Abstract
Adenovirus (Ad) vectors are widely used in human clinical trials. However, at higher dosages, Ad vector–triggered innate toxicities remain a major obstacle to many applications. Ad interactions with the complement system significantly contribute to innate immune responses in several models of Ad-mediated gene transfer. We constructed a novel class of Ad vectors, genetically engineered to “capsid-display” native and retro-oriented versions of the human complement inhibitor decay-accelerating factor (DAF), as a fusion protein from the C-terminus of the Ad capsid protein IX. In contrast to conventional Ad vectors, DAF-displaying Ads dramatically minimized complement activation in vitro and complement-dependent immune responses in vivo. DAF-displaying Ads did not trigger thrombocytopenia, minimized endothelial cell activation, and had diminished inductions of proinflammatory cytokine and chemokine responses. The retro-oriented display of DAF facilitated the greatest improvements in vivo, with diminished activation of innate immune cells, such as dendritic and natural killer cells. In conclusion, Ad vectors can capsid-display proteins in a manner that not only retains the functionality of the displayed proteins but also potentially can be harnessed to improve the efficacy of this important gene transfer platform for numerous gene transfer applications.
Introduction
Gene transfer via Adenovirus (Ad)–based vectors has proven to be extremely effective for both gene therapy and vaccine basic research, as well as for potential use in specific human clinical applications. Ad-mediated gene transfer is a rapidly developing field that has resulted in initiation of more than 385 human clinical trials (http://www.wiley.co.uk/wileychi/genmed/clinical/). Despite this fact, reduction of Ad vector–associated innate immunogenic toxicities will significantly broaden the utility of this bio-platform for use in multiple gene therapy or vaccine applications.
It has been shown that many Ad vector–induced innate immune responses are due to the Ad capsid activating the complement system.1-6 The complement system, which includes more than 30 fluid-phase and membrane-bound proteins, is an important first line of defense against invading foreign pathogens. In general, the classical complement pathway is activated subsequent to specific antibody interactions with previously encountered pathogens, whereas the alternative complement pathway is activated when spontaneously produced “C3b-like” molecules stably bind to the surface of newly encountered pathogens.7,8 Complement activation not only facilitates rapid clearance of pathogens but also enhances the adaptive immune response to the same pathogen(s).9 Excessive complement activation can also be detrimental, resulting in anaphylactoid reactions, systemic inflammatory response syndrome, adult respiratory distress syndrome, hypotensive shock, and/or death.10 These same toxicities have been observed after high-dose Ad administrations into rodents, nonhuman primates, and humans.11 Direct interaction of the Ad capsid with complement components (murine or human) has been directly and indirectly associated with these toxicities; many of these toxicities can be avoided when Ad vectors are injected into complement deficient (C3-KO) mice.1-5
On the basis of these considerations, we hypothesized that genetic engineering of the native Ad capsid in a manner that minimized its capacity to activate the complement system would reduce or mitigate Ad capsid–induced, complement-dependent, immune responses. In this study, we confirm that the native Ad capsid can “capsid-display” the natural complement inhibitor decay-accelerating factor (DAF; CD55) as a C-terminal fusion protein with the Ad-capsid protein, pIX. Ad capsid display of DAF can minimize the induction of the complement system and complement-dependent innate immune responses. Furthermore, we demonstrate that capsid-displaying the retro-oriented form of the human DAF protein (thereby displaying the primary DAF amino acid sequence in a more native conformation relative to the Ad capsid surface) further improves the ability of the modified Ads to minimize induction of the complement system in vivo. As a result of minimized complement activation, “DAF-displaying” Ads can efficiently transduce genes in vivo, while simultaneously minimizing the induction of several Ad-triggered immune responses.
Methods
Adenovirus vector construction: incorporation of DAF in the C-terminus of protein IX
The N-terminal cDNA coding for the N-terminal domain (entire 320 amino acids: 35-354, DAF–complement control protein repeats 1-4 [:CCPR1-4]:) of the human DAF gene was subcloned in-frame into the C-terminus of pIX. CCPR1 to CCPR4 of DAF was derived by polymerase chain reaction (PCR) with the following amplification primers tailed with NheI sites: (DAF forward, 5′-gctagcgactgtggccttcccccagatgtacc-3′; DAF reverse, 5′-gctagcacctgaagtggttccacttcctttatttgg-3′). The NheI-tailed PCR product, amplified from a human DAF cDNA clone (ATCC no. MGC-5192), was subcloned in-frame into the C-terminus of viral protein IX into pShuttle-IX/NheI, the latter constructed in our laboratory by introducing NheI recognition site at the C-terminus of capsid protein IX (just upstream of normal pIX stop codon) as previously described.12 The plasmid so obtained we refer to as pShuttle-IX-DAF, was linearized with PmeI restriction enzyme and homologously recombined with the rest of the Ad5 vector genome present in the plasmid pAdEasyI as previously described,13 yielding pAd-IX-DAF.
We have also displayed DAF in a more native context (DAF_REO): N-terminus-pIX-C-terminus-fusion/C-terminus-DAF-N-terminus. This required synthetic production (Geneart) of the DNA molecule encoding for the DAF amino acid sequence corresponding to 3′-5′ DAF (ie, amino acid sequence was reversed and displayed in the C-terminus of pIX) as described in the previous paragraph. A green fluorescent protein (GFP) expression cassette was inserted into the multiple cloning site of the pShuttle-IX-DAF (or pShuttle-IX-DAF_REO) as previously described.14 All viruses were found to be RCA free both by RCA PCR (E1 region amplification) and direct sequencing, methods as previously described.15 All Ads have also been tested for the presence of bacterial endotoxin as previously described15 and were found to contain less than 0.15 EU/mL.
Ad vector production and characterization
Ad vector production and characterization was performed as fully described in supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Animal procedures
Adult C57BL/6 WT and B6.129S4-C3tmlCrr (C3-KO) mice were purchased from The Jackson Laboratory. Ad5 vectors were injected intravenously (via the retro-orbital sinus, total volume 200 μL) into 8-week-old male mice after performing proper anesthesia with isoflurane. A total of 0.75 × 1011 vector particle (vp; medium dose equals 3 × 1012 vp/kg) or 2 × 1011 vp (high dose equals 8 × 1012 vp/kg) in 200 μL of phosphate-buffered saline (PBS) was injected per mouse intravenously. Several groups of mice (in C57BL/6 background) were analyzed in this study: (1) WT_Mock and C3-KO_Mock (PBS-injected mice, baseline), (2) WT_Ad5-GFP and C3-KO_Ad5-GFP (control groups injected with conventional Ad), (3) WT_Ad5-IX-dGFP (control group, injected with Ad displaying irrelevant peptide), (4) WT_Ad-GFP-IX-dDAF, and (5) WT_Ad-GFP-IX-dDAF_REO (the latter 2 experimental groups were injected with DAF-displaying Ads).
Control and experimental mice were sacrificed at different times after mock or virus treatment: 6, 24, and 72 hours after injection (n = 6 for virus-injected groups, n = 4 for Mock-injected groups, unless otherwise specified).
Plasma and tissue samples were collected and processed at the indicated time points in accordance with Michigan State University Institutional Animal Care and Use Committee. All procedures with recombinant Ads were performed under Biosafety Level 2, and all vector-treated animals were maintained in Animal Biosafety Level 2 conditions. All animal procedures were reviewed and approved by the Michigan State University Office of Radiation, Chemical, and Biological Safety and Institutional Animal Care and Use Committee. Care for mice was provided in accordance with standards of the Public Health Service and Association for Assessment and Accreditation of Laboratory Animal Care International.
Electron microscopy of purified Ad vectors
Negative staining of CsCl-purified Ad vectors was performed as follows. Ads diluted to 1012 vp/mL in 10mM Tris [:tris(hydroxymethyl)aminomethane]: were adsorbed to Formvar/Carbon film 300-mesh Copper grids (Electron Microscopy Sciences) and stained with freshly prepared 1% solution of phosphotungstic acid (1 g, 50 μL of fetal bovine serum [:FBS]:, 50 mL of miliQ water, pH 6.0, adjusted by KOH) for 30 seconds and examined with the use of transmission electron microscope (Philips EM410). Photographs were taken from representative areas from each sample.
Experimental gel-based liquid chromatographic tandem mass spectrometry
Experimental gel-based liquid chromatographic tandem mass spectrometry was performed as described in supplemental Methods.
Complement activation alternative pathway 50 normal human serum–based assay and C3a-desArg enzyme-linked immunoabsorbent assay
Cytokine/chemokine/endothelial cells activation assessments
To determine the effect of DAF-displaying Ads on Ad-mediated release of proinflammatory mediators, plasma levels of cytokine/chemokines at 6 hours after injection were measured in all groups of mice with the use of a multiplex bead array system exactly as previously described.5,15 The measurement of soluble intercellular adhesion molecule 1 (ICAM-1) and E-selectin molecules (endothelial cell ([:EC]: activation markers) in murine plasma (collected at 6 hours after injection) was performed with the use of mouse cardiovascular disease panel LINCOplex kit (Millipore) as per the manufacturer's instructions.
Platelet enumeration
To access the effect of DAF-displaying Ads on thrombocytopenia typically induced in C57BL/6 mice after administration of conventional Ad vectors, platelets were measured 24 and 72 hours after systemic Ad injection with the use of Unopette (Fisher Scientific) system as previously described2,15 as per the manufacturer's recommendations. Platelets were subsequently manually counted with the use of a Neubauer hemocytometer.
Cell staining and flow cytometry
Early activation of natural killer (NK) cells, T cells, and dendritic cells was studied by flow cytometric–based methods: 0.75 × 1011 vp/mouse of Ad5-GFP or Ad5-GFP-IX-dDAF_REO were injected intravenously into C57BL/6 mice. Six or 48 hours after Ad5 injections, splenocytes from individual mice were harvested, processed, and stained as described in supplemental Methods.
Hematoxylin and eosin staining
Hematoxylin and eosin (H&E) staining of mouse liver samples was performed as previously described.5,15 All the slides were scored on a scale from 0 to 3 in a blind manner, and the averages of their scores were taken. The sum of scores (10 slides) for each mouse was taken, and individual category scores were averaged for each group. Total inflammation index was computed by averaging the sum of individual category scores for each mouse. Mock-injected animals had scores of 0, indicating no inflammation in livers of these mice.5,15
Quantitative reverse transcription PCR analysis
Ad genome copy number per liver or spleen cell
To determine the number of Ad genome copies per liver or spleen cell at different time points after transduction, tissues (< 0.1 g) were snap-frozen in liquid nitrogen and crushed to a fine powder, and total DNA was extracted as previously described.3,15 Ad genome copy numbers were assessed using real-time PCR–based quantification as previously described.3,5,15
Plasma alanine aminotransferase levels as a measure of liver toxicity
Evidence of liver toxicity was quantified by measuring plasma alanine aminotransferase (ALT) activity levels in plasma collected at 6, 24, and 72 hours after injection. ALT activity was determined spectrophotometrically with the use of Infinity-ALT from Thermoelectron Corp per the manufacturer's protocol.
