In this issue of Blood, Séïté and colleagues report their findings on human tonsil B cells and human Ramos B-cell lymphoma line apoptosis, induced by sialic acid–intravenous immunoglobulin (SA-IVIg) fraction.1
IVIg is used for treatment of many inflammatory, autoimmune, and immune-deficiency disorders (reviewed in Tha-In et al,2 Schwartz-Albiez et al,3 and Nimmerjahn and Ravetch4 ). Because IVIg contains antibodies (Abs) toward idiotypes (Ids) specific for autoAbs, it modulates autoreactive B cells. Several mechanisms have been proposed to explain IVIg's activity, including blocking of phagocytic Fcγ receptors (Fcγ), up-regulation of inhibitory FcγR (FcγRIIb), modulating cytokine secretion, inhibiting cell proliferation, priming dendritic cell regulatory activity, and induction of T regulatory cells.2-4 B lymphocytes harbor IVIg-targeted molecules that set up the bone marrow B-cell repertoires, negative signaling through FcγRIIb, selective down-regulation of Ab production, and neutralization of circulating autoAbs by anti-Ids. Anti-Id–specific IVIg was found to be an efficient approach in treating animal models of human autoimmune diseases, such as systemic lupus erythematosus (SLE), antiphospholipid syndrome, and myasthenia gravis (MG).5,6 Manipulation of B cells by IVIg is not only passive (with neutralization of autoAbs and FcγR blockade) but also active (with effect on B-cell antigen receptor [BCR] signaling). This is regulated either positively or negatively by various B-cell membrane molecules (see figure).
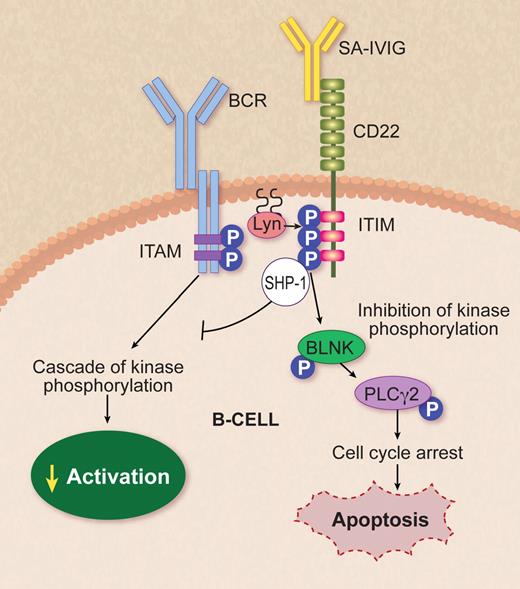
SA-IVIg targeting CD22 on B cells. BCR ligation initiates the activation of protein tyrosine kinases including Lyn, an Src family kinase that phosphorylates the ITAMs, leading to B-cell activation through cascade of kinase phosphorylation. Séïté et al1 proved that SA-IVIg coligation to CD22 promotes apoptosis via inhibiting the cascade of kinase phosphorylation in mature human tonsil B lymphocytes and in human Ramos lymphoma B-cell lines by inducing phosphorylation of ITIM. IVIg-CD22 decreases BCR-mediated signaling through down-modulation of tyrosine phosphorylation of Lyn and recruitment and activation of phosphatase SHP-1, which can then down-modulate BCR-dependent pathways, regulating BLNK and PLCγ2 activation. The latter results in cell-cycle arrest and up-regulation of cascade, leading to apoptosis. BCR indicates B-cell receptor; ITAM, immunoreceptor tyrosine-based activation motif; ITIM, immunoreceptor tyrosine-based inhibitory motif; P, phosphorylation; SHP-1, Src homology 2 domain-containing phosphatase-1; BLNK, B-cell linker protein; and PLCγ, phospholipase Cγ2. Professional illustration by Debra T. Dartez.
SA-IVIg targeting CD22 on B cells. BCR ligation initiates the activation of protein tyrosine kinases including Lyn, an Src family kinase that phosphorylates the ITAMs, leading to B-cell activation through cascade of kinase phosphorylation. Séïté et al1 proved that SA-IVIg coligation to CD22 promotes apoptosis via inhibiting the cascade of kinase phosphorylation in mature human tonsil B lymphocytes and in human Ramos lymphoma B-cell lines by inducing phosphorylation of ITIM. IVIg-CD22 decreases BCR-mediated signaling through down-modulation of tyrosine phosphorylation of Lyn and recruitment and activation of phosphatase SHP-1, which can then down-modulate BCR-dependent pathways, regulating BLNK and PLCγ2 activation. The latter results in cell-cycle arrest and up-regulation of cascade, leading to apoptosis. BCR indicates B-cell receptor; ITAM, immunoreceptor tyrosine-based activation motif; ITIM, immunoreceptor tyrosine-based inhibitory motif; P, phosphorylation; SHP-1, Src homology 2 domain-containing phosphatase-1; BLNK, B-cell linker protein; and PLCγ, phospholipase Cγ2. Professional illustration by Debra T. Dartez.
BCR ligation regulates B cells from an immunoreceptor tyrosine-based activation motif (ITAM) through a cascade of kinase phosphorylation. Signals initiated at the BCR regulate transcriptional, posttranscriptional, and posttranslational events, determining the fate of the B cell—whether it will survive, die, proliferate, or differentiate. Regulation of BCR signaling is mediated by the association of the BCR complex with coreceptors such as CD22, CD19, CD21, and FcγRIIB.
CD22 is a transmembrane adhesion molecule that belongs to the sialic acid (SA)–binding Ig-like lectin (Siglec) superfamily, with 7 Ig-like extracellular domains and an amino-terminal Ig domain. CD22 modulates the BCR signaling cascade by binding SA-modified glycoproteins. SA binding to CD22 is required for negative regulation of BCR signaling. Upon stimulation of the BCR, the cytoplasmic tail of CD22 is phosphorylated on tyrosine residues of Ig-like tyrosine-based inhibitory motifs (ITIMs). Different intracellular signaling proteins bind to the phosphorylated tyrosines of the CD22 tail. BCR signaling is inhibited through recruitment of the Src homology 2 domain-containing phosphatase-1 protein tyrosine phosphatase via ITIMs in the CD22 cytoplasmic tail, followed by the dephosphorylation cascade of other signal proteins.
Séïté et al, in the current issue, used SA-IVIg as a ligand molecule for CD22. The SA-enriched IVIg fraction, which is an IVIg-glycan specific for CD22, was affinity-purified from commercial IVIg using a Sambucus nigra agglutinin agarose column.1 The SA-IVIg was used to prove that B-cell membranous CD22 is a key receptor in IVIg-mediated BCR signaling. SA-IVIg through IVIg-CD22 promotes apoptosis in mature human tonsil B lymphocytes and in human Ramos lymphoma B-line cells. The IVIg-CD22 association involves several BCR-signaling pathways including inhibition of the phospholipase Cγ2 cascade, sustained activation of Erk1/2, p38, and down-regulation of PI3K. These changes are associated with the induction of cyclin-dependent kinase inhibitor p27Kip1, which inhibits cell-cycle progression at the G1 phase and thus promotes apoptosis.
B cell–depleted therapy by SA-IVIg has a strong potential for treating systemic and organ-specific autoimmune diseases including SLE, MG, multiple sclerosis, and other autoimmune conditions where their activity is mediated by BCR cell function. The efficacy of SA-IVIg was previously reported by Ravetch et al to be involved in the treatment inflammatory state.7 They demonstrated that the anti-inflammatory activity of IVIg is mainly mediated by Abs that harbor terminal α2,6-sialic acid linkages at the Asn297-linked glycan of the Fc region. This SA-rich IVIg fraction exerts an anti-inflammatory effect by induction of the expression of the inhibitory Fc-IIB. This receptor is exclusively expressed on B cells and serves as a negative regulator inhibiting BCR-elicited activation.7
A monoclonal Ab (mAb) targeting CD22 (Siglec-2; epratuzumab) is currently in clinical trials for treatment of B-cell non-Hodgkin lymphomas and autoimmune diseases. Whether SA-IVIg mimics the BCR-signaling pathway as anti-CD22 mAbs needs further study. The SA-IVIg binds CD22 via the SA on the Fc portion of the Ig, whereas the humanized anti-CD22 binds the CD22 via its Fab sites. One may assume that the binding site reproduces the SA-IVIg binding site. The advantage that SA-IVIg anti-CD22 is a natural compound with minimal side effects in contrast to the humanized anti-CD22 mAbs needs to be tested in animal models.
As a novel approach for B cell–targeted therapy, Chen et al introduced doxorubicin-loaded liposomal nanoparticles displaying high-affinity glycan ligands to CD22 of patients with B-cell lymphoma.8 The targeted liposomes are actively bound and endocytosed by CD22 on B cells, and significantly extend life in a xenograft model of human B-cell lymphoma. Like a Trojan horse, the α2-6–linked sialylated glycan ligands deliver the drug to the cells. Moreover, they bind and kill malignant B cells from peripheral blood samples obtained from patients with hairy cell leukemia, marginal zone lymphoma, and chronic lymphatic leukemia (CLL).8
The potential of IVIg for cancer therapy was previously proposed and summarized (Shoenfeld and Krause9 ). The fact that SA-IVIg induces apoptosis in the Ramos lymphoma B-cell line raises the possibility of future therapy of cell lymphomas and CLL that express high levels of CD22, with SA-IVIg. The up-regulation of ITIM expression by coligation of IVIg-CD22 may lead to tumor apoptosis in these cells. As shown previously by Besa,10 IVIg could serve successfully as a supportive therapy to chemotherapy in CLL. However, the mechanism was not delineated, although classical explanations were proposed.
In conclusion, the Séïté et al study provides for the first time the mechanism by which SA-IVIg may promote apoptosis-controlling B-cell malignancies, and the therapeutic future potential of SA-IVIg in attenuating B-cell activity in autoimmune disorders.
Conflict-of-interest disclosure: The authors declare no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal