasing expression of these ligands, which are important in triggering NK-cell responses on infected cells. In particular, we observed that primary CD4+ T cells responded to HIV-1 infection by selectively augmenting cell-surface expression of ULBP-2 in a Vpr-dependent manner (Figure 1). Furthermore, expression of Vpr alone was sufficient to enhance ULBP-2 expression in human CD4+ T lymphocytes (Figure 3A). Our results showing a lack of MICA and MICB cell-surface up-regulation by HIV-1 concur with recent reports.25-27 However, in contrast to the study of Ward et al,25 we did not detect any significant up-regulation of ULBP-1 and ULBP-3 in HIV-infected cells relative to uninfected cells. Interestingly, CEM.NKR T cells expressing solely Vpr showed an up-regulation of all tested NKG2D ligands, with the strongest effect observed with ULBP-2 (Figure 3B). Aside from the implication that HIV replication and expression of other virus proteins are not required for this activity of Vpr, this latter finding suggests that, perhaps in the context of a replicating virus, another viral function may limit the cell-surface expression of some NKG2D ligands. In this regard, recent evidence suggests that the HIV-1 Nef accessory protein down-regulates the cell-surface expression of NKG2D ligands as a means to evade NK-cell recognition.26 This activity of Nef may explain why Vpr-mediated up-regulation of NKG2D ligands is selective in the context of HIV-1 infection where Nef is expressed compared to cells that only express Vpr. In addition, it may also account for the discrepancies observed between our results and those of Ward et al25 because different Nef variants were found to down-regulate NKG2D ligands selectively and with different efficiencies.26 However, we cannot exclude the possibility that differences in viral strain and MOI may explain these discrepancies. Importantly, despite the presence of Nef, HIV infection, in a Vpr-dependent manner, increased the cell-surface expression of ULBP-2 in infected primary cells and triggered NK cell–mediated killing, at least in part, via the NKG2D receptor (Figure 2). Studying the regulation of NKG2D ligand expression by Vpr and other viral products, including Nef, should help understand how these ligands are modulated during HIV-1 infection.
Our results suggest that Vpr increases NKG2D ligand expression by a process that relies on the engagement of the DDB1-CUL4A (VprBP) E3 Ub ligase complex and on the activation of the ATR-mediated DNA damage checkpoint (Figure 5). Specifically, mutants of Vpr that were unable to induce a G2 cell-cycle arrest, either because they lost the ability to interact efficiently with the E3 ligase or were presumably unable to target putative host-cell substrate(s) for polyubiquitination and degradation,15 were found to have a reduced capability in up-regulating NKG2D ligand expression (Figure 5A). A role of ATR in this activity of Vpr was supported by experiments, which demonstrated that Vpr-mediated ULBP-2 up-regulation was efficiently blocked by caffeine, an inhibitor of ATR and ATM, but not by the ATM inhibitor KU55933 (Figure 5B-C). In contrast, ligand augmentation in response to APC, a known activator of DNA damage pathways,22 was inhibited by both inhibitors (Figure 5B-C). Our findings are consistent with earlier evidence showing that expression of NKG2D ligands is up-regulated by activation of the DNA damage/stress pathways initiated by ATR or ATM22 and that Vpr activates specifically the ATR-mediated DNA damage pathway.14
Our results support a model where virion-associated Vpr could also up-regulate NKG2D ligands after its delivery into target cells and, as a result, would trigger NK-cell cytotoxic responses (Figure 7). Studies have revealed that the ratio of defective to infectious viral particles may be relatively high. These defective viral particles, which are estimated to be in the range of 8 to 20 to 1, still contain packaged Vpr and therefore could induce expression of NKG2D ligands at the cell surface of noninfected CD4+ T cells and promote their killing by NK cells via NKG2D. These Vpr-containing defective particles may explain some of the bystander killing observed in acute HIV-1 and simian immunodeficiency virus infections when, for instance, more than 50% of CD4+ T cells in the gastrointestinal lamina propria are depleted,36-38 and yet only 7% of gastrointestinal CD4+ T cells are found to be infected.37
HIV-1 appears to have developed several strategies to offset the capability of activating NK cells efficiently. The selective preservation of cell-surface expression of HLA-C and -E molecules39 and the down-modulation of NKG2D ligands26 and ligands for the NTB-A (NK-T-B cell antigen) and 2B4 (CD244) activating coreceptors25 by Nef represent strategies used by HIV-1 virus to blunt NK-cell recognition. Nevertheless, HIV-1, through Vpr, still enhances the expression of some NKG2D ligands and triggers nonactivated NK cells to kill HIV-1–infected cells. A role of Vpr in increasing expression of NKG2D ligands and promoting NK-cell cytotoxic responses would seem at first counterproductive to the virus because it would make the infected cells more potent targets for NK cells. However, Vpr provides the virus with a selective replication and propagation advantage because it promotes infection of monocyte-derived macrophages,40,41 a cell type that is refractory to ATR activation by Vpr13 and consequently would not be expected to express ligands for the activating NKG2D receptor during infection. Furthermore, this activity of Vpr on modulating NKG2D ligand expression may not only contribute to HIV-1–induced CD4+ T-cell depletion but may also play a role in the perturbation of NK-cell functions observed during HIV infection. Increasing evidence indicates that NK-cell function as a whole is compromised during HIV infection through poorly understood mechanisms. HIV infection is associated with significant changes in NK-cell subset distribution in the peripheral circulation that are partially attributable to the emergence of a novel subset of NK cells that is rare in healthy persons, the CD3− CD56− CD16+ NK cells. These cells not only lack the majority of NK-cell effector functions, including killing, cytokine secretion, and antibody-dependent cellular cytotoxicity, but also exhibit aberrant dendritic cell editing.42-44 The observation that the redistribution of NK cells toward this anergic subset of cells is directly correlated with viral load suggests that the presence of viral antigens has a role in NK-cell dysfunction. Indeed, in viremic patients, NK cells display several phenotypic and functional alterations,27 including a slightly decreased expression of the NKG2D receptor,43 although no significant alteration in the percentage of NKG2D+ cells was observed.27 Interestingly, down-regulation of NKG2D also occurs in some cancer patients as a consequence of a soluble form of MICA released by tumor cells on proteolytic cleavage.45,46 Furthermore, sustained localized expression of ligands for the activating NKG2D receptor was reported to down-regulate NKG2D and to impair NK-cell cytotoxic activity in vitro and in mouse models, thus reducing tumor immunosurveillance.47,48 Hence, chronic exposure to NKG2D ligand-expressing tumor cells or to soluble NKG2D ligands shedded by tumor cells induces alteration of NKG2D function in NK cells.48,49 By analogy, the activity of Vpr on NKG2D ligands could contribute to NK-cell dysfunction because of sustained effector activation, thus effectively promoting viral immune evasion. A role of Vpr in HIV-1–induced NK-cell dysfunction is consistent with recent data, which showed that NK cells derived from human PBMCs infected in vitro with HIV-1 Vpr+ virus or exposed to recombinant Vpr protein exhibited reduced target-cell killing and reduced production of γ-interferon compared with their Vpr− counterparts.50 This NK-cell defect was not the result of direct infection of NK cells but rather resulted in part from the presence of membrane-associated factors on infected or recombinant Vpr-exposed nonmyeloid target cells. Hence, assessing whether the activity of Vpr on NKG2D ligands desensitizes the NKG2D receptor and evades NKG2D-mediated immune surveillance will further improve our understanding of the immune evasive strategies used by HIV to disarm innate immune responses and will help in the development of immunotherapeutic options for HIV-1–infected persons.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Didier Trono, Yukihito Ishizaka, and Heinrich Göttlinger for kindly providing reagents; Éric Massicotte, Martine Dupuis, and Andrea Kessous for technical assistance; Andrew Makrigiannis and David Favre for helpful discussions; and Dr Pierre Larochelle, the Institut de Recherches Clinique de Montreal clinic staff, and all donors for providing us with blood samples. The following reagents were obtained through the AIDS Research and Reference Reagent Program, Division of AIDS: pNL4-3 from Dr Malcolm Martin and human rIL-2 from Dr Maurice Gately, Hoffmann-La Roche Inc.
J.R. is the recipient of a Frederick Banting and Charles Best scholarship from the Canadian Institutes of Health Research (CIHR). J.-P.B. is the recipient of a CIHR studentship. E.A.C. holds the Canada Research Chair in Human Retrovirology. This work was supported by grants from CIHR and the Fonds de recherche en santé du Québec AIDS network (E.A.C.).
Authorship
Contribution: J.R., T.N.Q.P., and E.A.C. conceived and designed the experiments and analyzed the data; J.R., S.S., T.N.Q.P., and J.-P.B. performed the experiments; and J.R., J.-P.B., and E.A.C. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Éric A. Cohen, Laboratory of Human Retrovirology, Institut de Recherches Cliniques de Montréal, 110 Avenue des Pins Ouest, Montreal, QC, Canada H2W 1R7; e-mail: eric.cohen@ircm.qc.ca.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal