Abstract
Although malaria is widely considered a major cause of death in young children born with sickle cell anemia (SCA) in sub-Saharan Africa, this is poorly quantified. We attempted to investigate this question through 4 large case-control analyses involving 7164 children living on the coast of Kenya. SCA was associated with an increased risk of admission to hospital both with nonmalaria diseases in general (odds ratio [OR] = 4.17; 95% confidence interval [CI], 1.95-8.92; P < .001) and with invasive bacterial diseases in particular (OR = 8.73; 95% CI, 4.51-16.89; P < .001). We found no evidence for a strongly increased risk of either uncomplicated malaria (OR = 0.43; 95% CI, 0.09-2.10; P = .30) or malaria complicated by a range of well-described clinical features of severity (OR = 0.80; 95% CI, 0.25-2.51; P = .70) overall; nevertheless, mortality was considerably higher among SCA than non-SCA children hospitalized with malaria. Our findings highlight both the central role that malaria plays in the high early mortality seen in African children with SCA and the urgent need for better quantitative data. Meanwhile, our study confirms the importance of providing all children living with SCA in malaria-endemic areas with effective prophylaxis.
Introduction
The common causes of morbidity and mortality in children living with sickle cell anemia (SCA) in developed countries have been well documented through projects, such as the Cooperative Study of Sickle Cell Disease in the United States and the Jamaican Cohort study. Nevertheless, surprisingly little research has been conducted in Africa,1-3 where more than 230 000 children with SCA, approximately 80% of the global burden, are born every year.4 Although malaria is widely considered a major cause of death in African children with SCA,1,5-7 this assumption is supported by surprisingly few data. Most reports have involved small, hospital-based studies and have lacked control data that enable comparisons of risk to be made with non-SCA subjects.1 Perhaps the strongest evidence comes from the Garki project, conducted in northern Nigeria during the 1970s, which reported a nonsignificant trend toward higher survival of children with SCA in an area exposed to intensive malaria control.8 However, studies suggesting that malaria is an important cause of death in children with SCA are balanced by others that show the opposite: children with SCA might even be less susceptible to malaria than those without the disease.6,9-13
Determining the true risk of death from malaria in subjects with SCA is important for several reasons. From a policy perspective, documenting an association between SCA and malaria death would provide strong justification for early-life SCA screening and the targeted prescription of effective malaria prophylaxis. Conversely, if the risk of malaria-specific death was not elevated, alternative approaches to prophylaxis and treatment might be considered. Recently, we reported that malaria was a rare cause of morbidity in patients with confirmed SCA in both Dar-es-Salaam, Tanzania13 and in the Kilifi District on the coast of Kenya.11 Nevertheless, the diagnosis of SCA is often delayed until the disease becomes clinically manifest, and these studies could not have recorded malaria deaths in undiagnosed children. Here, we have approached this question using an alternative design. Instead of confining our study exclusively to subjects with an established diagnosis of SCA, we have taken as a starting point a representative sample of children presenting to hospital with a range of acute illnesses, including malaria. Admission blood samples collected from case children were tested retrospectively for Hemoglobin S (HbS) and their genotype frequencies compared with those of healthy controls recruited from the same community.
Methods
Ethics statement
The aims of the study were explained to the parents of all study participants who gave their written informed consent in accordance with the Declaration of Helsinki. The study was approved by the Kenya Medical Research Institute/National Ethical Review Committee.
Study area
The study was conducted in the Kilifi District on the coast of Kenya, one of the poorest districts nationwide where the economy is dominated by subsistence farming. Health facilities are limited and sick children are normally referred to Kilifi District Hospital (KDH), which acts as both a primary facility and a first-referral center for a population of more than 600 000. Facilities at the hospital include both a 50-bed general pediatric ward and a 10-bed high-dependency unit.7 Common health problems affecting children admitted to KDH include malaria, gastroenteritis, malnutrition, and lower respiratory tract infections.7 The prevalence of HbAS in the area surrounding KDH is 15%.14 Medical services for children with SCA are scarce in the Kilifi District: there is no program of antenatal or early life screening, and the only specialist clinic is in the KDH where only a small proportion of all anticipated cases are registered. As a result, only a small but unknown fraction of all children born with SCA in the study area receive specific antimalarial or antimicrobial prophylaxis. In 2000, we established a demographic surveillance system (the Kilifi Epi-DSS) in a defined area surrounding KDH,15 which we refer to as the study area. Approximately 80% of all children who are admitted to KDH are residents of this area, which includes 5 administrative divisions, covers a total of 891 km2, and is home to more than 100 000 children younger than 14 years. A system of routine clinical surveillance has operated on the pediatric wards at KDH since 1989.16 Data are recorded on all admitted children, including their location of residence, details of a standardized clinical history and examination, admission and discharge diagnoses, and the results of routine blood tests, including a full blood count, blood culture, and blood film examination for malaria parasites.16 In addition, a sample of blood from each admitted child is routinely stored at −80°C. The current study was conducted retrospectively using data and samples collected through this framework.
Study participants
We studied the relationship between SCA and malaria in 2 groups of case patients. The first consisted of a random selection of 1087 children younger than 5 years who were residents of our study area and were admitted to the general pediatric ward at KDH with uncomplicated slide-positive Plasmodium falciparum malaria between January 1, 2000 and December 31, 2004. For the purpose of this report, a case of uncomplicated malaria was defined as a child admitted to the general ward with P falciparum parasites in the peripheral blood, no clinical or laboratory features to support an alternative diagnosis and with no clinical signs of prostration (Blantyre coma score 3-4), coma (Blantyre coma score ≤ 2), or deep breathing.17 The second group included 1718 children younger than 5 years who were admitted from the study area to the high-dependency unit between September 1996 and February 2008 with malaria complicated by 1 or more of the following clinical features: prostration, coma, or deep breathing.17 To put our observations into context relative to other diseases not caused by malaria, we also studied 2 additional groups of case patients. The first included a random sample of 1008 children younger than 5 years of age who were admitted from the study area to the general pediatric ward during the same period as those admitted with uncomplicated malaria. All children in this case group had a negative blood test for malaria at the time of their admission and constituted a representative sample of nonmalaria diagnoses. The second consisted of 1872 children admitted between August 1998 and March 2008 with bacteremia defined by the growth of a pathogenic bacterium from a blood culture sample collected at admission.16 Controls were children younger than 5 years who were recruited by random sampling to studies conducted throughout the study area between September 1998 and November 2005 as described in detail previously.7,18,19
Sampling and laboratory methods
Routine hematology, blood culture, and malaria parasite data were collected on all admitted children as described in detail previously.16,17 Case patients were typed for HbS using a single-tube polymerase chain reaction method20 on DNA extracted from stored blood samples (QIAGEN DNA Blood Mini Kit) and controls were typed by hemoglobin electrophoresis on cellulose acetate gels following standard methods. All cases of SCA that were diagnosed by electrophoresis were confirmed by polymerase chain reaction. Although we did not test formally for β0thalassemia and, as a result, we cannot exclude a diagnosis of HbS/β0thalassemia in some subjects, it is probable that β0thalassemia is rare in the study population and that most patients will have had sickle cell disease-SS. As a result, we have elected to use the term SCA rather than sickle cell disease throughout this paper.
Statistical methods
We determined the odds ratios (ORs) for SCA between each group of case patients and controls by both univariate and multivariable logistic regression analysis, adjusting for the declining prevalence of SCA with age. We compared proportions using the χ2 or Cornfield exact test as appropriate and the means of normally distributed continuous data using the Student t tests. Nonparametric data were compared using the Mann-Whitney Rank Sum test. All analyses were conducted using STATA, Version 10.0.
Results
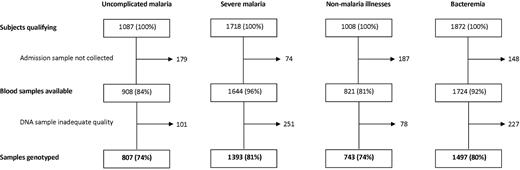
The sample flow and genotype results are summarized in Figure 1 and Table 1, respectively, and the clinical features of case patients, stratified by HbS genotype, are summarized in Table 2. Overall, of the severe malaria group, 468 of 1393 (33%) were prostrated, 620 (52%) were in coma, and 536 (41%) had signs of deep breathing. There were no significant differences in the distributions of these features between genotypic groups. Of those admitted with nonmalaria illnesses, the most common primary diagnoses at the time of discharge were lower respiratory tract infection (234; 31.4%), gastroenteritis (164; 22%), and malnutrition (61; 8.2%). There were no significant differences in the distributions of these diagnoses by genotype.
Success rates for HemoglobinS genotyping. Typing of controls was by electrophoresis using fresh blood and was successful in all subjects.
Success rates for HemoglobinS genotyping. Typing of controls was by electrophoresis using fresh blood and was successful in all subjects.
HbS genotype frequencies in cases and controls
| . | Overall, no. (%) . | HbAA, no. (%) . | HbAS, no. (%) . | SCA, no. (%) . |
|---|---|---|---|---|
| Controls | 1480 (100) | 1252 (84.59) | 218 (14.73) | 10 (0.80) |
| Uncomplicated malaria | 807 (100) | 770 (95.42) | 35 (4.34) | 2 (0.26)* |
| Severe malaria | 1393 (100) | 1357 (97.42) | 31 (2.23) | 5 (0.37)† |
| Nonmalaria illnesses | 743 (100) | 636 (85.60) | 86 (11.57) | 21 (3.30)‡ |
| Bacteremia | 1497 (100) | 1280 (85.50) | 134 (8.95) | 83 (6.48)§ |
| Total | 5919 (100) | 5295 (89.46) | 504 (8.51) | 120 (2.27) |
| . | Overall, no. (%) . | HbAA, no. (%) . | HbAS, no. (%) . | SCA, no. (%) . |
|---|---|---|---|---|
| Controls | 1480 (100) | 1252 (84.59) | 218 (14.73) | 10 (0.80) |
| Uncomplicated malaria | 807 (100) | 770 (95.42) | 35 (4.34) | 2 (0.26)* |
| Severe malaria | 1393 (100) | 1357 (97.42) | 31 (2.23) | 5 (0.37)† |
| Nonmalaria illnesses | 743 (100) | 636 (85.60) | 86 (11.57) | 21 (3.30)‡ |
| Bacteremia | 1497 (100) | 1280 (85.50) | 134 (8.95) | 83 (6.48)§ |
| Total | 5919 (100) | 5295 (89.46) | 504 (8.51) | 120 (2.27) |
Overall, the diagnosis of SCA was already established at the time of admission in 16 patients: *1, †1, ‡2, and §12.
The clinical and laboratory characteristics of case patients
| . | All genotypes . | HbAA . | HbAS . | SCA . | P‖ . |
|---|---|---|---|---|---|
| Uncomplicated malaria | |||||
| n | 807 (100) | 770 (95.4) | 35 (4.34) | 2 (0.25) | |
| Males | 421 (52.2) | 397 (51.5) | 24 (68.6) | 0 | .24# |
| Age, mo* | 23 (12-37) | 23 (12-37) | 18 (13-31) | 55 (54-55) | .02 |
| Hemoglobin† | 7.6 (7.4-7.7) | 7.5 (7.4-7.7) | 8.6 (7.9-9.5)‡ | 3.6 (2.0-6.5) | < .001** |
| Hemoglobin < 5 g/dL | 95 (11.8) | 90 (11.7) | 3 (8.6) | 2 (100) | .014†† |
| Fatal outcome | 3 (0.37) | 3 (0.39) | 0 | 0 | 1.00# |
| Severe malaria | |||||
| n | 1393 (100) | 1357 (97.4) | 31 (2.2) | 5 (0.4) | |
| Males | 699 (50.1) | 681 (50.1) | 16 (51.6) | 2 (40.0) | .68# |
| Age, mo* | 26 (15-37) | 26 (15-37) | 28 (13-41) | 9 (7-41) | .25‡‡ |
| Hemoglobin† | 6.1 (6.0-6.2) | 6.1 (6.0-6.2) | 6.7 (5.7-8.0) | 3.2 (2.0-5.0) | < .001** |
| Hemoglobin < 5 g/dL | 423 (30.4) | 412 (30.4) | 7 (22.6) | 4 (80.0) | .03# |
| Fatal outcome | 141 (10.1) | 136 (10.0) | 1 (3.2) | 4 (80.0) | .0005# |
| Nonmalaria illnesses | |||||
| n | 743 (100) | 636 (85.6) | 86 (11.6) | 21 (3.3) | |
| Males | 435 (58.5) | 376 (58.9) | 45 (52.3) | 14 (66.7) | .65# |
| Age, mo* | 10 (2-10) | 10 (2-19) | 10 (1.75-18) | 19 (15-31) | .002‡‡ |
| Hemoglobin† | 9.6 (9.3-9.8) | 9.6 (9.3-9.9) | 10.3 (9.8-10.9) | 5.2 (4.3-6.4) | < .001** |
| Hemoglobin < 5 g/dL | 40 (5.4) | 30 (4.7) | 1 (1.2) | 9 (43.9) | < .001# |
| Fatal outcome | 48 (6.5) | 41 (6.1) | 6 (7.0) | 1 (4.8) | 1.00# |
| Bacteremia | |||||
| n | 1497 (100) | 1280 (85.5) | 134 (9.0) | 83¶ (5.5) | |
| Males | 861 (57.5) | 722 (56.4) | 86 (64.2) | 53 (63.9) | .18 |
| Age, mo* | 8 (1-21) | 9 (2-22) | 6.5 (1-19) | 14 (7-31) | < .001‡‡ |
| Hemoglobin† | 8.4 (8.2-8.5) | 8.2 (8.0-8.4) | 9.0 (8.5-9.6) | 6.4 (5.8-7.0) | <.001 |
| Hemoglobin < 5 g/dL | 155 (10.4) | 128 (10.0) | 4 (3.0)§ | 23 (27.7) | <.001 |
| Fatal outcome | 366 (24.5) | 312 (24.4) | 36 (26.9) | 18 (21.7) | 0.58 |
| . | All genotypes . | HbAA . | HbAS . | SCA . | P‖ . |
|---|---|---|---|---|---|
| Uncomplicated malaria | |||||
| n | 807 (100) | 770 (95.4) | 35 (4.34) | 2 (0.25) | |
| Males | 421 (52.2) | 397 (51.5) | 24 (68.6) | 0 | .24# |
| Age, mo* | 23 (12-37) | 23 (12-37) | 18 (13-31) | 55 (54-55) | .02 |
| Hemoglobin† | 7.6 (7.4-7.7) | 7.5 (7.4-7.7) | 8.6 (7.9-9.5)‡ | 3.6 (2.0-6.5) | < .001** |
| Hemoglobin < 5 g/dL | 95 (11.8) | 90 (11.7) | 3 (8.6) | 2 (100) | .014†† |
| Fatal outcome | 3 (0.37) | 3 (0.39) | 0 | 0 | 1.00# |
| Severe malaria | |||||
| n | 1393 (100) | 1357 (97.4) | 31 (2.2) | 5 (0.4) | |
| Males | 699 (50.1) | 681 (50.1) | 16 (51.6) | 2 (40.0) | .68# |
| Age, mo* | 26 (15-37) | 26 (15-37) | 28 (13-41) | 9 (7-41) | .25‡‡ |
| Hemoglobin† | 6.1 (6.0-6.2) | 6.1 (6.0-6.2) | 6.7 (5.7-8.0) | 3.2 (2.0-5.0) | < .001** |
| Hemoglobin < 5 g/dL | 423 (30.4) | 412 (30.4) | 7 (22.6) | 4 (80.0) | .03# |
| Fatal outcome | 141 (10.1) | 136 (10.0) | 1 (3.2) | 4 (80.0) | .0005# |
| Nonmalaria illnesses | |||||
| n | 743 (100) | 636 (85.6) | 86 (11.6) | 21 (3.3) | |
| Males | 435 (58.5) | 376 (58.9) | 45 (52.3) | 14 (66.7) | .65# |
| Age, mo* | 10 (2-10) | 10 (2-19) | 10 (1.75-18) | 19 (15-31) | .002‡‡ |
| Hemoglobin† | 9.6 (9.3-9.8) | 9.6 (9.3-9.9) | 10.3 (9.8-10.9) | 5.2 (4.3-6.4) | < .001** |
| Hemoglobin < 5 g/dL | 40 (5.4) | 30 (4.7) | 1 (1.2) | 9 (43.9) | < .001# |
| Fatal outcome | 48 (6.5) | 41 (6.1) | 6 (7.0) | 1 (4.8) | 1.00# |
| Bacteremia | |||||
| n | 1497 (100) | 1280 (85.5) | 134 (9.0) | 83¶ (5.5) | |
| Males | 861 (57.5) | 722 (56.4) | 86 (64.2) | 53 (63.9) | .18 |
| Age, mo* | 8 (1-21) | 9 (2-22) | 6.5 (1-19) | 14 (7-31) | < .001‡‡ |
| Hemoglobin† | 8.4 (8.2-8.5) | 8.2 (8.0-8.4) | 9.0 (8.5-9.6) | 6.4 (5.8-7.0) | <.001 |
| Hemoglobin < 5 g/dL | 155 (10.4) | 128 (10.0) | 4 (3.0)§ | 23 (27.7) | <.001 |
| Fatal outcome | 366 (24.5) | 312 (24.4) | 36 (26.9) | 18 (21.7) | 0.58 |
Values are the number of subjects with percentages in parentheses with the following exceptions:
median (interquartile range); and
geometric mean concentration (g/dL; 95% CI).
P = .007.
P < .001.
Compared with HbAA children.
The organisms associated with bacteremia among the SCA group were as follows: Streptococcus pneumoniae, 33 (40%); Staphylococcus aureus, 6 (7%); other Gram-positive organisms, 3 (4%); non-typhi Salmonella species, 14 (17%); Haemophilus influenzae, 13 (16%); Escherichia coli, 6 (7%); Acinetobacter species, 4 (5%); and other Gram-negative organisms 4 (5%).
χ2 test.
Two-tailed Student t test.
Cornfield exact test.
Mann-Whitney rank-sum test.
Overall, 218 of 1479 (14.7%) controls were heterozygous and 10 (0.7%) were homozygous for HbS (allele frequency = 0.08) and were in Hardy-Weinberg equilibrium. The prevalence of SCA decreased from 8 of 782 (1.0%) in children younger than 12 months to 2 of 697 (0.3%) in children 12 to 59 months of age. We therefore calculated the ORs for HbS genotypes for the 4 clinical case groups both with and without adjustments for age. Although we found no evidence of a strongly increased risk of either uncomplicated or severe P falciparum malaria in children with SCA (OR = 0.48 and 0.80, respectively), we did find that a high proportion (6 of 7) of those admitted with malaria who tested positive for SCA were severely anemic (Table 2) and, furthermore, that mortality was considerably higher in the SCA (4 of 7) than non-SCA groups (140 of 2193; P < .001; Table 2). SCA was significantly associated with a 4-fold increase in admission to hospital with nonmalaria illnesses (OR = 4.17) and a greater than 8-fold increase in bacteremia (OR = 8.73; Table 3). The ratio of SCA compared with HbAA children was significantly different across the 4 case groups (χ2 3 df = 107; P < .001). Because the time period for sample collection differed between the case conditions, we conducted a secondary analysis restricted only to cases collected during the 5-year period of January 1, 2000 to December 31, 2004. These results were not significantly different from the ORs reported here (data not shown). As expected, we found protective associations among children with HbAS against bacteremia (OR = 0.60), uncomplicated malaria (OR = 0.24), and severe malaria (OR = 0.12) and borderline evidence for protection against nonmalaria illnesses (OR = 0.78; Table 3).
Age-adjusted ORs for disease-specific admission by HbS genotype
| Case category . | HbAA . | HbAS . | P . | SCA . | P . |
|---|---|---|---|---|---|
| Uncomplicated malaria | 1 | 0.24 (0.17-0.36) | < .001 | 0.43 (0.09-2.10) | .30 |
| Severe malaria | 1 | 0.12 (0.08-0.18) | < .001 | 0.80 (0.25-2.51) | .70 |
| Nonmalaria illnesses | 1 | 0.78 (0.60-1.01) | .066 | 4.17 (1.95-8.92) | < .001 |
| Bacteremia | 1 | 0.60 (0.48-0.76) | < .001 | 8.73 (4.51-16.89) | < .001 |
| Case category . | HbAA . | HbAS . | P . | SCA . | P . |
|---|---|---|---|---|---|
| Uncomplicated malaria | 1 | 0.24 (0.17-0.36) | < .001 | 0.43 (0.09-2.10) | .30 |
| Severe malaria | 1 | 0.12 (0.08-0.18) | < .001 | 0.80 (0.25-2.51) | .70 |
| Nonmalaria illnesses | 1 | 0.78 (0.60-1.01) | .066 | 4.17 (1.95-8.92) | < .001 |
| Bacteremia | 1 | 0.60 (0.48-0.76) | < .001 | 8.73 (4.51-16.89) | < .001 |
Values are ORs (95% CI) compared with the reference group (HbAA) adjusted for age group (0-23 months, 24-59 months) by logistic regression. Case categories are as defined in “Study participants.” In a subanalysis restricted to bacteremia caused by the rapidly progressive condition Streptococcus pneumoniae, the OR for SCA in cases versus controls was 15.3 (95% CI, 7.3-35.1; P < .001).
Discussion
Each year, approximately 13 000 children are born with SCA in the north4 where, as a result of intensive research over the last 50 years, their prognosis is constantly improving.21 In Africa, however, where more than 230 000 affected children are born each year,4 the picture is very different. The majority of these children die in early life before the diagnosis of SCA has ever been established,3,5,8,22 such that currently SCA is responsible for more than 6% of all deaths in African children younger than 5 years.4 This proportion is set to rise as governments strive to meet their Millennium Development Goals and all-cause under-5 mortality begins to fall.23 Nevertheless, developing appropriate strategies for the identification and management of these children is impossible without quality data on the common causes of morbidity and mortality in the African context.2
Although malaria is widely viewed as a major problem in African children with SCA,1,22,24 the supportive evidence is inconclusive. There is no doubt that such subjects can develop severe and fatal malaria24-29 ; however, their risk relative to those without the disease remains unknown. Most existing studies have been small and uncontrolled and have focused on children whose SCA has already been confirmed, and the many biases of this approach make the results of such studies difficult to interpret. For example, in most parts of Africa, SCA is rarely diagnosed before the condition becomes manifest clinically and studies based entirely on children with a confirmed diagnosis will naturally under-report early events. In the current study, we have attempted to avoid this bias by adopting a case-control design in which our entry point was admission to hospital, an approach unbiased by preconceptions regarding the SCA status of admitted children.
As anticipated, we found that SCA was strongly associated with bacteremia, a diagnosis that was accompanied by an overall mortality of more than 20%. Exploring this in more detail elsewhere,30 we have estimated the annual incidence of bacteremia among young children with SCA in our study population at 4% to 10%, confirming that bacteremia makes a significant contribution to their overall mortality. Similarly, we found a strong association between SCA and admission to hospital with nonmalaria illnesses. As expected, a high proportion of these children were severely anemic, a condition that in less well-resourced facilities is probably associated with significant mortality. Although we found no evidence for a strong positive association between SCA and admission to hospital with either uncomplicated or severe P falciparum malaria, of critical importance we found that both severe anemia and death were considerably more common among SCA than non-SCA patients who were hospitalized with malaria. This observation alone emphasizes how essential it is that all children born with SCA in malaria-endemic environments are provided with effective prophylaxis.
Our failure to detect a marked increase in the overall risk of malaria in patients with SCA through our case-control approach is open to various interpretations. First, we might easily have failed to detect a true-positive association between SCA and admission to hospital with clinical malaria through a lack of power. Although our study was more than 80% powered to detect an OR = of 3.0, it was poorly powered to detect effect sizes of lower magnitude that might still be important at the individual level. Second, and probably more importantly, our failure to detect a positive association might simply relate to bias. There are multiple theoretical reasons why malaria might follow a particularly fulminant course in children with SCA that could result in rapid death and, therefore, to under-ascertainment in a hospital-based study such as ours. First, the spleen plays an important role in malaria immunity31,32 and asplenia, which develops functionally in all children with SCA, is a well-described risk factor for severe disease. It seems probable, therefore, that susceptibility to malaria in subjects with SCA will increase as splenic function deteriorates with age. In cohort studies conducted in the north, it has been shown that abnormal splenic function (indicated by the presence of pitted red cells in the peripheral circulation) is common by 6 months of age and affects almost half of children by the age of 2 years.33 Although splenomegaly has been shown to persist until later life in African studies than is seen in the north,34-36 an observation that has been linked to the presence of malaria,34,35,37 there are no data on splenic function in the African context where the role of the spleen in malaria protection therefore remains unknown. Second, even without malaria, children with SCA are already anemic, and a sudden decline in hemoglobin from such a low baseline value could easily be catastrophic. The current study and an earlier study in which we also found that both severe anemia and death were significantly more common among parasitemic than nonparasitemic SCA patients presenting to hospital in Dar-es-Salaam,13 both suggest that malaria is probably an important cause of community death from anemia among African children with SCA. On the same basis, it is also probable that malaria was the precipitant in a high proportion of patients with SCA who presented with anemia in our “nonmalaria” grouping but that parasites were present at such low densities that they were not detected through routine microscopy.
