Abstract
Leukocyte-associated immunoglobulin-like receptor-1 (LAIR-1), one of the most widely spread immune receptors, attenuates immune cell activation when bound to specific sites in collagen. The collagen-binding domain of LAIR-1 is homologous to that of glycoprotein VI (GPVI), a collagen receptor crucial for platelet activation. Because LAIR-1 and GPVI also display overlapping collagen-binding specificities, a common structural basis for collagen recognition would appear likely. Therefore, it is crucial to gain insight into the molecular interaction of both receptors with their ligand to prevent unwanted cross-reactions during therapeutic intervention. We determined the crystal structure of LAIR-1 and mapped its collagen-binding site by nuclear magnetic resonance (NMR) titrations and mutagenesis. Our data identify R59, E61, and W109 as key residues for collagen interaction. These residues are strictly conserved in LAIR-1 and GPVI alike; however, they are located outside the previously proposed GPVI collagen-binding site. Our data provide evidence for an unanticipated mechanism of collagen recognition common to LAIR-1 and GPVI. This fundamental insight will contribute to the exploration of specific means of intervention in collagen-induced signaling in immunity and hemostasis.
Introduction
The leukocyte receptor complex (LRC) on human chromosome 19 encodes 2 receptors for collagens that are structurally related: leukocyte-associated immunoglobulin (Ig)–like receptor-1 (LAIR-1) and glycoprotein VI (GPVI). Their genomic location and structural homology suggest a common origin for these 2 collagen-binding receptors. However, their function is in 2 completely different biologic systems and on mutually exclusive cell subsets.
LAIR-1 belongs to the family of immune inhibitory receptors, which are involved in controlling the balance of the immune system to prevent improper activation or overactivation, which may result in tissue damage or autoimmune diseases. Upon interaction with their ligands, these receptors attenuate the signals provided by activating receptors, thereby increasing the threshold for activation. LAIR-1 is one of the most widely distributed inhibitory receptors, expressed on most immune cells, including natural killer cells, T cells, B cells, monocytes, and CD34+ hematopoietic progenitor cells.1-5
LAIR-1 is a type I glycoprotein of 287 amino acids containing a single extracellular Ig-like domain followed by a stalk region connected to the single transmembrane domain and 2 cytoplasmic immunoreceptor tyrosine-based inhibitory motifs that relay the inhibitory signal.2 LAIR-1 binds both transmembrane and extracellular matrix collagens.6,7 Under physiologic conditions, immune cells present in the bloodstream do not encounter matrix collagens. However, their extravasion results in exposure to the collagen-rich subendothelium, where LAIR-1 transduces an inhibitory signal to the cell, resulting in abrogation or inhibition of immune cell function.6
As opposed to LAIR-1, GPVI is an activating receptor, with 2 instead of 1 extracellular Ig-like domain. GPVI is expressed by megakaryocytes and platelets and plays a central role in hemostasis by activating platelets upon binding to collagen, along with von Willebrand factor and the collagen-binding integrin α2β1.8,9
Collagens play crucial roles in the development, morphogenesis, and growth of many tissues. Their role in hemostasis is well established and the recent identification of LAIR-1 as an inhibitory collagen receptor reveals a novel role for collagen as immune regulatory protein.1,6 All collagens are composed of 3 polypeptide chains that are characterized by a repeating Gly-X-X′ sequence, where X is often proline and X′ frequently 4-R-hydroxyproline (Hyp, O). The GPO triplets are an almost exclusive feature of collagens and allow the formation of the characteristic triple-helical collagen structure. Although GPVI and LAIR-1 are functionally different, they are similar in their collagen-binding properties. Both receptors can bind GPO repeats.10 When expressed on the same cell, LAIR-1 is capable of inhibiting collagen-induced GPVI signaling.11 Recently, collagen-binding properties of GPVI and LAIR-1 have been characterized using collagen II– and collagen III–derived synthetic Toolkit peptides.7,12 LAIR-1 appears to have several binding sites on collagens II and III and its binding spectrum on the latter is overlapping, although not identical, with that of GPVI.13
LAIR-1 and GPVI are structurally related to several other inhibitory and activating LRC receptors. These include leukocyte inhibitory receptors, such as LIR-1, LIR-2 and LILRB5, the killer-cell Ig-like receptors (KIRs), and the IgA receptor FcαRI. The extracellular domain of LRC receptor proteins consists of 1 (LAIR-1), 2 (GPVI, LIR-5, FcαRI, p58 KIRs), 3 (p70 KIRs), or 4 (LIR-1, LIR-2) Ig-like domains, termed D1 to D4. The ligand-binding sites of these receptors are limited to the D1 domain and the D1D2 hinge region. The crystal structures of the D1 to D2 region of several LRC receptors in complex with their respective ligands have been solved.14-22 Despite large structural overlaps, LRC receptors display a remarkable versatility in the organization of their ligand recognition site (supplemental Figure 1, available on the Blood website; see the Supplemental Materials link at the top of the online article).
Manipulation of either thrombosis through GPVI or the immune system through LAIR-1 can be envisaged using reagents that interfere with collagen binding of either one of these receptors. However, to prevent unwanted side effects resulting from interference with the other receptor, it is important to precisely characterize their collagen-binding sites. The large variation in ligand interaction sites of LRC receptor proteins and the limited data on the GPVI/collagen interaction make it difficult to predict the LAIR-1 collagen-binding site and emphasize the need for more structural information for this family of proteins.
In this paper, we present the 1.8-Å crystal structure of the human LAIR-1 collagen-binding domain. We mapped the collagen-binding site to the structure using nuclear magnetic resonance (NMR) titrations and site-directed mutagenesis. The LAIR-1 collagen-binding site is shown to be surprisingly different from the proposed GPVI collagen-binding site.