Statistical analysis
For every experiment, pilot trials were performed with n = 3 per group. This allowed us to determine effect size and sample variance so that power analysis could be performed to correctly determine the number of subjects per group required to achieve a statistical power greater than 0.8 at the 95% confidence level. Statistically significant differences in toxicities associated with innate immune responses (ie, platelet counts, gene induction, etc) were determined with the use of 1-way analysis of variance with a Student-Newman-Keuls post hoc test (P < .05). All graphs in this article are presented as mean of the average plus or minus SD. GraphPad Prism software was used for statistical analysis.
Results
Construction and validation of novel Ad vectors, displaying complement regulatory peptides on the surface of virions
The Ad5 capsid contains 9 proteins.17 The Ad proteins fiber, penton base, protein IX, and hexon have all been exploited for insertion of foreign peptides, as in-frame fusion proteins either embedded within hypervariable loop regions (fiber, penton, hexon) or at the C-terminus (pIX, fiber) of these capsid proteins. If viable, these maneuvers generate fusion proteins variously displayed from an infectious Ad capsid.18,19 Only the pIX protein allows for viable incorporation of peptides of substantial size (maximal insertion achieved to date: 1018 amino acids20 ) into the Ad5 capsid. We have used the C-terminus of protein IX as an insertion site for capsid display of the functional domain, (consisting of CCPR1-4; 320 amino acids]:) of the human DAF protein.
Two different viruses displaying this domain of DAF were constructed. These E1- and E3-deleted Ad5 vectors were also engineered to express a GFP transgene, to allow for visual or molecular confirmation of successful gene transduction by the novel, DAF-displaying, [:E1-, E3-]: Ad-GFP vectors.
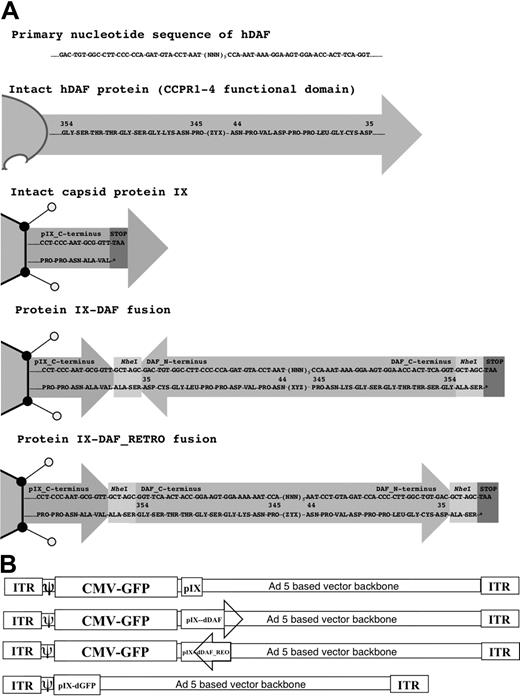
It is known that the C-terminus of pIX is the portion of the protein that is exposed on the exterior of the Ad5 capsid.17 One DAF-displaying Ad5 construct had the DAF cDNA sequence inserted in-frame at the C-terminus of protein IX (Ad5-GFP-IX-dDAF). This orientation potentially posed a problem, because endogenous DAF is normally inserted into the cell membrane at its C-terminus, and its N-terminus is exposed to the extracellular milieu (Figure 1A). To take these caveats into consideration, we also constructed a second virus, designed to display the CCPR1 to CCPR4 of DAF in a more native context relative to the Ad5 capsid. We produced a synthetic cDNA encoding the primary amino acid sequence of DAF in reverse order relative to the native DAF gene sequence that, when subcloned into the C-terminus of the pIX open reading frame, resulted in a re-orientation of the human DAF relative to the Ad capsid (ie, retro-oriented form of DAF protein; Figure 1A). This construct, (Ad5-GFP-IX-dDAF_REO) has the C-terminal amino acids of DAF fused with the C-terminus of pIX, thereby allowing the N-terminal amino acids of DAF to be displayed from the Ad capsid in a manner similar to its native display from human cells. Retro-orientation of primary amino acid sequences of other proteins has been shown to allow for retainment of the enzymatic capacity of these proteins.21-23 Finally, as an important control for nonspecific shielding of the Ad capsid by display of any large pIX protein fusion, we also constructed an Ad vector capsid-displaying the irrelevant GFP protein at the C-terminus of protein IX.24 The schematic diagram of all vectors constructed is shown in Figure 1B.
