Abstract
We examined 6 different FMS-like tyrosine kinase-3 (FLT3) inhibitors (lestaurtinib, midostaurin, AC220, KW-2449, sorafenib, and sunitinib) for potency against mutant and wild-type FLT3, as well as for cytotoxic effect against a series of primary blast samples obtained from patients with acute myeloid leukemia (AML) harboring internal tandem duplication (FLT3/ITD) mutations. We found that inhibition of FLT3 autophosphorylation in a FLT3/ITD specimen does not always induce cell death, suggesting that some FLT3/ITD AML may not be addicted to FLT3 signaling. Relapsed samples and samples with a high mutant allelic burden were more likely to be responsive to cytotoxicity from FLT3 inhibition compared with the samples obtained at diagnosis or those with a low mutant allelic burden. These FLT3 inhibitors varied to a considerable degree in their selectivity for FLT3, and this selectivity influenced the cytotoxic effect. These results have important implications for the potential therapeutic use of FLT3 inhibitors in that patients with newly diagnosed FLT3-mutant AML might be less likely to respond clinically to highly selective FLT3 inhibition.
Introduction
Internal tandem duplication mutations of the FMS-like tyrosine kinase-3 receptor (FLT3/ITD mutations) are one of the most common molecular abnormalities found in de novo acute myeloid leukemia (AML) and have a strong negative prognostic impact.1 Given the obvious and sometimes dramatic clinical benefits achieved using kinase inhibitors for other malignancies, efforts have been underway for the past decade to identify and clinically test small-molecule FLT3 inhibitors for use in improving the clinical outcome of FLT3-mutant AML.2,3 At the present time, at least 5 such agents are in active clinical development, including phase 3 trials.4-8
We have studied the majority of these compounds in the laboratory, using model cell lines either engineered to express FLT3 mutant constructs, cell lines derived from patients with AML harboring FLT3/ITD mutations, and, perhaps most importantly, primary blasts obtained directly from patients with AML harboring FLT3/ITD mutations. In addition, we have participated in several the clinical trials of these drugs, and have had the opportunity to perform correlative studies on leukemia samples obtained from trial patients. We have observed that the in vitro cytotoxic response of primary AML blasts to FLT3 inhibitors was predictive of clinical response.9-11 When we investigated the cytotoxic effects of FLT3 inhibitors on a larger series of FLT3/ITD blasts derived from nontrial patients, we noted that a given sample could be resistant in vitro to one inhibitor and responsive to another.10 Others have reported similar findings.12 Because in vitro cytotoxic responses have correlated with clinical response to these drugs, we wished to identify the factors influencing the cytotoxic responses of primary blasts to FLT3 inhibitors and thereby potentially develop a predictive model for clinical activity.
To this end, we have conducted a systematic comparison of 6 different FLT3 inhibitors, derived from 5 distinct chemical classes, for potency and selectivity against FLT3, as well as for relative cytotoxic effect against a series of FLT3/ITD AML primary samples. For this study, we chose to use the indolocarbazoles lestaurtinib (previously referred to as CEP-701) and midostaurin (previously referred to as PKC-412), as well as KW-2449, sorafenib, sunitinib, and AC220. Each of these agents is or has been under study as a FLT3 inhibitor.13-17
In our study, we have found that the clinical status of patients with AML was a significant predictor of cytotoxic response to the more selective FLT3 inhibitors. Our findings have important implications both for the potential clinical application of FLT3 inhibitors, as well as for underlying biologic differences between newly diagnosed and recurring AML.
Methods
FLT3 inhibitors
FLT3 inhibitors were obtained as powder and dissolved in dimethyl sulfoxide (DMSO) at stock concentrations of 10 mM. Stocks were aliquoted into 10 μL volumes and stored at −80°C and thawed immediately before use. Lestaurtinib was supplied by Cephalon Inc. AC220 was supplied by Ambit Biosciences Inc. KW-2449 was supplied by Kyowa Hakko Kirin Co Ltd. Midostaurin, sorafenib, and sunitinib were obtained from LC Laboratories Inc. All samples in any given experiment contained identical concentrations of DMSO.
Patient samples
Leukemia cell specimens were provided by the Sidney Kimmel Cancer Center at Johns Hopkins Tumor and Cell Procurement Bank, supported by the Regional Oncology Research Center Grant No. 2 P30 CA 006973-44. All patients gave informed consent according to the Declaration of Helsinki under a protocol approved by the Johns Hopkins institutional review board. The criteria for selecting a sample was that it had to have been obtained from a patient with de novo AML (ie, no antecendent myelodysplasia, no treatment-related AML) and there needed to be sufficient vials in storage to perform a large number of assays at least in duplicate. The blasts needed to have sufficient viability after thawing such that an optical density (OD) of greater than 0.15 (after subtraction of background) was obtained in the MTT assay. The samples had to be obtained before any treatment with chemotherapy (including hydroxyurea). All whole-blood samples were collected directly from the patient into heparin tubes. After Ficoll-Hypaque centrifugation, the blast monolayer was collected, washed, and frozen in fetal bovine serum (FBS)/DMSO on the day of collection. The mononuclear cells were aliquoted and stored frozen in liquid nitrogen in bovine serum with 10% DMSO for repeated use. Before each use, aliquots of these blasts were thawed rapidly into warm culture medium, incubated for 12 hours, and then recentrifuged over Ficoll to eliminate cells dying from the freeze-thaw process. Using this method, we obtained blasts that maintained satisfactory viability (as determined by Trypan blue exclusion) and responsiveness to cytotoxic agents in culture over 72 hours.
Cell lines and culture methods
All cell lines and primary blast samples were cultured in RPMI/10% FBS (Millipore) at 37°C in 5% CO2. Ficoll-Hypaque was obtained from GE Healthcare. Molm-14 and SEMK2 cells were obtained from the DSMZ (Deutsche Sammlung von Mikroorganismen und Zellkulturen, Germany). The BaF3/ITD cell line (kindly provided by Dr Donald Small, Johns Hopkins University, Baltimore, MD) was derived by transfecting interleukin-3 (IL-3)–dependent Ba/F3 cells with an expression vector containing the FLT3 coding sequence for an ITD mutation from a patient with AML, as described. The resultant BaF3/ITD line is growth factor–independent and expresses constitutively phosphorylated FLT3. The IL-3 rescue assay was performed as described.13 IL-3 was obtained from Peprotech.
Cytotoxicity assays
Cytotoxicity was assessed using an MTT (dimethyl-thiazol diphenyl tetrazolium bromide) assay. In selected cases, we also used an annexin V binding apoptosis assay to confirm that the cytotoxic effect observed (or lack thereof) was associated with an equivalent degree of apoptosis. MTT (Roche, Indianapolis, IN) and Annexin V (Pharmingen) assays were performed as described previously.18
FLT3 phosphorylation
Leukemia cells were washed in phosphate-buffered saline (PBS), then lysed by resuspending them in lysis buffer (20 mM Tris, pH 7.4; 100mM NaCl; 1% Igepal [Sigma-Aldrich]; 1mM EDTA; 2mM NaVO4; plus Complete protease inhibitor [Roche]) for 30 minutes while rocking. The extract was clarified by centrifugation at 16 000g, and the supernatant was assayed for protein (Bio-Rad). A 50-μg aliquot was removed as whole-cell lysate for analysis of STAT5, and the remainder was used for immunoprecipitation with anti-FLT3. Anti-FLT3 antibody (S18; Santa Cruz Biotechnology) was added to the extract for overnight incubation; protein A sepharose (Upstate Biotechnology) was then added for 2 additional hours. Separate SDS-PAGE (sodium dodecyl sulfate–polyacrylamide gel electrophoresis) gels for whole-cell lysate and immunoprecipates were run in parallel. After transfer to Immobilon membranes (Millipore), immunoblotting was performed with antiphosphotyrosine antibody (4G10; Upstate Biotechnology) to detect phosphorylated FLT3 or, for the whole-cell lysate gels, with a rat monoclonal antibody against phosphorylated STAT5 (residue Y694; kindly provided by Kyowa Hakko Kirin Co Ltd), then stripped and reprobed with anti-FLT3 antibody to measure total FLT3. Proteins were visualized using electrochemiluminescence (ECL; GE Healthcare), exposed on Kodak BioMax XAR Film, developed, and scanned using a Bio-Rad GS800 densitometer. The concentration of drug for which the phosphorylation of FLT3 was inhibited to 50% of its baseline (IC50) was determined using linear regression analysis of the dose-response curves after linear transformation using an exponential model (CalcuSyn software; Biosoft Inc).
FLT3-mutant allelic burden
For qualitative analysis of FLT3/ITD mutations, genomic DNA was isolated using a QIamp Mini Kit (QIAGEN), and polymerase chain reaction (PCR) was performed using the Platinum PCR Supermix (Invitrogen) with primers flanking the juxtamembrane coding region: 13F (GTAAAACGACGGCCAGTGCAGAACTGCCTATTCCTA) and 15R (CAGGAAACAGCTATGACCTGTCCTTCCACTATACTGT). A total of 35 cycles of amplification were performed at 94°C for 30 seconds for denaturation, at 55°C for 30 seconds for annealing, and at 72°C for 1 minute for extension (DNA iCycler; Bio-Rad). PCR products were resolved on 2.5% agarose gels and visualized under ultraviolet light after ethidium bromide staining. For quantitative analysis of FLT3/ITD mutations, the PCR reactions were carried out using Taq Gold (Applied Biosystems) with fluorochrome-labeled primers, followed by capillary electrophoresis on an ABI 3100 capillary electrophoresis instrument (Applied Biosystems), as described previously.19 All FLT3 mutations were confirmed by DNA sequencing. Sequences were analyzed and allelic ratios were calculated using GeneScan software (Applied Biosystems). For quantitative analysis of RNA transcripts of mutant versus wild-type FLT3, RNA was prepared from the blasts using RNeasy Mini Kit (QIAGEN). After generation of cDNA, the PCR reactions and quantitative analyses were carried out as for the genomic DNA. For confirmation of the expression ratios in select cases, we performed quantitative PCR with patient-specific primers prepared using a 20-bp primer overlapping the insertion site, after the method of Stirewalt et al.20
Flow cytometric analysis
Fluorescence-activated cell sorter (FACS) analysis was performed using a FACScalibur cytometer and CellQuest software from Becton Dickinson. Fluoroscene isothiocyanate (FITC)–, phycoerythrin (PE)–, and allophycocyanin (APC)–conjugated antibodies to human Annexin V, CD3, CD19, CD34, and CD33 were obtained from BD Biosciences PharMingen.
Results
FLT3 inhibitors
For each of the inhibitors used in this study (lestaurtinib, midostaurin, sunitinib, KW-2449, sorafenib, and AC220), we prepared dose-response curves for inhibition of wild-type and mutant FLT3 autophosphorylation. For FLT3 activated by an ITD mutation, we used Molm-14 cells, which harbor a 21-bp insertion in the juxtamembrane domain.21 Wild-type FLT3 was more problematic for our study. In general, wild-type FLT3 in cultured AML cell lines requires the addition of exogenous FLT3 ligand (FL) for activation.22 We have recently determined that exogenous FL impedes the effects of FLT3 inhibitors (manuscript in preparation; results to be presented at the 2009 meeting of the American Society of Hematology23 ). Therefore, to study the effects of these inhibitors on nonmutated FLT3, we used the SEMK2 cell line, in which FLT3 is activated through overexpression due to genomic amplification of the locus.24 Leukemia cells in culture medium treated with increasing concentrations of each compound were lysed and subject to FLT3 immunoprecipitation, SDS-PAGE, and immunoblotting with antiphosphotyrosine. After densitometric analysis of the gel bands, we prepared dose-response curves for inhibition of wild-type and mutant FLT3 autophosphorylation. To provide an estimate of in vivo potency, and given that the activity of these agents is significantly influenced by plasma protein binding, we carried out parallel experiments in which Molm-14 cells in were exposed to the drugs in 100% human plasma. Table 1 lists the IC50 values for FLT3 inhibition in SEMK2 and Molm-14 cells in culture medium and Molm-14 cells in plasma. Because FLT3 inhibition must be sustained to induce cytotoxicity refill, the plasma half-lives derived from early phase clinical trials is also included.5,11,25-28 All 6 drugs potently inhibited ITD-mutated FLT3 in culture medium, with IC50 values ranging from 1 to 10 nM, and, in general, very close to the values reported by others. AC220 was the most potent inhibitor, and KW-2449 was the least potent. Interestingly, all 6 drugs were 3- to 9-fold less potent at inhibiting wild-type FLT3. This predilection for the FLT3/ITD-mutant receptor over the wild-type receptor has been previously reported for lestaurtinib and midostaurin.12 Although the drugs were of similar potency in culture medium, potency in plasma varied across 2 orders of magnitude (Table 1). Taking the reported plasma half-lives into consideration, lestaurtinib would be predicted to be the least effective at achieving sustained FLT3 inhibition in vivo, whereas AC220 is predicted to be the most effective.
Inhibition of ITD-mutant (FLT3/ITD) and wild-type (WT) FLT3 autophosphorylation
| Drug . | WT FLT3 IC50, medium, nM . | FLT3/ITD IC50, medium, nM . | FLT3/ITD IC50, plasma, nM . | In vivo t1/2, h . |
|---|---|---|---|---|
| Lestaurtinib | 10 | 2 | 700 | 825 |
| Midostaurin | 30 | 3 | 1700 | 24+26 |
| Sorafenib | 28 | 3 | 484 | 24+27 |
| Sunitinib | 2 | 1 | ND | 24+28 |
| KW-2449 | 36 | 10 | 144 | 411 |
| AC220 | 5 | 1 | 18 | 24+5 |
| Drug . | WT FLT3 IC50, medium, nM . | FLT3/ITD IC50, medium, nM . | FLT3/ITD IC50, plasma, nM . | In vivo t1/2, h . |
|---|---|---|---|---|
| Lestaurtinib | 10 | 2 | 700 | 825 |
| Midostaurin | 30 | 3 | 1700 | 24+26 |
| Sorafenib | 28 | 3 | 484 | 24+27 |
| Sunitinib | 2 | 1 | ND | 24+28 |
| KW-2449 | 36 | 10 | 144 | 411 |
| AC220 | 5 | 1 | 18 | 24+5 |
SEMK2 cells (wild-type FLT3) and Molm-14 cells (FLT3/ITD) were incubated with increasing concentrations of inhibitor. R values for all experiments were greater than 0.98. Sunitinib was not analyzed in plasma, as there are no active FLT3 AML trials with this agent. The plasma half-lives (In vivo t1/2) were obtained from early-phase clinical trial data (see references). ND indicates not determined.
Cytotoxic response of primary AML samples
Based on the dose-response curves for inhibition of FLT3 autophosphorylation, we chose to test the dose range of 0 to 100nM for all inhibitors against primary samples. The maximum concentration of 100nM for each of these drugs would be predicted to result in near complete inhibition of autophosphorylation of both mutant and wild type FLT3 in culture medium. Pharmacokinetic studies in humans have been performed for all 6 compounds. A concentration of 100nM in culture medium, equivalent to approximately 1 to 10μM in plasma, corresponds to roughly achievable trough concentration for each of these drugs, based on dosing regimens evaluated in early-phase clinical trials. We previously carried out a comparison of the indolocarbazole FLT3 inhibitors lestaurtinib and midostaurin (previously referred to as PKC-412), and its metabolite CGP52421, using primary samples, and determined that the off-target activity of these compounds were important to their cytotoxic effects on AML.10 We decided not to include midostaurin in this portion of the study, in part because we have already compared it directly to lestaurtinib, but also because its pharmacokinetics, particularly in regards to its metabolites, are complex, rendering a simple study of the parent drug (PKC-412) alone of questionable validity. In a broad sense, midostaurin, together with its metabolite CGP52421, can be considered very similar to lestaurtinib, as it is of the same chemical class (indolocarbazole) and has a similar cytotoxic profile against primary blasts.10
For this screen of inhibitors, we used a total of 27 primary AML samples from our tissue bank—13 with FLT3/ITD mutations and 14 with wild-type FLT3. For each ITD sample, the individual ITD mutation was sequenced directly. Mutant allelic burden was determined as described in “FLT3-mutant allelic burden.” The characteristics of these samples are summarized in Table 2.
Characteristics of AML patient samples used in inhibitor screen
| Sample no. . | Age, y/sex . | Status . | Cytogenetics . | ITD length, bp . | Mutant, % . |
|---|---|---|---|---|---|
| 1 | 53/F | Diagnostic | INV16 | 78 | 5 |
| 2 | 75/M | Diagnostic | Normal | 39 | 31 |
| 3 | 58/M | Diagnostic | Normal | 96 | 35 |
| 4 | 28/M | Diagnostic | Normal | 75 | 37 |
| 5 | 58/F | Diagnostic | Normal | 33 | 42 |
| 6 | 69/F | Diagnostic | Normal | 33 | 43 |
| 7 | 54/F | Diagnostic | Normal | 51 | 70 |
| 8 | 26/M | Diagnostic | Normal | 51 | 9 |
| 9 | 64/M | Relapse | Normal | 21 | 95 |
| 10 | 53/M | Relapse | Normal | 18 | 91 |
| 11 | 53/F | Relapse | +8,13,22 | 39 | 55 |
| 12 | 34/M | Relapse | t(7;11) | 201 | 55 |
| 13 | 55/M | Relapse | Normal | 30 | 96 |
| 14 | 59/F | Diagnostic | Normal | — | — |
| 15 | 62/M | Diagnostic | t(11;19) | — | — |
| 16 | 69/F | Diagnostic | Normal | — | — |
| 17 | 23/F | Diagnostic | INV16 | — | — |
| 18 | 71/M | Diagnostic | Normal | — | — |
| 19 | 70/M | Diagnostic | Normal | — | — |
| 20 | 78/F | Diagnostic | Normal | — | — |
| 21 | 68/F | Diagnostic | Normal | — | — |
| 22 | 28/M | Diagnostic | t(11;19) | — | — |
| 23 | 40/M | Diagnostic | Normal | — | — |
| 24 | 25/F | Relapse | t(9;11) | — | — |
| 25 | 60/F | Relapse | Normal | — | — |
| 26 | 50/M | Relapse | Normal | — | — |
| 27 | 55/F | Relapse | Normal | — | — |
| Sample no. . | Age, y/sex . | Status . | Cytogenetics . | ITD length, bp . | Mutant, % . |
|---|---|---|---|---|---|
| 1 | 53/F | Diagnostic | INV16 | 78 | 5 |
| 2 | 75/M | Diagnostic | Normal | 39 | 31 |
| 3 | 58/M | Diagnostic | Normal | 96 | 35 |
| 4 | 28/M | Diagnostic | Normal | 75 | 37 |
| 5 | 58/F | Diagnostic | Normal | 33 | 42 |
| 6 | 69/F | Diagnostic | Normal | 33 | 43 |
| 7 | 54/F | Diagnostic | Normal | 51 | 70 |
| 8 | 26/M | Diagnostic | Normal | 51 | 9 |
| 9 | 64/M | Relapse | Normal | 21 | 95 |
| 10 | 53/M | Relapse | Normal | 18 | 91 |
| 11 | 53/F | Relapse | +8,13,22 | 39 | 55 |
| 12 | 34/M | Relapse | t(7;11) | 201 | 55 |
| 13 | 55/M | Relapse | Normal | 30 | 96 |
| 14 | 59/F | Diagnostic | Normal | — | — |
| 15 | 62/M | Diagnostic | t(11;19) | — | — |
| 16 | 69/F | Diagnostic | Normal | — | — |
| 17 | 23/F | Diagnostic | INV16 | — | — |
| 18 | 71/M | Diagnostic | Normal | — | — |
| 19 | 70/M | Diagnostic | Normal | — | — |
| 20 | 78/F | Diagnostic | Normal | — | — |
| 21 | 68/F | Diagnostic | Normal | — | — |
| 22 | 28/M | Diagnostic | t(11;19) | — | — |
| 23 | 40/M | Diagnostic | Normal | — | — |
| 24 | 25/F | Relapse | t(9;11) | — | — |
| 25 | 60/F | Relapse | Normal | — | — |
| 26 | 50/M | Relapse | Normal | — | — |
| 27 | 55/F | Relapse | Normal | — | — |
Peripheral blood blasts were obtained through an institutional review board–approved protocol at diagnosis or relapse. F indicates female; M, male; and —, wild-type FLT3.
Each of the 5 drugs was tested individually at identical concentrations (0-100nM) against each of the 13 FLT3/ITD samples. Dose-response curves were generated, comparing the percentage OD to untreated control after 72 hours of incubation. The 65 individual dose-response curves are displayed in supplemental Figure 1 (available on the Blood website; see the Supplemental Materials link at the top of the online article). The cytotoxic response to any single inhibitor varied widely from sample to sample.
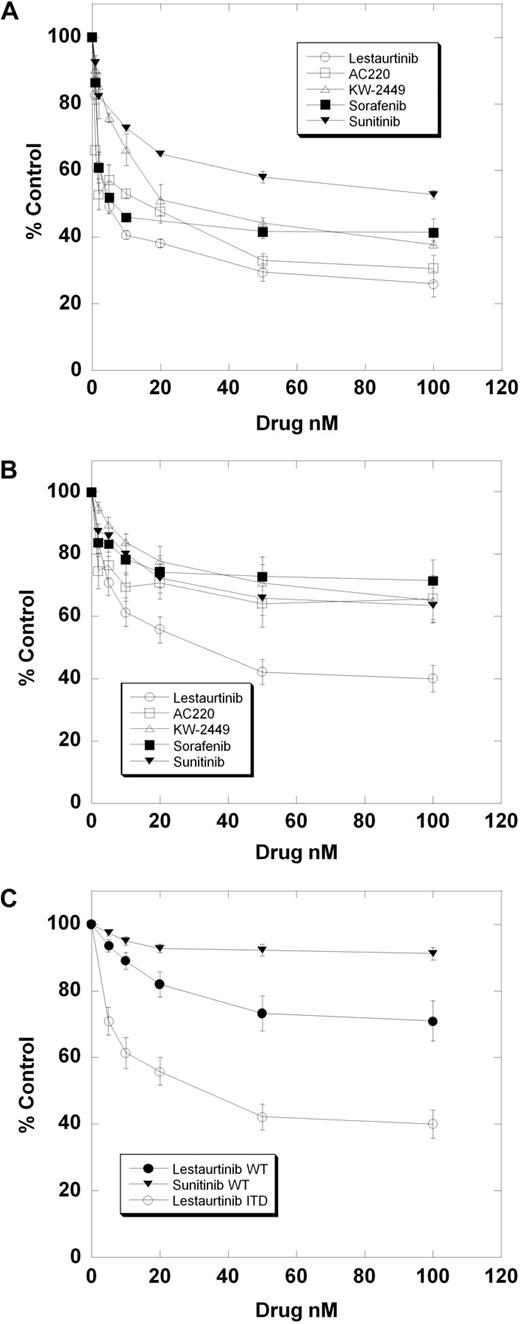
There was also variation in the response of a given sample (Sample 10; Table 2) to each of the 5 inhibitors individually (Figure 1A). To discern any broad patterns of response that might exist, we combined the individual dose-response curves for each inhibitor. For each drug concentration, the percentage control for all 13 samples was expressed as a mean value, with error bars representing SEMs. Thus, each of the 5 dose-response curves shown in Figure 1B represents the mean dose-response curve for all 13 samples against an individual drug. What is apparent from examining these curves is that lestaurtinib is significantly more cytotoxic over a similar range of FLT3 inhibition than the other 4 inhibitors. This difference between lestaurtinib and the others extends to samples with wild-type FLT3. Shown in Figure 1C are the composite curves derived from dose-response experiments using 14 primary AML samples with wild-type FLT3 treated with lestaurtinib or sunitinib. For comparison, the composite curve of the lestaurtinib-treated FLT3/ITD samples from Figure 1B is included. Lestaurtinib has a very modest cytotoxic effect against these 14 wild-type samples, whereas sunitinib (and KW-2449, sorafenib, and AC220; not shown) have no effect whatsoever. However, lestaurtinib is still distinctly more cytotoxic to the FLT3-mutant samples than to the wild-type samples.
Cytotoxicity assays of FLT3/ITD primary AML samples. Peripheral blood blasts isolated from 13 patients with AML harboring FLT3/ITD mutations were incubated in increasing concentrations of each of the 5 FLT3 inhibitors indicated and analyzed using the MTT assay as described in “Cytotoxicity assays.” The experiment was repeated, and the data from both experiments was combined. (A) Dose-response curves for a single FLT3/ITD sample (No. 10 from Table 2) exposed to each of 5 FLT3 inhibitors. Error bars represent standard deviations. (B) Composite dose-response curves for all 5 inhibitors and all 13 FLT3/ITD samples. For each drug concentration, the percentage control for all 13 samples was expressed as a mean value, with error bars representing the SEMs. Each individual data point on these curves represents the mean of roughly 7 OD values for each of 13 different AML samples (ie, 7 × 13 = 91 OD values). (C) Composite dose-response curves for 14 wild-type FLT3 samples exposed to sunitinib (top curve) or lestaurtinib (middle curve).
Cytotoxicity assays of FLT3/ITD primary AML samples. Peripheral blood blasts isolated from 13 patients with AML harboring FLT3/ITD mutations were incubated in increasing concentrations of each of the 5 FLT3 inhibitors indicated and analyzed using the MTT assay as described in “Cytotoxicity assays.” The experiment was repeated, and the data from both experiments was combined. (A) Dose-response curves for a single FLT3/ITD sample (No. 10 from Table 2) exposed to each of 5 FLT3 inhibitors. Error bars represent standard deviations. (B) Composite dose-response curves for all 5 inhibitors and all 13 FLT3/ITD samples. For each drug concentration, the percentage control for all 13 samples was expressed as a mean value, with error bars representing the SEMs. Each individual data point on these curves represents the mean of roughly 7 OD values for each of 13 different AML samples (ie, 7 × 13 = 91 OD values). (C) Composite dose-response curves for 14 wild-type FLT3 samples exposed to sunitinib (top curve) or lestaurtinib (middle curve).
To examine these differences in cytotoxicity more closely, we directly compared lestaurtinib with the most potent of the inhibitors, AC220, in 2 of the samples. As shown in Figure 2A, lestaurtinib is cytotoxic to both Sample 3 and Sample 13, whereas AC220 (and KW-2449, sorafenib, and sunitinib; not shown) is cytotoxic only to Sample 13. To confirm that FLT3 autophosphorylation was fully inhibited by both drugs in both samples, we performed a dose-response immunoblot (Figure 2B). STAT5 is an important downstream protein in the FLT3 signaling pathway that has been correlated with survival. The level of total and phosphorylated STAT5 was noticeably less in Sample 3 compared with Patient 13, although it was fully inhibited in both samples by both drugs. While we have shown previously that the MTT assay of primary blasts correlates directly with the induction of apoptosis, we noted that the dose-response curve for Sample 3 against lestaurtinib was still relatively shallow, descending to 61% of untreated control (Figure 2A). Flow cytometric analysis of the Sample 3 blasts treated with DMSO alone, AC220, or lestaurtinib (Figure 2C), however, confirmed that the dose-response curve to lestaurtinib was due to the induction of apoptosis. At thawing (Figure 2C; Day 0 DMSO control), the sample contains 12% dead cells (gate A), 75% viable blasts (gate B), and 4% lymphocytes (gate C). We confirmed that the cells in gate A are annexin V–positive, the cells in gate B are annexin V–negative, CD33+, CD3− and CD19−, and that the cells in gate C are CD3+ or CD19+ (data not shown). This confirms that the cytotoxic effect seen in the MTT assay is due to the induction of apoptosis, rather than inhibition of proliferation, which we have reported previously.18 The relatively high level of residual MTT metabolism is presumably due to the presence of a population of viable lymphocytes, together with a large amount of cellular debris and/or dead cells after 3 days of incubation. A significant number of these blasts, therefore, die over a 3-day period under control conditions, and lestaurtinib adds to the rate of apoptosis (Figure 2C; 29.92% to 3.54%). Strikingly, however, and in complete accordance with the MTT dose-response curves, AC220 is very similar to the DMSO control cells (29.92% to 25.52%), having very little cytotoxic effect.
Cytotoxic effect is not exclusively dependent on inhibition of FLT3 autophosphorylation. (A) MTT assay dose-response curves for Sample 3 (circles) and Sample 13 (squares) incubated with AC220 (open symbols) and lestaurtinib (filled symbols). Error bars represent standard deviations. (B) Blasts from Sample 3 (top blots) and Sample 13 (bottom blots) were incubated for 1 hour in culture medium with the indicated concentrations of lestaurtinib (left) and AC220 (right). The blasts were then analyzed for phospho-FLT3 and phospho-STAT5 as described in “FLT3 phosphorylation.” (C) Blasts from Sample 3 were thawed and resuspended in culture medium. An aliquot was removed (Day 0), fixed, and stained with anti-CD33, anti-CD3, anti-CD19, and annexin V for flow cytometric analysis, and then the remaining blasts were divided into 3 cultures: DMSO control, lestaurtinib 50 nM, and AC220 50 nM. After 72 hours of culture, the blasts were fixed, stained, and analyzed for comparison with the day 0 blasts. Shown are the forward (x-axis) and side (y-axis) scatter plots. Gate A represents dead cells (annexin V+); gate B, viable blasts (annexin V−, CD33+); gate C, lymphocytes (CD3+ or CD19+).
Cytotoxic effect is not exclusively dependent on inhibition of FLT3 autophosphorylation. (A) MTT assay dose-response curves for Sample 3 (circles) and Sample 13 (squares) incubated with AC220 (open symbols) and lestaurtinib (filled symbols). Error bars represent standard deviations. (B) Blasts from Sample 3 (top blots) and Sample 13 (bottom blots) were incubated for 1 hour in culture medium with the indicated concentrations of lestaurtinib (left) and AC220 (right). The blasts were then analyzed for phospho-FLT3 and phospho-STAT5 as described in “FLT3 phosphorylation.” (C) Blasts from Sample 3 were thawed and resuspended in culture medium. An aliquot was removed (Day 0), fixed, and stained with anti-CD33, anti-CD3, anti-CD19, and annexin V for flow cytometric analysis, and then the remaining blasts were divided into 3 cultures: DMSO control, lestaurtinib 50 nM, and AC220 50 nM. After 72 hours of culture, the blasts were fixed, stained, and analyzed for comparison with the day 0 blasts. Shown are the forward (x-axis) and side (y-axis) scatter plots. Gate A represents dead cells (annexin V+); gate B, viable blasts (annexin V−, CD33+); gate C, lymphocytes (CD3+ or CD19+).
In summary, for Sample 3, both AC220 and lestaurtinib fully inhibit FLT3 autophosphorylation and suppress downstream STAT5 activation, but only lestaurtinib induces apoptosis in conjunction with FLT3 inhibition. If a cytotoxic effect in the MTT assay is defined by a drop in the dose-response curve over the FLT3 inhibitory concentration (1-10nM), down to 75% or less of the control, then it can be concluded that, at least for Sample 3, inhibition of FLT3 autophosphorylation, (together with STAT5 activation) by itself is insufficient to induce meaningful cytotoxicity. In fact, as the data in supplemental Figure 1A-E show, several of the 13 FLT3/ITD samples we used in this study showed minimal or no cytotoxic response to FLT3 inhibitory concentrations of these drugs.
No effect of FLT3 or ITD sequence on response to inhibitors
Samples 1, 3, and 8 all displayed minimal or no cytotoxic effect from AC220, KW-2449, sorafenib, and sunitinib. In light of recently published data demonstrating cytotoxic resistance to FLT3 inhibition despite inhibition of FLT3 autophosphorylation associated with a mutation at residue 627,29 we sequenced the entire transmembrane and cytoplasmic portions (including the juxtamembrane and kinase domains) of the FLT3 cDNA from each of these samples. No additional molecular abnormalities were present other than the ITD mutations. In addition, there was no correlation with ITD length and response to inhibitors (data not shown).
FLT3 inhibitor selectivity
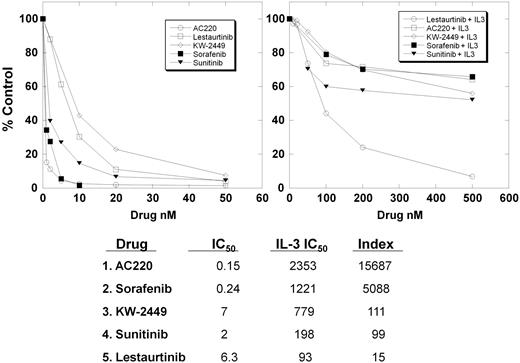
The distinct difference in cytotoxic effect exerted by lestaurtinib compared with the other inhibitors appeared to be at least in part independent of FLT3 inhibition. Lestaurtinib is very closely related in structure to staurosporine, which has been characterized as a highly promiscuous kinase inhibitor.30,31 We hypothesized that a significant proportion of the cytotoxicity of lestaurtinib could be due to its nonselectivity (and resultant inhibition of other kinases or other ATP-binding proteins). We have previously used an IL-3 rescue assay to compare the selectivity of lestaurtinib with midostaurin.10 The difference between the IC50 values in the absence and presence of IL-3 provide an index of a compound's selectivity for FLT3.
Shown in Figure 3 are the results of IL-3 rescue assays for the 5 FLT3 inhibitors tested in the cytotoxicity assays. The IL-3 index values are tabulated below the graphs. As expected, lestaurtinib has the lowest index of selectivity, and AC220 is the most selective. The inhibitors can be ranked, from most to least selective, as AC220, sorafenib, sunitinib, KW-2449, and lestaurtinib. In a rough fashion, AC220 and sorafenib are highly selective, KW-2449 and sunitinib are of intermediate selectivity, and lestaurtinib is relatively nonselective. Of note, this order of selectivity corresponds to selectivity scores found with these compounds using an in vitro competition binding assay: AC220, 0.067; sorafenib, 0.18; sunitinib, 0.58; and lestaurtinib, 0.81 (except for KW-2449, which was not evaluated).15,31
IL-3 rescue assay. BaF3/ITD cells were incubated in quadruplicate wells of a 96-well plate in culture medium with increasing concentrations of the inhibitors in the absence (left) or presence (right) of 1 ng/mL IL-3. After 48 hours, the MTT reagent was added and OD values were obtained. The dose-response curves were prepared as percentage control (DMSO only), and regression analysis was used to obtain IC50 values. The ratio of the IC50 in the presence of IL-3 divided by the IC50 in the absence of IL-3 is the IL-3 index, tabulated below the graphs.
IL-3 rescue assay. BaF3/ITD cells were incubated in quadruplicate wells of a 96-well plate in culture medium with increasing concentrations of the inhibitors in the absence (left) or presence (right) of 1 ng/mL IL-3. After 48 hours, the MTT reagent was added and OD values were obtained. The dose-response curves were prepared as percentage control (DMSO only), and regression analysis was used to obtain IC50 values. The ratio of the IC50 in the presence of IL-3 divided by the IC50 in the absence of IL-3 is the IL-3 index, tabulated below the graphs.
Therefore, as determined by 2 different assay methods, lestaurtinib can be considered a broadly multitargeted kinase inhibitor that also happens to inhibit FLT3. At the opposite end of the spectrum, AC220 appears to be a highly selective FLT3 inhibitor, but is less broadly cytotoxic to FLT3/ITD primary samples in vitro.
FLT3-mutant allelic burden correlates with response to selective inhibitors
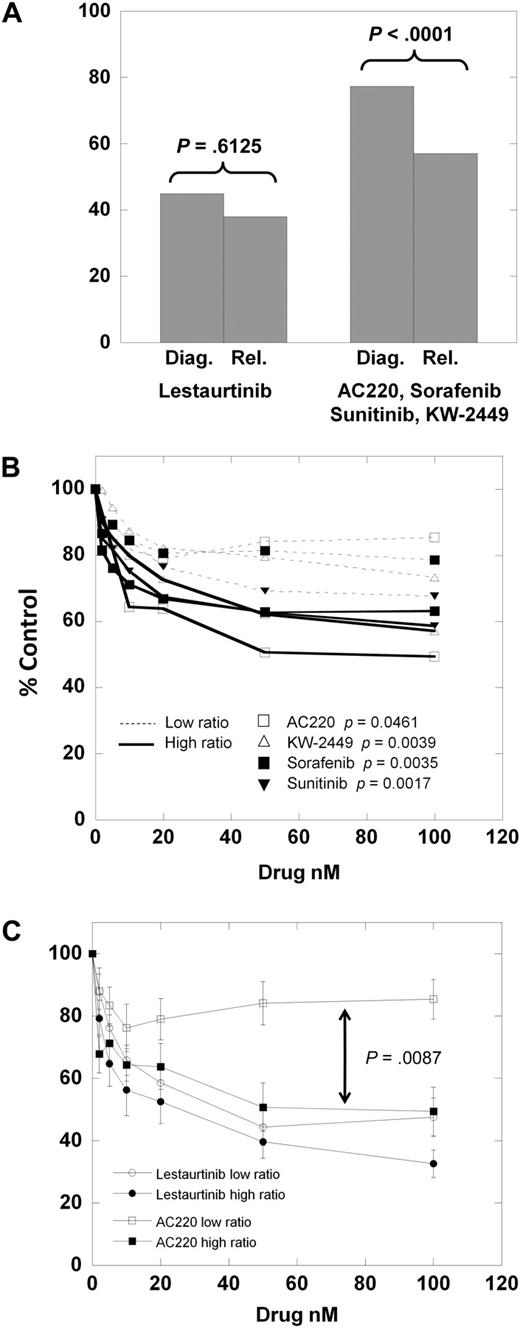
We next divided the samples into those obtained at diagnosis and those obtained at relapse. For lestaurtinib, the cytotoxicity dose-response curves of the diagnostic versus relapsed samples were very similar. However, for the other 4 inhibitors, the dose-response curves (supplemental Figure 1A-E) reveal the relapsed samples to be distinctly more responsive than the diagnostic samples. As shown in Figure 4A, at 100nM lestaurtinib, the mean MTT results for diagnostic versus relapsed samples are not significantly different (P = .613). However, the mean results for the other 4 (more selective) inhibitors are significantly different (P < .001).
Cytotoxic response according to clinical status. (A) The results of the MTT assay for the 8 diagnostic samples versus the 5 relapsed samples exposed to 50 nM of either lestaurtinib (left) or 50 nM of the other 4 inhibitors averaged (right). Comparison was made using the Student 2-tailed t test. (B) Composite dose-response curves for 4 inhibitors (AC220, KW-2449, sorafenib, sunitinib) against low mutant ratio (Table 1; Samples 1-6, 8; thin dashed lines) and high mutant ratio (Table 1; Samples 7, 9-13; solid lines) FLT3/ITD samples. For each drug concentration, the percentage control for all samples was expressed as a mean value. Error bars, representing the SEMs, were all less than 3%, and were omitted for graph clarity. Comparison was made using the Student 2-tailed t test. (C) Composite dose-response curves for AC220 and lestaurtinib against low and high mutant ratio samples (as in panel B). For each drug concentration, the percentage control for all samples tested was expressed as a mean value, with error bars representing the SEMs. Comparison was made using the Student 2-tailed t test, with the arrow denoting a P value for AC220 low versus high allelic ratio samples.
Cytotoxic response according to clinical status. (A) The results of the MTT assay for the 8 diagnostic samples versus the 5 relapsed samples exposed to 50 nM of either lestaurtinib (left) or 50 nM of the other 4 inhibitors averaged (right). Comparison was made using the Student 2-tailed t test. (B) Composite dose-response curves for 4 inhibitors (AC220, KW-2449, sorafenib, sunitinib) against low mutant ratio (Table 1; Samples 1-6, 8; thin dashed lines) and high mutant ratio (Table 1; Samples 7, 9-13; solid lines) FLT3/ITD samples. For each drug concentration, the percentage control for all samples was expressed as a mean value. Error bars, representing the SEMs, were all less than 3%, and were omitted for graph clarity. Comparison was made using the Student 2-tailed t test. (C) Composite dose-response curves for AC220 and lestaurtinib against low and high mutant ratio samples (as in panel B). For each drug concentration, the percentage control for all samples tested was expressed as a mean value, with error bars representing the SEMs. Comparison was made using the Student 2-tailed t test, with the arrow denoting a P value for AC220 low versus high allelic ratio samples.
One possible explanation for this finding is that relapsed FLT3-mutant AML is more “addicted” to FLT3 signaling than at diagnosis. A direct way to test such a conclusion would be to use matched diagnostic and relapsed samples. Our tissue bank did not have sufficient amounts of such samples for this comparison. However, others have reported direct comparisons of matched FLT3/ITD samples, examining the FLT3 mutant–wild-type ratio.32-34 From this body of data, it is clear that at relapse, in most patients with AML, the FLT3 mutation is retained, and usually at a higher mutant allelic burden compared with diagnosis. For each of the samples used in our series, we directly sequenced the FLT3/ITD mutation and performed gene scan analysis to provide a quantitative estimate of the mutant allelic burden, expressed as a percentage of total alleles (Table 2). Because there are invariably contaminating nonmalignant cells in primary samples (Figure 2C), most primary AML samples heterozygous for a FLT3/ITD mutation would be expected to have allelic burdens of 30% to 45%. We noted that all of the relapsed FLT3/ITD samples (Samples 9-13) had allelic burdens greater than 50%, and also noted that the one diagnostic sample with a burden greater than 50% (Sample 7) was generally responsive to all inhibitors. We hypothesized that the increased mutant allelic burden conferred a greater degree of dependence of a sample on FLT3 for survival. Using the same approach displayed in Figure 1, we plotted the mean dose-response curves for the 4 more selective inhibitors against the 7 samples with low mutant allelic burden (Samples 1-6, 8) versus the 6 samples with mutant allelic burden greater than 50%. (Samples 7, 9-13) As shown in Figure 4B, the samples with a mutant allelic burden greater than 50% are more responsive to the FLT3 inhibitors compared with the low ratio samples. Furthermore, the dose-response curves for the low burden samples (thin dashed lines in Figure 4B) fall into the exact same order as the selectivity scores shown in Figure 3 (AC220, sorafenib, KW-2449, sunitinib). If we consider AC220 as the most selective inhibitor, and lestaurtinib as the least selective, it is instructive to compare the curves for these 2 drugs against low- versus high-allelic burden samples (Figure 4C).