Methods
Expression and purification
hLAIR-1a cloned into the pMX retroviral vectors was described previously.6 Site-specific mutations were introduced using the QuikChange method (Stratagene) and confirmed by sequencing. Wild-type and mutant hLAIR-1 were stably expressed in human erythroleukemia K562 cells by retroviral transduction as described previously.6 The isolated collagen-binding domain of hLAIR-1 (hLAIR1-CBD), amino acids 22 to 122, was expressed in Escherichia coli and purified by affinity chromatography and size exclusion chromatography as described in the supplemental data.
Crystallization and crystal structure determination
Crystals of hLAIR1-CBD were grown using vapor diffusion at 18°C in 25% to 30% polyethylene glycol-1500, 100mM succinic acid, 100mM sodium dihydrogen phosphate, 100mM glycine, 10mM CoCl2, pH 4.1 to 4.4. Crystals appeared within 0.5 to 2 days. Protein concentration before setting up the drops was 20 to 30 mg/mL. For cryoprotection, crystals were transferred to the crystallization solution supplemented with 20% (vol/vol) glycerol.
Diffraction data were collected at 100 K at beamline ID23-1 of the European Synchrotron Radiation Facility and processed with Mosflm.23 The structure was solved by molecular replacement with the programs PHASER and MolRep, which are part of the CCP4 suite,23 using a model generated by the Swiss-model server24 based on an alignment of LAIR-1 with GPVI, LILRA5, LIR-1, and LIR-2 (Protein Data Bank entries 2GI7, 2D3V, 1UGN, and 2GW5, respectively).25 Initial rounds of refinement were done using Refmac5,23 followed by twin least squares-refinement in Phenix.26
The final model consists of 3 molecules hLAIR1-CBD and contains 1 glycine and 1 glycerol molecule. Stereochemistry was checked using Molprobity.27 Data collection and refinement statistics are listed in supplemental Table 1. Graphics were generated using PYMOL (DeLano Scientific). The final coordinates were deposited at the Protein Data Bank under accession code 3KGR.25
NMR experiments
15N-labeled hLAIR1-CBD was produced as described in the supplemental data. The collagen III–derived synthetic peptide III-30 was synthesized as described previously.28 The amino acid sequence of a single chain of the peptide is GPC(GPP)5GAOGLRGGAGPOGPEGGKGAAGPO-GPO(GPP)5GPC-NH2 with bold residues denoting the collagen III–derived sequence. The free cysteines were blocked by S-carboxymethylation using 0.50mM iodoacetamide at 37°C. The peptide was transferred to 10mM potassium phosphate, pH 7.1, using a High-Trap desalting column (GE Healthcare) and concentrated to 3.1mM.
NMR experiments were performed on a Bruker Avance III spectrometer operating at 600-MHz proton resonance frequency. For spectral assignment 15N-NOESY–heteronuclear single quantum coherence (HSQC) and 15N-TOCSY-HSQC spectra were recorded at 25°C with 0.8mM 15N-hLAIR in 10mM potassium phosphate buffer, pH 7.2, containing 10% D2O. For the titration,{1H;15N}-HSQC spectra at 30°C followed the stepwise addition of small amounts of the collagen III-30 peptide to 0.1mM 15N-LAIR-1. hLAIR-1/triple-helical collagen III-30 ratios varied between 1:0.1 and 1:3.7.
Collagen interaction
Interaction of hLAIR1-CBD with collagen was confirmed in a solid-state binding assay. Microtiter wells were coated with collagen I or collagen III (50 μg/mL) in phosphate-buffered saline (PBS; pH 7.4) for 16 hours at 4°C. After washing, wells were blocked with 3% bovine serum albumin (BSA) and subsequently incubated with serial dilutions of hLAIR1-CBD in PBS containing 0.01% Tween-20 and 2 mg/mL BSA for 2 hours at room temperature (RT). After washing, anti–human LAIR-1 antibody (clone DX26) was added to the plates for 1 hour at RT. After washing 3 times with PBS/0.01% Tween-20, wells were incubated with horseradish peroxidase–conjugated polyclonal goat anti–mouse antibodies for 1 hour at RT. After 3 washes with PBS/0.01% Tween-20, bound antibodies detecting LAIR-1 were visualized via incubation with 2,2′-azino-bis(3-ethylbenzthiazoline-6-sulphonic acid.
LAIR-1/collagen interactions in solution were assayed by incubating K562 cells transfected with wild-type or mutant hLAIR-1a with fluorescein isothiocyanate (FITC)–conjugated collagen I or collagen III (conjugated as described in Van de Walle et al31 ) or Oregon green 488–conjugated collagen IV (Molecular Probes) plus phycoerythrin-conjugated anti–LAIR-1 monoclonal antibody (BD Biosciences). Cells were incubated for 30 minutes, washed, and analyzed by flow cytometry (FACSCalibur; BD).
Binding of K562 transfectants to plate-bound collagen was analyzed by adhesion of calcein-labeled cells; 96-well MAXIsorp flat-bottom plates were coated overnight at 4°C with collagen I, III, or IV (10 μg/mL; Sigma-Aldrich) or BSA (5 μg/mL) in 100 μL of PBS supplemented with 2mM acetic acid. Wild-type K562 or K562 cells (5 × 106/mL), stably transfected with hLAIR-1a, were assayed for their capacity to adhere to the collagens in the 96-well plates as described before.6
Docking calculations
Three-dimensional models of the LAIR-1/peptide III-30 complex were calculated using the HADDOCK web server (http://haddock.chem.uu.nl/Haddock/haddock.php, Utrecht University). Details of model construction and the docking procedure are described in the supplemental Data.