Schematic diagram of all Ad vectors constructed and used in our study. (A) Cloning strategy to design protein IX-DAF and pIX-DAF_REO is shown. Nucleotide (top) and amino acid (bottom) sequences are depicted, corresponding to 5 C-terminal amino acids of protein IX fused in-frame (using NheI enzyme, recognition sequence of which adds 2 amino acids “ALA-SER” flanking DAF or DAF_REO sequence), followed by DAF or DAF_REO sequence and stop codon. Only first and last 10 amino acids of human DAF or DAF_REO are shown. Note that natural display of human DAF protein from cell surface allows N-terminus to be exposed; however, when DAF is displayed from pIX in its natural form, C-terminus is protruding from Ad5 capsid and therefore oppose natural orientation of DAF. To overcome this limitation we have designed Ad5 vector capsid-displaying retro form of DAF (amino acids 35-354), which mimic natural orientation of DAF with N-terminus protruding from Ad5 capsid. Note that N-terminal amino acids for DAF become C-terminal for DAF_REO and vice versa. Numbers represent amino acids of native human DAF protein. (B) Genome maps of all Ads constructed are shown. Ad vectors were designed as described in “Adenovirus vector construction: incorporation of DAF in the C-terminus of protein IX.” Capsid protein IX is outlined as Ad capsid protein used for fusion with DAF or DAF_REO. Letter “d” before DAF or GFP defines that this peptide is capsid-displayed. DAF orientation is depicted; arrowhead represents DAF C-terminus. Note that naturally DAF N-terminus is protruding from cell membrane (DAF_REO orientation). Genomes are not drawn to scale. ITR indicates inverted terminal repeat; and CMV, cytomegalovirus.
Schematic diagram of all Ad vectors constructed and used in our study. (A) Cloning strategy to design protein IX-DAF and pIX-DAF_REO is shown. Nucleotide (top) and amino acid (bottom) sequences are depicted, corresponding to 5 C-terminal amino acids of protein IX fused in-frame (using NheI enzyme, recognition sequence of which adds 2 amino acids “ALA-SER” flanking DAF or DAF_REO sequence), followed by DAF or DAF_REO sequence and stop codon. Only first and last 10 amino acids of human DAF or DAF_REO are shown. Note that natural display of human DAF protein from cell surface allows N-terminus to be exposed; however, when DAF is displayed from pIX in its natural form, C-terminus is protruding from Ad5 capsid and therefore oppose natural orientation of DAF. To overcome this limitation we have designed Ad5 vector capsid-displaying retro form of DAF (amino acids 35-354), which mimic natural orientation of DAF with N-terminus protruding from Ad5 capsid. Note that N-terminal amino acids for DAF become C-terminal for DAF_REO and vice versa. Numbers represent amino acids of native human DAF protein. (B) Genome maps of all Ads constructed are shown. Ad vectors were designed as described in “Adenovirus vector construction: incorporation of DAF in the C-terminus of protein IX.” Capsid protein IX is outlined as Ad capsid protein used for fusion with DAF or DAF_REO. Letter “d” before DAF or GFP defines that this peptide is capsid-displayed. DAF orientation is depicted; arrowhead represents DAF C-terminus. Note that naturally DAF N-terminus is protruding from cell membrane (DAF_REO orientation). Genomes are not drawn to scale. ITR indicates inverted terminal repeat; and CMV, cytomegalovirus.
All Ad vectors constructed and CsCl purified in our studies were confirmed to be viable and to have similar infectivities, as quantified by several independent techniques, all as previously described.15,16 Furthermore, DNA sequencing confirmed the presence of the DAF encoding DNA sequences in the respective vector preparations. Electron microscopy verified the lack of abnormal numbers of damaged capsids or free capsid proteins found in any of the CsCl-purified Ad preparations.16 Viral particle/transducing unit/tissue culture infectious dose ratios of DAF-displaying Ads were not dramatically different from those of control Ads (supplemental Table 1). To confirm the incorporation of pIX-DAF_REO fusion proteins into purified Ad virions, we separated the Ad capsid proteins by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and performed mass spectrometry of pIX-containing bands. We were able to identify multiple, DAF_REO-specific peptides by this method in the virus preparations, derived from DAF_REO-displaying Ads.
Capsid display of DAF minimizes Ad-dependent complement activation in vitro
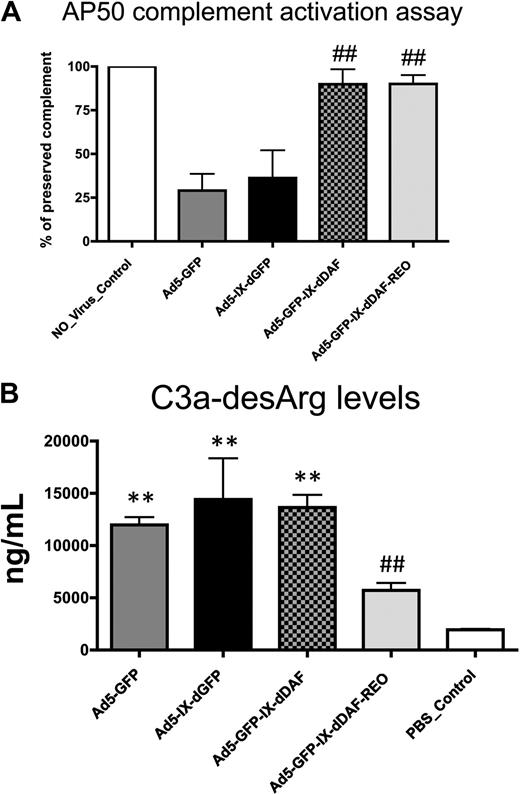
To investigate the potential of DAF-displaying Ads to diminish activation of the human complement system, we used 2 different NHS-based assays. To measure the activation of the alternative complement pathway, control and DAF-displaying Ads were incubated with NHS in the presence of EGTA (ethylene glycol tetraacetic acid; thereby blocking the activation of classical complement pathway, despite the presence of Ad antibodies in pooled NHS), and residual complement activity was then measured (alternative pathway 50 assay). NHS preincubated with conventional Ad5-based vectors showed significant complement consumption (∼ 75%), reconfirming significant activation of the alternative complement pathway by Ad5-based vectors1,16 (Figure 2A). In contrast, the amount of complement activation induced after NHS incubation of either of the DAF-displaying Ads was below the detection limits of the assay. Importantly, the control virus capsid-displaying the GFP (Ad5-IX-dGFP) protein from pIX activated complement to levels similar to conventional Ad5 vectors, suggesting that Ad5 capsid display of the DAF protein sequences specifically prevented alternative pathway activation on exposure of the novel vectors to NHS.
Novel DAF-displaying Ads minimize Ad-mediated complement activation in vitro. (A) Activation of complement alternative pathway mediated by control and DAF-displaying Ads was performed. Residual complement activity was normalized to NHS incubated with media (no_virus control). Data from a representative experiment are shown. The error bars represent ± SD. Statistical analysis was completed using 1-way analysis of variance with a Student-Newman-Keuls post hoc test. ## indicates statistically different values from Ad5-IX-dGFP and Ad5-GFP, P < .001. (B) Complement activation mediated by control and DAF-displaying Ads was determined by C3a-desArg–specific enzyme-linked immunoabsorbent assay (ELISA). Negative control was incubated with NHS and with PBS before ELISA. Data from a representative experiment are shown. The error bars represent ± SD. Statistical analysis was completed using 1-way analysis of variance with a Student-Newman-Keuls post hoc test. Complement activation by DAF-displaying Ads was compared with corresponding controls. Values statistically different from negative control, **P < .001; statistically different values from Ad5-IX-dGFP and Ad5-GFP, ##P < .001, respectively. Note that Ad5-GFP-IX-dDAF_REO virus did not significantly activate complement. AP50 indicates alternative pathway 50.
Novel DAF-displaying Ads minimize Ad-mediated complement activation in vitro. (A) Activation of complement alternative pathway mediated by control and DAF-displaying Ads was performed. Residual complement activity was normalized to NHS incubated with media (no_virus control). Data from a representative experiment are shown. The error bars represent ± SD. Statistical analysis was completed using 1-way analysis of variance with a Student-Newman-Keuls post hoc test. ## indicates statistically different values from Ad5-IX-dGFP and Ad5-GFP, P < .001. (B) Complement activation mediated by control and DAF-displaying Ads was determined by C3a-desArg–specific enzyme-linked immunoabsorbent assay (ELISA). Negative control was incubated with NHS and with PBS before ELISA. Data from a representative experiment are shown. The error bars represent ± SD. Statistical analysis was completed using 1-way analysis of variance with a Student-Newman-Keuls post hoc test. Complement activation by DAF-displaying Ads was compared with corresponding controls. Values statistically different from negative control, **P < .001; statistically different values from Ad5-IX-dGFP and Ad5-GFP, ##P < .001, respectively. Note that Ad5-GFP-IX-dDAF_REO virus did not significantly activate complement. AP50 indicates alternative pathway 50.
Overall complement activation was also quantified by assessment of C3a-desArg levels in NHS after exposure to the DAF-displaying vectors (in the absence of EGTA). C3a-desArg is a stable protein byproduct produced after C3 cleavage; therefore, its measurement in samples can serve as a measure of overall complement activation. With the use of this method, conventional Ad5 vectors significantly activated complement, because the levels of C3a-desArg produced after these exposures were 5 to 6 times higher than those observed in NHS preincubated with PBS (Figure 2B). We also found that capsid display of the native DAF also resulted in detection of elevated C3a-desArg, suggesting that not all aspects of complement activation could be mitigated by simple display of human DAF-derived peptides from the Ad capsid protein pIX. In contrast, equivalent amounts of the retro-oriented DAF-displaying Ad vector did not result in significantly increased C3a-desArg levels when incubated with NHS (Figure 2B), suggesting that this orientation of DAF, as displayed from a viral capsid, may facilitate improved functionality of DAF's decay-accelerating properties. Retro-orientation of primary amino acid sequences can facilitate retainment of the enzymatic capacity of the retro-oriented peptide or protein sequence; our results confirm that these notions can be expanded to include capsid display of retro-oriented DAF and, potentially, other proteins from the surface of a complex structure such as the Ad capsid.21-23
DAF-displaying Ads minimize induction of innate immune responses in vivo
Several innate immune responses occur subsequent to administration of Ad vectors.2,4,15,25-28 Many of these responses are induced by Ads via interactions with the complement system in general and with the C3 protein in particular.2-6
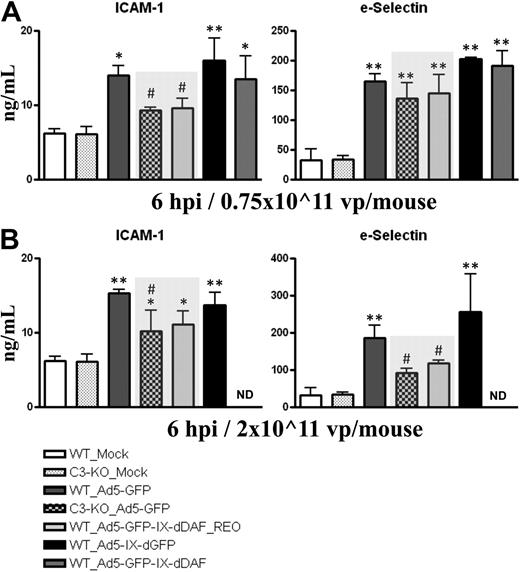
Activation of vascular endothelium is a critical step during initiation of immune responses, because many inflammatory cells (ie, platelets, neutrophils, macrophages, mast cells) use activated ECs to localize to damaged tissues. Systemic administration of Ads activates ECs, including liver sinusoidal ECs.28 EC activation can be facilitated by activation of the complement system.29,30 As shown in Figure 3, plasma levels of soluble forms of ICAM-1 and sE-selectin rise after medium and high-dose injection of conventional Ad5 vectors. In contrast, at some doses, wild-type mice identically treated with the retro-DAF-displaying Ad5 vector had significantly lower inductions of sICAM-1 (medium dose) and sE-selectin (high dose), approximating the diminished levels achieved after injections of conventional Ad5 vectors into complement-deficient (C3-KO) mice. The level of these differences become especially relevant, given that similar differences have been reported in assessing chronic disease states also associated with EC activation, such as chronic liver disease.31,32 Furthermore, ICAM-1 mRNA transcripts in murine liver at 6 hours after systemic injection of 0.75 × 1011 vp/mouse of Ad5-based vectors were also found to be significantly decreased in wild-type mice treated with the retro-DAF-displaying Ad5, compared with wild-type mice injected with a conventional Ad5 vector (Table 1).
Ad5 vectors capsid-displaying retro-DAF complement inhibitor significantly reduce Ad-dependent activation of ECs in C57BL/6 mice. C57BL/6 WT and C3-KO mice were intravenously injected with (A) 0.75 × 1011 vp/mouse (medium dose) or (B) 2 × 1011 vp/mouse (high dose) of Ad5-based control and experimental vectors. Plasma samples, collected at 6 hours after injection (n = 6 for virus-treated groups, n = 4 for Mock-injected groups) were analyzed with the use of a multiplexed bead array–based quantitative system. The bars represent mean ± SD. Statistical analysis was completed with the use of 1-way analysis of variance with a Student-Newman-Keuls post hoc test. Values statistically different from those in Mock-injected animals of both genotypes, *P < .05, **P < .001, respectively; significant reduction of EC activation compared with both WT_Ad5-GFP and WT_Ad5-IX-dGFP injected mice, #P < .05. Note that levels of EC activation triggered by Ad5-GFP-IX-dDAF_REO novel Ad closely parallel the levels observed in C3-KO mice treated with conventional Ad5-GFP vector (shaded bars).
Ad5 vectors capsid-displaying retro-DAF complement inhibitor significantly reduce Ad-dependent activation of ECs in C57BL/6 mice. C57BL/6 WT and C3-KO mice were intravenously injected with (A) 0.75 × 1011 vp/mouse (medium dose) or (B) 2 × 1011 vp/mouse (high dose) of Ad5-based control and experimental vectors. Plasma samples, collected at 6 hours after injection (n = 6 for virus-treated groups, n = 4 for Mock-injected groups) were analyzed with the use of a multiplexed bead array–based quantitative system. The bars represent mean ± SD. Statistical analysis was completed with the use of 1-way analysis of variance with a Student-Newman-Keuls post hoc test. Values statistically different from those in Mock-injected animals of both genotypes, *P < .05, **P < .001, respectively; significant reduction of EC activation compared with both WT_Ad5-GFP and WT_Ad5-IX-dGFP injected mice, #P < .05. Note that levels of EC activation triggered by Ad5-GFP-IX-dDAF_REO novel Ad closely parallel the levels observed in C3-KO mice treated with conventional Ad5-GFP vector (shaded bars).
Ad5-induced gene expression in a liver of C57BL/6_WT mice (fold over Mock, 6 hours after injection)
| . | Mock . | Ad5-GFP . | Ad5-IX-dGFP . | Ad5-GFP-IX-dDAF4 . | Ad5-GFP-IX-dDAF4_REO . |
|---|---|---|---|---|---|
| ADAR* | 1.0 ± 0.3 | 2.2 ± 0.4† | 2.6 ± 0.2† | 1.9 ± 0.5† | 2.4 ± 0.8† |
| CD14* | 1.0 ± 0.2 | 6.0 ± 1.5† | 10.0 ± 5.4‡ | 6.2 ± 3.2† | 3.4 ± 2.4 |
| CXCL-9 | 1.0 ± 0.2 | 10.7 ± 3.0† | 17.4 ± 5.7‡ | 10.6 ± 1.2† | 5.1 ± 1.3†§ |
| DAF | 1.0 ± 0.1 | 1.5 ± 0.5 | 1.2 ± 0.2 | 1.5 ± 0.3 | 1.1 ± 0.2 |
| e-Selectin | 1.0 ± 0.4 | 4.0 ± 0.6† | 5.7 ± 1.0‡ | 3.7 ± 1.3† | 3.5 ± 0.8† |
| ICAM* | 1.0 ± 0.3 | 4.2 ± 0.4† | 3.8 ± 0.8† | 3.6 ± 0.7† | 2.2 ± 0.3†§ |
| IFNα | 1.0 ± 0.4 | 1.3 ± 0.4 | 1.4 ± 0.1 | 0.8 ± 0.5 | 1.5 ± 0.5 |
| IFNβ | 1.0 ± 0.4 | 1.2 ± 0.4 | 1.5 ± 0.3 | 0.8 ± 0.5 | 1.7 ± 0.7 |
| IRF-7 | 1.0 ± 0.4 | 16.4 ± 3.6† | 19.6 ± 2.7† | 16.5 ± 5.0† | 10.9 ± 2.2†§ |