Taken together, these considerations suggest that it is highly probable that our failure to detect a strong overall association between SCA and malaria in our current study relates to study design. Nevertheless, from a biologic perspective, it remains possible that malaria is not such a major cause of morbidity in children with SCA as is commonly thought. Carriers of HbS are strongly protected from all forms of clinical malaria7,38,39 through mechanisms that probably include the premature removal by the spleen of malaria-infected red blood cells,40-43 and in vivo, the degree of protection in carriers is correlated with HbS, being virtually complete in those with the highest intracellular concentrations.14 Moreover, in a number of in vitro studies, the mechanisms that have been proposed as potential explanations for the malaria protection afforded by HbS have been more pronounced in homozygotes.40,41,44-46 For these reasons, it is still possible that children with SCA enjoy a greater degree of innate malaria protection than heterozygotes, an interpretation that is supported by a number of studies that have noted both a reduced prevalence9,10,28 and a reduced incidence6,11 of malaria in children with SCA. Nevertheless, our study was too small to find anything other than a very large protective effect: for example, given a control prevalence of 0.06 and more than 1700 cases of severe malaria, our study was 80% powered to show an OR of 0.1 at the .05 significance level, an effect that seems implausibly large. More importantly, even if the overall incidence of clinical malaria were lower in SCA than non-SCA children, any advantage would be more than offset by the massively increased risk of death seen in such children when they do develop the disease.
Given the huge burden of SCA in Africa, the continuing lack of clear quantitative data regarding the relative importance of malaria as a cause of morbidity and mortality more than 50 years after this question was first debated39,47-49 is unhelpful. As the Global Burden of Disease Study enters its first major revision,50 the causes of death in African children with SCA remain poorly documented and the lack of data make it impossible to evaluate the relative benefits of various interventions. Such data are critical if African governments are to make informed decisions regarding alternative approaches. At one extreme, the introduction of neonatal screening for SCA, which allows services to be targeted toward affected children, has been explored in a number of countries.51-53 However, this is expensive, logistically complicated, and difficult to sustain,51-53 and the relative difficulties with which screening can be introduced in rural versus urban communities raises the issue of equity. At the other extreme, a number of countries in sub-Saharan Africa are now implementing nontargeted programs at the national level that will probably result in marked improvements in the survival of children with SCA. For example, in Kenya, recent initiatives have seen the introduction of both Haemophilus influenzae type b vaccine (which has led to a sustained reduction in hemophilus disease15 ) and of free access to insecticide-treated bed nets for children and pregnant women (to which major declines in malaria incidence have been attributed54 ). Moreover, Kenya will soon introduce a 10-valent conjugate vaccine against Streptococcus pneumoniae that covers 80% of the prevailing S pneumoniae serotypes.30 Under these circumstances, a range of interventions will be delivered at population level that will be of particular benefit to children with SCA, a strategy that will probably translate to a rapid improvement in survival. Under this new paradigm, the additional value of targeted malaria prophylaxis becomes a critical question for health planners evaluating the potential benefits of screening.
The current study highlights the urgent need for better data regarding the overall role that malaria plays in the ill health and death of African children with SCA. Given the importance of this question, a definitive and well-powered intervention study seems long overdue. In the mean time, the high risk of death seen in children with SCA admitted to hospital with malaria in our study underscores how important it is that effective prophylaxis is made freely available to all children living with SCA in malaria-endemic countries.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the field, laboratory, medical, and nursing staff of the Centre for Geographic Medicine Research–Coast for their help in conducting this study; Charles Newton, Jay Berkley, and Bob Snow for helpful discussions; Greg Fegan for statistical support; and a number of anonymous reviewers for their help in revisions of this manuscript.
This work was supported by the Wellcome Trust, United Kingdom (grant 076934; T.N.W.) and (grant 077092; K.M.) and the European Union Network 6 BioMalpar Consortium Network of Excellence. This paper is published with the permission of the Director of Kenya Medical Research Institute.
Wellcome Trust
Authorship
Contribution: C.F.M. performed the research, analyzed the data, and wrote the first draft of the paper; C.W., J.M., A.M., S.U., D.H.O., A.N., and K.M. helped perform the research and commented on draft manuscripts; C.N. prepared the data, helped with data analysis, and commented on draft manuscripts; J.A.G.S. helped with the interpretation and analysis of the data and commented on draft manuscripts; and T.N.W. designed the research, wrote the final draft of the paper, had full access to all of the data in the study, and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Thomas N. Williams, KEMRI/Wellcome Trust Programme, Centre for Geographic Medicine Research-Coast, PO Box 230, Kilifi, Kenya; e-mail: twilliams@kilifi.kemri-wellcome.org.