Results
The isolated collagen-binding domain of LAIR-1 retains its functionality
The collagen-binding domain of human LAIR-1 (hLAIR1-CBD) was expressed in E coli. The purified CBD bound to immobilized human collagens I and III with apparent dissociation constants of 147nM (± 16nM) and 750nM (± 71nM), respectively (supplemental Figure 2). The hLAIR1-CBD also displayed significant binding to GPO10, albeit, like the native protein,6 with much reduced affinity compared with collagen (data not shown). These apparent dissociation constants are lower than the previously determined dissociation constant of 26μM for collagen I binding of hLAIR1-CBD fused to rat CD4 domains 3 and 4,32 whereas a hLAIR-1-IgG fusion displays even lower dissociation constants of 14.2nM (± 2.7nM) and 16.2nM (± 3.8nM) for collagens I and III, respectively.6 Stronger binding of the hLAIR-1-IgG fusion is explained by its dimeric nature, arising from disulfide bridge formation between the IgG parts. The relatively high affinity of our monomeric E coli–expressed hLAIR1-CBD for collagens I and III strongly suggests that it is properly folded and shows that the single N-glycosylation site at residue N692 that remains unglycosylated upon expression in E coli is not essential for collagen binding.
Crystal structure
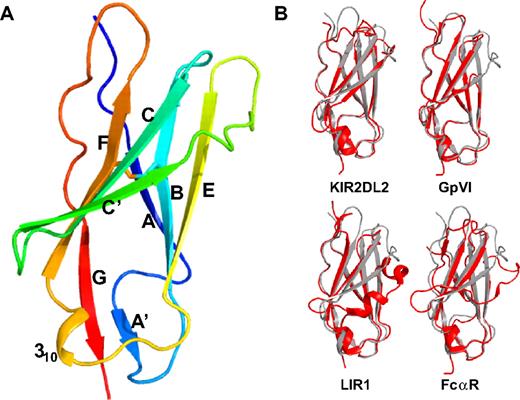
The hLAIR-1 collagen-binding domain was crystallized and its structure was solved to 1.8-Å resolution using molecular replacement (supplemental Table 1). The asymmetric unit of the crystal contains 3 hLAIR1-CBDs that were built and refined independently. The 3 CBDs are essentially identical with main chain root mean square difference (rmsd) values ranging from 0.28 to 0.39 Å. The LAIR1-CBD forms a single I-type Ig-fold consisting of a sandwich of 2 β-sheets composed of β-strands ABE and A′GFCC′ (Figure 1A). A disulfide bond between β-strands B and F connects the 2 β-sheets. The A′ strand is created by a cis-proline (P35) that introduces a sharp bend after the first β-strand. The loop between β-strands E and F contains a short 310-helix. The N-linked glycosylation site at N69 is located in β-strand C′ and is fully exposed to the solvent.
LAIR-1 overall structure and comparison with other LRC-family members. (A) Ribbon drawing of the LAIR-1 ectodomain structure in rainbow colors from N-terminus (blue) to C-terminus (red). The disulfide bond between β-strands B and F, characteristic of Ig-like domains, is indicated in stick representation. (B) Superposition of the LAIR-1 ectodomain (gray) with the D1 domains of LRC-family members KIR2DL2, GPVI, LIR1, and the Fc-alpha receptor, respectively (red).
LAIR-1 overall structure and comparison with other LRC-family members. (A) Ribbon drawing of the LAIR-1 ectodomain structure in rainbow colors from N-terminus (blue) to C-terminus (red). The disulfide bond between β-strands B and F, characteristic of Ig-like domains, is indicated in stick representation. (B) Superposition of the LAIR-1 ectodomain (gray) with the D1 domains of LRC-family members KIR2DL2, GPVI, LIR1, and the Fc-alpha receptor, respectively (red).
The overall structure of the hLAIR1-CBD is similar to the D1 domains of other LRC receptors with Cα rmsd values ranging from 1.1 to 2.1 Å (Figure 1B and supplemental Table 2). The largest differences between these proteins are found in the CC′-loop, the C′-strand, and the C′E-loop region. In LIR-1 and LIR-2 strand, C′ is replaced by a 310-helix. In GPVI, there is a 12–amino acid deletion in this region, including part of β-strands C′ and E and most of loop C′E. The closest structural matches to LAIR-1 are the KIR2DL2, LILRA5, and GPVI D1 domains with Cα rmsd values of 1.34 Å over 93 residues, 1.36 Å over 91 residues, and 1.09 Å over 85 residues, respectively.
LAIR-1 signaling is most likely induced by multimerization as cross-linking using monoclonal antibodies results in receptor signaling and cell down-regulation.2,3,6,33 Inspection of the crystal packing reveals that 2 of the 3 CBD molecules in the asymmetric unit form an antiparallel dimer, whereas the third molecule does not share an extended interface with any other molecule in the crystal (supplemental Figure 3). We analyzed the potential biologic significance of the dimer using the PISA web server (European Bioinformatics Institute).34 On the basis of the relatively small size of the dimer interface (439 Å2) and the nature of the interaction surface, PISA suggests that the dimer observed in the crystal is not likely to be of physiologic relevance.
LAIR-1 and GPVI bind to an overlapping set of synthetic collagen peptides.7,12 It is therefore tempting to speculate that their collagen-binding sites are similar. Based on the GPVI crystal structure, mutagenesis data and rigid body docking calculations, it has been proposed that the collagen-binding site in GPVI is a shallow hydrophobic groove formed by β-strands C′ and E.14 Although these strands are more extended in LAIR-1, also here a groove is found at this position. Interestingly, R65, a residue recently shown to be important for LAIR-1 collagen binding,35 is located at one end of this groove. Furthermore, rigid body docking calculations using the PatchDock web server (Tel Aviv University) placed a collagen peptide in this groove. To obtain experimental data on the location of the collagen-binding site of LAIR-1, we performed NMR spectroscopy and mutagenesis studies.