To summarize, of the 13 total FLT3/ITD samples we studied, all 6 relapsed samples and all 7 samples with high mutant allelic burdens were responsive to selective FLT3 inhibition. Only 2 of 7 diagnostic samples with low mutant allelic burdens were responsive to these same drugs, despite those drugs' abilities to completely inhibit FLT3 authophosphorylation. The nonselective inhibitor lestaurtinib (and, by inference, midostaurin with its metabolite CGP52421) seems to be equally effective at killing all types of FLT3/ITD samples.
Discussion
This is the first direct comparison of the in vitro cytotoxic response patterns of a series of FLT3/ITD patient samples to several different small-molecule FLT3 inhibitors. These results, in combination with our previous comparison of the indolocarbazole inhibitors lestaurtinib and midostaurin,10 have important implications for the potential therapeutic use of FLT3 inhibitors.
From our data we can make several observations. First, inhibition of FLT3 autophosphorylation in a FLT3/ITD specimen does not by itself guarantee cell death. That is, some FLT3/ITD AML is not truly addicted to FLT3 signaling. This phenomenon appears confined (at least in our small series) to samples obtained at diagnosis. Second, relapsed FLT3/ITD AML is more responsive to cytotoxicity from FLT3 inhibition compared with the disease at diagnosis. Third, FLT3 inhibitors vary in their selectivity for FLT3, and this selectivity influences the cytotoxic effects induced by these drugs. Fourth, FLT3/ITD AML with a high mutant allelic burden is more responsive to selective FLT3 inhibition compared with cases with a low mutant allelic burden.
The 5 inhibitors we studied (6, if midostaurin is considered to be in the same category as lestaurtinib) can be broadly separated into highly selective (AC220 and sorafenib), intermediate selective (sunitinib and KW-2449), and less selective (lestaurtinib and midostaurin) classes. Selective inhibition of FLT3 autophosphorylation is by itself insufficient for induction of in vitro cytotoxicity in some cases of FLT3/ITD AML. This failure of a FLT3/ITD sample to be “addicted” to FLT3 signaling seems confined to samples obtained at diagnosis. Because the response rate for diagnostic specimens is clearly higher for the least selective of the drugs, we can derive a model that may potentially predict the clinical response of a patients with FLT3/ITD AML to a given FLT3 inhibitor. A newly diagnosed case may best be treated with lestaurtinib or midostaurin, whereas the blasts from a relapsed patient would be predicted to respond to any drug that can completely inhibit FLT3. The clinical utility of this model, of course, is predicated on the correlation between in vitro response and in vivo response. Although this correlation has been observed for both lestaurtinib and KW-2449, it obviously needs to be established in larger numbers of patients and for the other FLT3 inhibitors as well.9,11
Complete in vivo inhibition of FLT3 has been difficult to achieve thus far with drugs such as lestaurtinib and midostaurin. Given that these are relatively nonselective inhibitors, this may highlight a difficulty in achieving profound FLT3 inhibition in a tolerable fashion with these agents. The more selective inhibitors might achieve complete and tolerable in vivo target inhibition, but this may be less effective at in the diagnostic setting. Therefore, one might consider using the indolocarbazoles (lestaurtinib or midostaurin) at diagnosis. In fact, in preliminary data, midostaurin, administered in combination with chemotherapy, has been reported to increase the complete remission rate for patients with newly diagnosed FLT3/ITD.7 At relapse, the presumably better-tolerated selective inhibitors could be used. Alternately, because patients with FLT3/ITD achieve remission at a similar rate to nonmutant cases, a selective agent could be used as maintenance to prevent outgrowth of the more addicted clone. KW-2449, an inhibitor with intermediate selectivity, potentially offers an intriguing compromise between nonselectivity and in vivo potency, if an effective dose can be identified for phase 2 studies.11
Our data imply that a high mutant allelic burden (as well as the relapsed state) is correlated with cytotoxic response to selective FLT3 inhibition. From this we might predict that only a portion of patients with newly diagnosed FLT3/ITD AML will have a clinical response to monotherapy with a FLT3 inhibitor. These findings may also speak to the underlying biology of FLT3-mutant AML, in that the disease seems to undergo an evolution at relapse to a state of greater dependency on FLT3 compared with that at diagnosis.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grants from the National Cancer Institute (NCI Leukemia SPORE P50 CA100632-06, R01 CA128864) and the American Society of Clinical Oncology (M.L.). M.L. is a Clinical Scholar of the Leukemia & Lymphoma Society.
National Institutes of Health
Authorship
Contribution: K.W.P. conducted experiments, helped design the study, and helped write the manuscript; T.S. helped conduct experiments; K.M.M. performed the allelic ratio experiments; A.S. performed the IL-3 rescue experiments; T.R. conducted the quantitative PCR studies; and M.L. designed the study, conducted experiments, and wrote the manuscript.
Conflict-of-interest disclosure: M.L. is a consultant for Cephalon Inc and Ambit Biosciences Inc. The remaining authors declare no competing financial interests.
Correspondence: Mark Levis, Kimmel Cancer Center at Johns Hopkins, 1650 Orleans St, Rm 243, Baltimore, MD 21231; e-mail: levisma@jhmi.edu.