| IRF-8 | 1.0 ± 0.2 | 3.7 ± 1.1† | 3.6 ± 0.8† | 3.2 ± 1.1† | 1.9 ± 0.4§ |
| Jak-1 | 1.0 ± 0.3 | 2.3 ± 0.6† | 1.6 ± 0.3 | 1.8 ± 0.6 | 1.3 ± 0.3§ |
| Jak-3 | 1.0 ± 0.2 | 2.8 ± 0.8† | 1.7 ± 0.2 | 1.9 ± 1.0 | 1.2 ± 0.4§ |
| MyD88* | 1.0 ± 0.3 | 7.0 ± 1.4† | 8.9 ± 1.3† | 6.9 ± 1.9† | 4.0 ± 0.9†§ |
| NFκB-RelA | 1.0 ± 0.2 | 2.1 ± 0.4† | 2.6 ± 0.6† | 1.7 ± 0.4† | 1.2 ± 0.2§ |
| NOD-1 | 1.0 ± 0.2 | 2.4 ± 0.4† | 2.6 ± 0.3† | 2.7 ± 0.5† | 1.9 ± 0.3† |
| NOD-2 | 1.0 ± 0.4 | 2.0 ± 0.4† | 2.3 ± 0.2† | 1.5 ± 0.4 | 2.4 ± 0.7† |
| OAS-1a* | 1.0 ± 0.5 | 12.1 ± 1.0† | 14.5 ± 1.4† | 12.9 ± 4.8† | 5.9 ± 1.6†§ |
| SOCS-1* | 1.1 ± 0.7 | 12.6 ± 4.0† | 12.7 ± 4.1† | 11.7 ± 4.7† | 6.7 ± 2.3† |
| SOCS-3* | 1.1 ± 0.6 | 5.4 ± 1.8† | 5.8 ± 1.4† | 4.6 ± 0.9† | 4.0 ± 0.9† |
| TBK-1 | 1.0 ± 0.2 | 6.5 ± 0.8† | 6.1 ± 1.7† | 6.7 ± 1.9† | 3.5 ± 0.7†§ |
| TLR-2* | 1.0 ± 0.4 | 45.2 ± 14.3† | 86.7 ± 30.0‡ | 48.4 ± 16.0† | 29.3 ± 7.6† |
| TLR-3* | 1.0 ± 0.2 | 19.2 ± 2.9† | 29.7 ± 4.0‡ | 25.3 ± 7.0† | 15.0 ± 6.0† |
| TLR-6* | 1.0 ± 0.3 | 6.0 ± 1.6† | 4.2 ± 0.6† | 6.0 ± 2.6† | 3.0 ± 0.9§ |
| TLR-9* | 1.0 ± 0.2 | 2.0 ± 0.4† | 2.5 ± 0.3† | 1.9 ± 0.4† | 2.5 ± 0.8† |
| TRAF2bp* | 1.1 ± 0.5 | 16.3 ± 7.8† | 36.2 ± 6.3‡ | 19.7 ± 6.3† | 8.2 ± 1.9†§ |
| VCAM | 1.0 ± 0.4 | 2.1 ± 0.8 | 1.9 ± 0.1 | 1.5 ± 0.7 | 1.7 ± 0.6 |
| . | Mock . | Ad5-GFP . | Ad5-IX-dGFP . | Ad5-GFP-IX-dDAF4 . | Ad5-GFP-IX-dDAF4_REO . |
|---|---|---|---|---|---|
| ADAR* | 1.0 ± 0.3 | 2.2 ± 0.4† | 2.6 ± 0.2† | 1.9 ± 0.5† | 2.4 ± 0.8† |
| CD14* | 1.0 ± 0.2 | 6.0 ± 1.5† | 10.0 ± 5.4‡ | 6.2 ± 3.2† | 3.4 ± 2.4 |
| CXCL-9 | 1.0 ± 0.2 | 10.7 ± 3.0† | 17.4 ± 5.7‡ | 10.6 ± 1.2† | 5.1 ± 1.3†§ |
| DAF | 1.0 ± 0.1 | 1.5 ± 0.5 | 1.2 ± 0.2 | 1.5 ± 0.3 | 1.1 ± 0.2 |
| e-Selectin | 1.0 ± 0.4 | 4.0 ± 0.6† | 5.7 ± 1.0‡ | 3.7 ± 1.3† | 3.5 ± 0.8† |
| ICAM* | 1.0 ± 0.3 | 4.2 ± 0.4† | 3.8 ± 0.8† | 3.6 ± 0.7† | 2.2 ± 0.3†§ |
| IFNα | 1.0 ± 0.4 | 1.3 ± 0.4 | 1.4 ± 0.1 | 0.8 ± 0.5 | 1.5 ± 0.5 |
| IFNβ | 1.0 ± 0.4 | 1.2 ± 0.4 | 1.5 ± 0.3 | 0.8 ± 0.5 | 1.7 ± 0.7 |
| IRF-7 | 1.0 ± 0.4 | 16.4 ± 3.6† | 19.6 ± 2.7† | 16.5 ± 5.0† | 10.9 ± 2.2†§ |
| IRF-8 | 1.0 ± 0.2 | 3.7 ± 1.1† | 3.6 ± 0.8† | 3.2 ± 1.1† | 1.9 ± 0.4§ |
| Jak-1 | 1.0 ± 0.3 | 2.3 ± 0.6† | 1.6 ± 0.3 | 1.8 ± 0.6 | 1.3 ± 0.3§ |
| Jak-3 | 1.0 ± 0.2 | 2.8 ± 0.8† | 1.7 ± 0.2 | 1.9 ± 1.0 | 1.2 ± 0.4§ |
| MyD88* | 1.0 ± 0.3 | 7.0 ± 1.4† | 8.9 ± 1.3† | 6.9 ± 1.9† | 4.0 ± 0.9†§ |
| NFκB-RelA | 1.0 ± 0.2 | 2.1 ± 0.4† | 2.6 ± 0.6† | 1.7 ± 0.4† | 1.2 ± 0.2§ |
| NOD-1 | 1.0 ± 0.2 | 2.4 ± 0.4† | 2.6 ± 0.3† | 2.7 ± 0.5† | 1.9 ± 0.3† |
| NOD-2 | 1.0 ± 0.4 | 2.0 ± 0.4† | 2.3 ± 0.2† | 1.5 ± 0.4 | 2.4 ± 0.7† |
| OAS-1a* | 1.0 ± 0.5 | 12.1 ± 1.0† | 14.5 ± 1.4† | 12.9 ± 4.8† | 5.9 ± 1.6†§ |
| SOCS-1* | 1.1 ± 0.7 | 12.6 ± 4.0† | 12.7 ± 4.1† | 11.7 ± 4.7† | 6.7 ± 2.3† |
| SOCS-3* | 1.1 ± 0.6 | 5.4 ± 1.8† | 5.8 ± 1.4† | 4.6 ± 0.9† | 4.0 ± 0.9† |
| TBK-1 | 1.0 ± 0.2 | 6.5 ± 0.8† | 6.1 ± 1.7† | 6.7 ± 1.9† | 3.5 ± 0.7†§ |
| TLR-2* | 1.0 ± 0.4 | 45.2 ± 14.3† | 86.7 ± 30.0‡ | 48.4 ± 16.0† | 29.3 ± 7.6† |
| TLR-3* | 1.0 ± 0.2 | 19.2 ± 2.9† | 29.7 ± 4.0‡ | 25.3 ± 7.0† | 15.0 ± 6.0† |