Mapping the collagen-binding site of hLAIR1-CBD by NMR
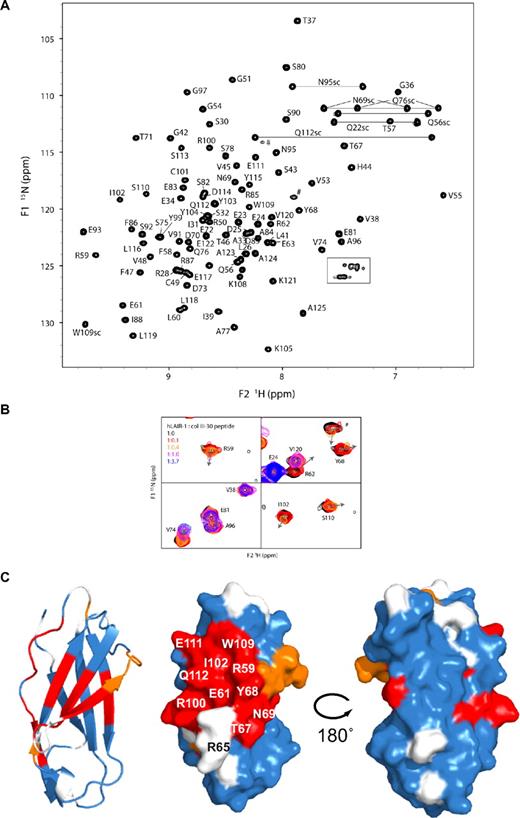
Previously, screening of collagen II and collagen III peptide libraries has identified several triple-helical peptides as functional ligands for LAIR-1.7 We used the most potent of these, peptide III-30 (see “NMR experiments”), to map the collagen-binding region in 15N-labeled hLAIR1-CBD by NMR titration experiments. {1H;15N}-HSQC spectra were recorded in the absence and in the presence of increasing amounts of peptide III-30. In absence of ligand, the CBD shows a well-dispersed spectrum of homogenous line-width, indicative of a single well-defined fold (Figure 2A). Most of the backbone and side chain amides were assigned unambiguously using 15N-TOCSY-HSQC and 15N-NOESY-HSQC spectra. The most N-terminal residues, G20-Q22, residues S64-R65-S66 in the C-C′ loop, and G94 remain unassigned. Two signals in the HSQC spectrum most likely belong to residues from the S64-S66 loop. However, their assignment remains ambiguous due to low signal intensity.
NMR analysis of the titration of unlabeled collagen III-30 peptide to 15N-labeled hLAIR1-CBD. (A) Assigned {1H;15N}-HSQC spectrum of the isolated LAIR-1 ectodomain in the absence of ligand. Gray lines connect the resonances of asparagine and glutamine side chain amides. The boxed region contains arginine side chain resonances. Side chain assignments are indicated by sc. Unassigned signals are indicated by #. (B) Selected regions of the overlay of {1H;15N}-HSQC spectra recorded at different peptide III-30 concentrations. Shown are representative examples of residues with large chemical shift changes that are in intermediate-to-slow exchange (R59, R62, Y68, I102, S110, #), residues that are in fast-to-intermediate exchange (V74), and residues that show no shift (E24, V120) or shifts smaller than a line-width (E81, A96). The arrows indicate the direction of peak displacement. Color coding reflects the relative peptide concentration as indicated. (C) Mapping of spectral changes on a ribbon representation of hLAIR1-CBD (left), a surface representation in the same orientation (middle), and a surface representation rotated by 180° around a vertical axis (right). White indicates no data due to lacking assignments or spectral overlap; blue, no spectral change or change smaller than one line-width; orange, residues in fast-to-intermediate exchange; and red, residues in intermediate-to-slow exchange. Residues of interest are labeled.
NMR analysis of the titration of unlabeled collagen III-30 peptide to 15N-labeled hLAIR1-CBD. (A) Assigned {1H;15N}-HSQC spectrum of the isolated LAIR-1 ectodomain in the absence of ligand. Gray lines connect the resonances of asparagine and glutamine side chain amides. The boxed region contains arginine side chain resonances. Side chain assignments are indicated by sc. Unassigned signals are indicated by #. (B) Selected regions of the overlay of {1H;15N}-HSQC spectra recorded at different peptide III-30 concentrations. Shown are representative examples of residues with large chemical shift changes that are in intermediate-to-slow exchange (R59, R62, Y68, I102, S110, #), residues that are in fast-to-intermediate exchange (V74), and residues that show no shift (E24, V120) or shifts smaller than a line-width (E81, A96). The arrows indicate the direction of peak displacement. Color coding reflects the relative peptide concentration as indicated. (C) Mapping of spectral changes on a ribbon representation of hLAIR1-CBD (left), a surface representation in the same orientation (middle), and a surface representation rotated by 180° around a vertical axis (right). White indicates no data due to lacking assignments or spectral overlap; blue, no spectral change or change smaller than one line-width; orange, residues in fast-to-intermediate exchange; and red, residues in intermediate-to-slow exchange. Residues of interest are labeled.
Titration of peptide III-30 resulted for all cross-peaks in a gradual increase in line-width due to the substantial increase in mass upon binding of the 11.5-kDa hLAIR1-CBD to the 16.0-kDa triple-helical peptide. Besides that, we observed clear shifts of several cross-peaks (Figure 2B). Signals of residues that have different chemical shifts in the free and bound state of hLAIR1-CBD experience additional line broadening due to continuous interconversion between the 2 states on the NMR time scale. For relatively small chemical shift differences (ie, limited influence of peptide binding), the residues are in fast-to-intermediate exchange and the peaks can be followed throughout the titration. For residues that show relatively large chemical shift differences (intermediate-to-slow exchange), line broadening is severe, and the signal is lost in the course of the titration. Overall, this observed spectral behavior is consistent with a collagen-binding affinity in the micromolar range.
The signals that shifted more than one line-width and displayed intermediate-to-slow exchange were considered to constitute the peptide III-30 binding interface on hLAIR1-CBD. Residues showing this behavior are R59-E63, T67-N69, A84-F86, R100-I102, W109-S113, Y115-L116, and L118. In addition, the side chain resonances of W109 and Q112 are affected by the presence of collagen III-30, and, finally, although this region could not be assigned, peptide binding affects probably one of the unassigned residues in the sequence S64-R65-S66. The residues that are perturbed by peptide addition cluster on β-strands C′, C, and F, and form a continuous area on the surface (Figure 2C). The location of the LAIR-1 collagen-binding surface as identified by NMR resembles the major histocompatibility complex I–HLA interaction sites of LIR-1 and LIR-2,15,19 rather than the proposed collagen-binding site in GPVI (compare Figure 2C and supplemental Figure 1).
Collagen-binding site mutants
Assignment of the hLAIR-1 collagen-binding surface was verified by site-directed mutagenesis. We designed 13 mutations, including 5 in the putative collagen-binding site as indicated by the NMR titration experiments, 2 in the unassigned loop S64-S66, and 6 outside the region with the largest chemical shift changes, of which 4 correspond to the putative collagen-binding site of GPVI.
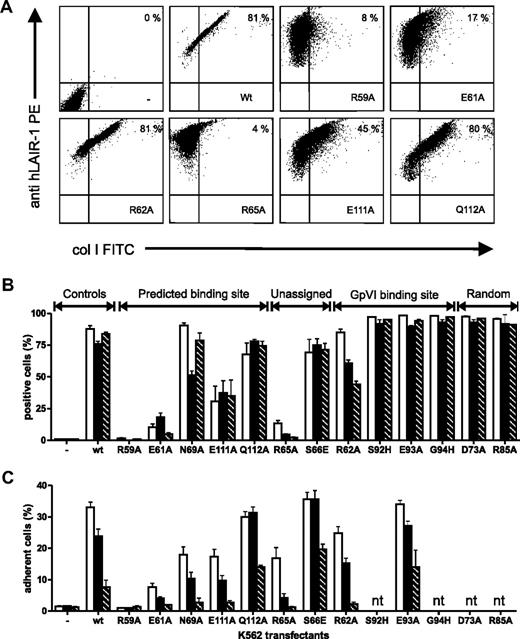
Mutant hLAIR-1 proteins were stably expressed in K562 cells and tested in a flow cytometric assay for binding to FITC-conjugated collagens I and III or Oregon green 488–conjugated collagen IV (Figure 3A-B). Surface expression levels of the mutant proteins, as determined by binding of LAIR-1 antibodies (clones DX26 and 8A8) in the flow cytometric experiments were comparable with the wild-type protein (Figure 3A), which is an indication of proper folding. Mutants R59A, E61A, and R65A showed severely reduced binding to all collagens tested. In addition, mutant E111A showed decreased collagen binding, albeit to a lesser extent. The R62A mutant displayed somewhat reduced binding to collagen III, even further reduced binding to collagen IV, and unaffected binding to collagen I. None of the mutations outside the area designated by the NMR experiments significantly affected collagen binding. We also measured the adhesion of transfected cells to immobilized collagens for a subset of LAIR-1 mutants (Figure 3C). The subset included all 5 mutants that showed decreased collagen binding in the flow cytometric assay and 4 randomly selected mutants that displayed unaffected collagen binding. Adhesion to immobilized collagens I, III, and IV was significantly reduced in the R59A, E61A, R65A, and E111A mutants, although the magnitude of the effect depended on the type of collagen tested. In addition, adhesion was somewhat reduced for mutants R62A and N69A, which showed near wild-type collagen binding in the flow cytometric assay.
Binding of K562 cells transfected with hLAIR-1a mutants to various collagens. (A) Flow cytometric analysis of LAIR-1 expression (y-axis) and FITC-conjugated collagen I binding (x-axis) on parent K562 cells (upper left panel) or K562 cells expressing wt or mutant LAIR-1a as indicated. Representative dot plots of at least 3 independent experiments are shown. Percentage of LAIR-1+ collagen-binding cells is indicated. (B) Summary flow cytometric analyses showing binding of soluble collagens I, III, and IV to parent K562 cells (−) and K562 cells expressing wt LAIR-1a or mutants as indicated. (C) Adhesion of parent K562 cells (−) and K562 cells expressing wt LAIR-1a or mutants as indicated to plate-bound collagens I, III, and IV. White bars indicate collagen I; black bars, collagen III; and striped bars, collagen IV (nt indicates not tested). Data represent mean ± SD of at least 3 independent experiments.
Binding of K562 cells transfected with hLAIR-1a mutants to various collagens. (A) Flow cytometric analysis of LAIR-1 expression (y-axis) and FITC-conjugated collagen I binding (x-axis) on parent K562 cells (upper left panel) or K562 cells expressing wt or mutant LAIR-1a as indicated. Representative dot plots of at least 3 independent experiments are shown. Percentage of LAIR-1+ collagen-binding cells is indicated. (B) Summary flow cytometric analyses showing binding of soluble collagens I, III, and IV to parent K562 cells (−) and K562 cells expressing wt LAIR-1a or mutants as indicated. (C) Adhesion of parent K562 cells (−) and K562 cells expressing wt LAIR-1a or mutants as indicated to plate-bound collagens I, III, and IV. White bars indicate collagen I; black bars, collagen III; and striped bars, collagen IV (nt indicates not tested). Data represent mean ± SD of at least 3 independent experiments.
Binding of hLAIR1 mutants to FITC-conjugated peptide III-30 in the flow cytometric assay was severely decreased for the R59A mutant, slightly reduced for the E61A and E111A mutant proteins, and unaffected for all other mutant LAIR-1 proteins except the N69A mutant. Comparable results were obtained with the adhesion to coated peptide III-30 (supplemental Figure 4). Surprisingly, the R65A and E61A mutations do not or only slightly affect peptide III-30 binding, although both mutations severely reduce binding to intact collagens. Apparently, recognition of a specific binding site in the context of the triple-helical peptide III-30 differs somewhat from binding to intact fibrous collagens (see “Discussion”).
The mutational analysis confirms the location of the LAIR-1 collagen-binding site as determined by the NMR titration (compare Figure 2B and Figure 4A) and establishes that residues R59, E61, R65, and E111 contribute significantly to the affinity of collagen binding.
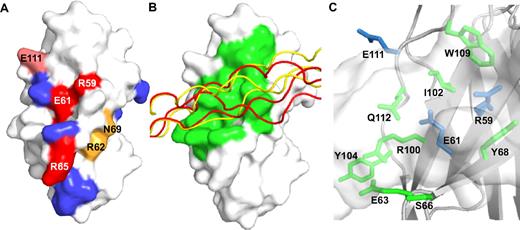
The LAIR-1/collagen complex. (A) Surface representation of LAIR-1 showing the effect of mutations on collagen binding: red indicates strong decrease; pink, moderate decrease; orange, moderate decrease in the plate adhesion assay only; and blue, no effect. (B) Surface representation of LAIR-1 with ribbon representations of collagen peptide III-30 models from the 2 highest scoring docking solutions (supplemental Table 3), shown in red and yellow. LAIR-1 residues that contact the docked peptides are shown in green. (C) Close-up of the putative collagen-binding site, showing in sticks LAIR-1 residues that are in contact with the docked collagen peptide. Residues that affect collagen binding when mutated are shown in blue. The collagen peptide is shown in semitransparent surface representation.
The LAIR-1/collagen complex. (A) Surface representation of LAIR-1 showing the effect of mutations on collagen binding: red indicates strong decrease; pink, moderate decrease; orange, moderate decrease in the plate adhesion assay only; and blue, no effect. (B) Surface representation of LAIR-1 with ribbon representations of collagen peptide III-30 models from the 2 highest scoring docking solutions (supplemental Table 3), shown in red and yellow. LAIR-1 residues that contact the docked peptides are shown in green. (C) Close-up of the putative collagen-binding site, showing in sticks LAIR-1 residues that are in contact with the docked collagen peptide. Residues that affect collagen binding when mutated are shown in blue. The collagen peptide is shown in semitransparent surface representation.
Docking of LAIR-1 and collagen model peptides
We combined our crystallographic, NMR, and mutagenesis data to generate models of the LAIR-1/collagen complex by data-driven docking using the program HADDOCK.36-38 Docking of the LAIR1-CBD crystal structure to a theoretic model of peptide III-30 was driven by intermolecular distance restraints between the collagen peptide and CBD residues that were identified by NMR and/or mutagenesis to be close to or in the binding site. As no experimental data on interacting residues from collagen are available, all surface-accessible residues of the collagen models were allowed to participate in docking.
The 2 best scoring solutions show the collagen peptide bound at an angle of roughly 60° to the F, C, and C′ β-strands of LAIR-1 (Figure 4B). These solutions have similar Haddock scores, well above the scores of other solutions (data for the best 10 solutions are presented in supplemental Table 3). Whereas the interaction site on LAIR-1 is very similar in both solutions, binding occurs to different regions of the collagen peptide: the C-terminal sequence KGAAGPOGPO or the more N-terminal sequence RGGAGPOGPE. Both putative binding sites comprise residues with short side chains including 2 and 1 GPO motif. Although this could reflect an intrinsic binding preference of LAIR1, we cannot exclude that it is an artifact of the docking procedure. Binding at 2 sites would be consistent with the suggested presence of 2 binding sites in peptide III-30,8 with the most important binding site for receptor signaling located toward the C-terminal 2 GPO triplets. However, due to the limited experimental data on collagen residues involved in LAIR binding, the docking results should be interpreted with caution.
Variations between solutions of comparable scores are considerable (Figure 4B), nevertheless, some general conclusions seem justified. The peptide binds across the β-sheet, burying a surface area of approximately 1300 Å2. The binding site comprises 3 collagen triplets, with LAIR-1 contacting 2 of the 3 collagen peptide chains. LAIR-1 residues E61, S66, Y68, I102, W109, and Y115 provide Van der Waals interactions, whereas hydrogen bonds to the ligand frequently involve LAIR-1 residues R59, E63, R100, E111, and Q112 (Figure 4C). In our docking solutions, we see no significant interactions involving the R65 side chain; however, it is in close enough proximity to the peptide to envisage an interaction upon a minor and local conformational change. The weak intensities of peaks attributed to residues S64-R65-S66 in the NMR experiments indicate the presence of slow dynamics in this loop region that would enable R65 to adopt multiple conformations, which may not be sampled during docking calculations. Our docking model gives insight into the mechanism by which LAIR-1 and collagen interact and provides a basis for further mutagenesis studies on both the collagen peptide and LAIR-1.
Discussion
Collagen interactions are essential in many biologic processes including immune suppression, cell migration, and hemostasis. Here we set out to explore the interaction of LAIR-1 with collagen using a combination of X-ray crystallography, NMR titrations, and mutagenesis. The crystal structure of the collagen-binding domain of hLAIR-1 was determined to 1.8-Å resolution and most closely resembles the KIR2DL2, LILRA5, and GPVI D1 domains. The NMR data define a continuous surface patch on the hLAIR1-CBD that is involved in the interaction with collagen. Probing of this interaction surface by site-directed mutagenesis shows residues R59 and E61 to be essential for collagen binding. The importance of R59 and E61 is further emphasized by their strict conservation in LAIR proteins from 9 different species (supplemental Figure 5). Residues R59 and E61, together with W109, form a patch of conserved surface-exposed residues in the core of the collagen-interaction surface as identified by NMR (Figures 2B and 5C). Although W109 was not probed by mutagenesis, the chemical environment of its side chain changes upon collagen binding, as is evident from the spectral shift of the side chain nitrogen-resonance in the NMR titration. In combination with its strict conservation, this is suggestive of an important role for W109 in ligand binding. Mutagenesis data show a limited contribution to collagen binding of residue E111, located at the periphery of the interaction surface. Accordingly, E111 is not conserved. Surprisingly, R65 is also not conserved, despite its essential role in collagen binding described previously35 and confirmed in this study. Its important role, but lack of conservation, indicates the existence of species-specific differences in collagen interactions. Also of note: mutation of R65 has no effect on binding to peptide III-30 (further discussed below). We postulate that residues R59, E61, and also W109 make up the core of the collagen-binding site, whereas more peripheral residues such as E111 contribute less to binding. The role of R65 in the LAIR-1/collagen interaction remains ambiguous.
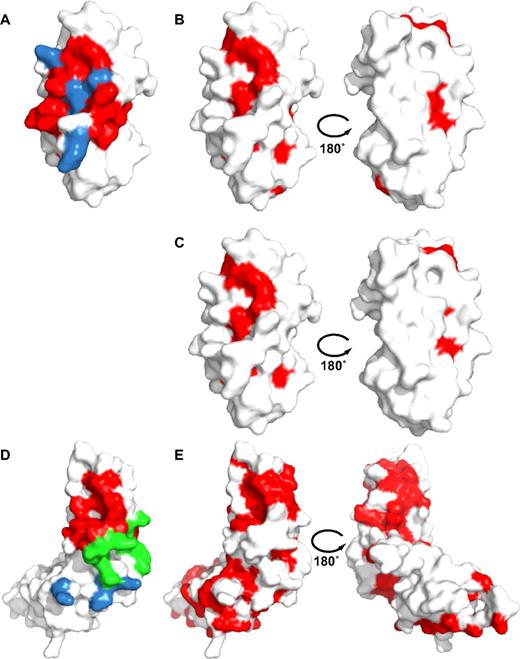
Conservation of putative collagen-binding residues in LAIR-1 and GPVI. (Left) Surface representations of the LAIR1-CBD (A) and the GPVI ectodomain (D) showing in blue residues that contribute to collagen binding according to mutagenesis data, in red residues that form the LAIR-1 collagen binding surface based on the NMR titrations, and in green residues that contact docked collagen peptides in GPVI. Data for LAIR-1 and GPVI are from this study and Horii et al,14 respectively. GPVI residues, L53 and F54 that are part of the docking interface as well as the equivalent of the LAIR-1/collagen interface, are shown in green. (Right) Surface representations in 2 orientations of LAIR-1 (B-C) and GPVI (E) showing in red residues that are strictly conserved within the LAIR-family (B), within both the LAIR and GPVI families (C) and, residues conserved within the GPVI family only (E). Note that putative collagen-binding residues of LAIR-1 are significantly conserved in both LAIR and GPVI families, whereas putative collagen-binding residues of GPVI are not at all conserved. Conservation is based on the alignments shown in supplemental Figures 5 and 6. The orientation of LAIR is identical to Figure 2B, whereas GPVI is depicted with its D1 domain in an orientation similar to LAIR-1.
Conservation of putative collagen-binding residues in LAIR-1 and GPVI. (Left) Surface representations of the LAIR1-CBD (A) and the GPVI ectodomain (D) showing in blue residues that contribute to collagen binding according to mutagenesis data, in red residues that form the LAIR-1 collagen binding surface based on the NMR titrations, and in green residues that contact docked collagen peptides in GPVI. Data for LAIR-1 and GPVI are from this study and Horii et al,14 respectively. GPVI residues, L53 and F54 that are part of the docking interface as well as the equivalent of the LAIR-1/collagen interface, are shown in green. (Right) Surface representations in 2 orientations of LAIR-1 (B-C) and GPVI (E) showing in red residues that are strictly conserved within the LAIR-family (B), within both the LAIR and GPVI families (C) and, residues conserved within the GPVI family only (E). Note that putative collagen-binding residues of LAIR-1 are significantly conserved in both LAIR and GPVI families, whereas putative collagen-binding residues of GPVI are not at all conserved. Conservation is based on the alignments shown in supplemental Figures 5 and 6. The orientation of LAIR is identical to Figure 2B, whereas GPVI is depicted with its D1 domain in an orientation similar to LAIR-1.
Comparison of ligand-binding sites of LRC-family members shows that they are diverse and located at different positions on the D1 and D2 domains, reflecting the diversity of ligands bound by the various family members (supplemental Figure 1). Surprisingly, the location of the LAIR-1 collagen-binding site, across the FCC′ β-sheet, most closely resembles the major histocompatibility complex–HLA interaction sites of LIR-1 and LIR-2 and not the proposed collagen-binding site of GPVI, which is located in a hydrophobic groove on the surface in between β-strands C′ and E.14
In view of their homology and overlapping ligand preferences,7,12 it is remarkable that the proposed LAIR-1 and GPVI collagen-binding surfaces differ so completely. Mapping of conserved surface residues reveals, however, significant conservation of the LAIR-1 collagen-binding site in GPVI (Figure 5 and supplemental Figures 5-6). Conserved residues include R59, E61, and W109, which are central to collagen binding in LAIR-1. The converse is not true: residues implicated in the collagen-binding site of human GPVI are not conserved in LAIR-1. Surprisingly, the putative GPVI collagen-binding site is not conserved in GPVI orthologues either (Figure 5B,E and supplemental Figure 6). This is unexpected because protein-protein interaction sites in general belong to the most conserved surface residues among orthologous proteins. Identification of the GPVI collagen-binding site has relied on rigid body docking of a collagen-like peptide on the crystal structure of human GPVI and is supported by mutagenesis data on GPVI residues, not conserved in LAIR-1: K59, R60, and R166.14,39,40 The collagen-binding mode that we propose for LAIR-1 would not place these GPVI residues in the proximity of collagen and thus cannot provide an alternative explanation for the GPVI mutagenesis data. It should be noted that some evidence suggests the existence of 2 collagen-binding sites on GPVI or at least a more extended collagen-binding site, including GPVI residues G30, V34, and L38.41 It is possible that LAIR-1 and GPVI have evolved different strategies for binding the same ligand, similar to the different collagen-binding modes of the von Willebrand factor–A3 domain and its structural homologue the integrin α2 I-domain.42,43 However, the observed partial conservation of the LAIR-1 collagen-binding site in GPVI, in combination with the absence of conservation of the proposed GPVI binding site in GPVI orthologues, is suggestive of similar collagen-binding modes for LAIR-1 and GPVI and warrants further studies into the GPVI-collagen interaction.
Libraries of overlapping synthetic triple-helical peptides encompassing the complete collagen II and III triple-helical domain have been instrumental in the identification of recognition sites for a range of collagen-binding proteins, including LAIR-1, GPVI, von Willebrand factor, SPARC, and integrin α2β1.7,12,26,44-46 Biophysical techniques such as polarimetry confirm the triple-helical nature of these peptides,28 whereas crystal structures, albeit of shorter synthetic peptides, show that they display the proper register with a 1-residue stagger between the 3 peptide strands.47-50 LAIR-1 was found to bind at least 6 peptides of the collagen III library.7 The existence of multiple binding sites on collagen is also evident from BIAcore data showing that on average approximately 10 LAIR-1 binding sites are present per collagen II or III triple helix.6 Our data reveal some intriguing differences between the binding of LAIR-1 mutants to whole collagen III and to collagen peptide III-30. Mutants E61A and R65A show a strong reduction in binding to collagen III, whereas binding to peptide III-30 is not affected for mutant R65A and only slightly reduced for mutant E61A (supplemental Figure 4). Other mutations, such as R59A and Q111A, reduce binding to peptide and collagen to similar extents. The observed differences between our peptide- and collagen-binding studies could arise from several factors including subtle differences in the conformation of the triple helix. Crystal structures of synthetic triple-helical peptides show a 7/2 helical twist in imino acid–rich regions going toward 10/3 or intermediate twist in the more amino acid–rich collagen regions.47-50 To date, there is no high-resolution collagen structure available and there is no consensus model for the collagen helical twist; both a 7/2 and a 10/3 helical twist of collagen have been proposed.51,52 Another factor that might be responsible for the observed differences between peptide and collagen binding is LAIR-1 contacting more than one triple-helix in fibrillar collagen simultaneously, a situation that cannot be reproduced in the model peptide. A similar situation exists for integrin α2β1 where the context of one specific binding site is important to determine binding affinity,28 suggesting adjacent residues in the collagen fiber may affect binding. Alternatively, the superstructure of the collagen microfibril could result in variable exposure of different segments of the collagen triple helix on the surface of the fiber53 ; some LAIR-1 binding sites present in collagen peptides may therefore not be exposed at the surface of a collagen fibril and these would be inaccessible for LAIR-1 binding. This situation could lead to different effects of mutations on binding to a collagen peptide and intact fibrous collagen. Thus, synthetic triple-helical peptides are invaluable tools for the identification of collagen-protein interaction sites, but at least in the case of LAIR-1, results obtained with these peptides will need to be validated in the context of whole collagen.
In summary, we have clearly defined a surface on LAIR-1 that is involved in the interaction with collagen and this surface is conserved across species. We have identified key binding-site residues that are important in the interaction of LAIR-1 with collagen. Although the LAIR-1 collagen-binding site is well conserved, there are some surprising differences between species. The LAIR-1 collagen-binding site is remarkably different from the proposed collagen-binding site of the LAIR-1 homologue, platelet protein GPVI. However, our analyses suggest that with the presently available data it cannot be excluded that GPVI binds collagen at a site similar to that of LAIR-1 suggesting a common structural basis for the recognition of collagen by both proteins. Further studies are needed into the GPVI-collagen interaction to clarify this issue. The available crystal structures will facilitate the development of compounds targeted at specifically modulating immune responses via the LAIR-1/collagen interaction or thrombosis through GPVI.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank the European Synchrotron Radiation Facility for providing data collection facilities and the beamline scientists at ID23-1 for their help with data collection. We thank Dr Alexandre Bonvin for his help and advice with regard to the docking experiments.
This work was supported by the Netherlands Heart Foundation (NHS2005B238), the Netherlands Foundation for Chemical Research NWO/CW, the Center for Biomedical Genetics, and the EU FP6 project EU-NMR (contract no. RII3-026145).
Authorship
Contribution: R.J.L., T.R., and L.M. initiated the work and performed the mutagenesis studies; T.H.C.B. and J.B. purified proteins and did the crystallographic studies; J.B., H.I., H.W., and R.B. performed and analyzed the NMR experiments; R.W.F. provided the synthetic peptide; T.H.C.B. performed the docking; and T.H.C.B., L.M., and E.G.H. designed the experiments and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Eric G. Huizinga, Crystal and Structural Chemistry, Bijvoet Center for Biomolecular Research, Utrecht University, Padualaan 8, Utrecht 3584 CH, The Netherlands; e-mail: e.g.huizinga@uu.nl.