| TLR-6* | 1.0 ± 0.3 | 6.0 ± 1.6† | 4.2 ± 0.6† | 6.0 ± 2.6† | 3.0 ± 0.9§ |
| TLR-9* | 1.0 ± 0.2 | 2.0 ± 0.4† | 2.5 ± 0.3† | 1.9 ± 0.4† | 2.5 ± 0.8† |
| TRAF2bp* | 1.1 ± 0.5 | 16.3 ± 7.8† | 36.2 ± 6.3‡ | 19.7 ± 6.3† | 8.2 ± 1.9†§ |
| VCAM | 1.0 ± 0.4 | 2.1 ± 0.8 | 1.9 ± 0.1 | 1.5 ± 0.7 | 1.7 ± 0.6 |
Medium dose of virus used for injection was 0.75 × 1011 vp/mouse. The numbers represent mean ± SD. Statistical analysis was completed using one-way analysis of variance with a Student-Newman-Keuls post hoc test; P < .05 was deemed a statistically significant difference. n = 4 for mock-injected groups, n = 5 for virus-injected groups.
Genes shown to be induced in C3-dependent manner after systemic Ad injection [2].
Significant differences compared to WT_Mock.
Significant induction of transcriptional activation compared to WT_Ad5-GFP group.
Significant reductions of transcriptional activation compared to WT_Ad5-GFP group.
After systemic administration of conventional Ad vectors, several cytokines are elaborated in a complement-dependent fashion.2-5,15,25 We found that mice injected with the retro-DAF-displaying Ad5 vector activated several cytokine/chemokines in a dose-dependent manner but, importantly, had significantly reduced inductions of the p40 subunit of interleukin-12 and monocyte chemoattractant protein 1 (MCP-1; at low dose) and macrophage-inflammatory protein 1β (at high dose) compared with conventional Ad5 vectors (Figure 4). In some instances, this level of reduction of complement-dependent Ad-triggered cytokine activation in retro-DAF-displaying Ad5-injected mice paralleled observations made when conventional Ad5 vectors were similarly injected into complement-deficient (C3-KO) mice; however, the activation of several other cytokines/chemokines, known to be triggered by Ads (granulocyte colony-stimulating factor, regulated on activation normal T cell expressed and secreted, keratinocyte-derived chemokine, interleukin-6), was not reduced (Figure 4; data not shown). Interestingly, capsid display of the native DAF protein (Ad5-GFP-IX-dDAF) did not alter cytokine responses, triggered by conventional Ad5 vectors (data not shown), further confirming the improved complement inhibition afforded by retro-display of DAF.
Systemic administration of Ad5 vectors capsid-displaying retro-DAF complement inhibitor significantly reduces Ad-mediated cytokine and chemokine release in C57BL/6 mice. C57BL/6 wild-type (WT) and C3-KO mice were intravenously injected with 0.75 × 1011 vp/mouse (medium dose) or 2 × 1011 vp/mouse (high dose) of Ad5-based control and experimental vectors. Plasma samples, collected at 6 hours after injection (n = 6 for virus-treated groups, N = 4 for Mock-injected groups) were analyzed with the use of a multiplexed bead array–based quantitative system. Statistical analysis was completed with the use of 1-way analysis of variance with a Student-Newman-Keuls post hoc test. The bars represent mean ± SD; plasma cytokine values that are statistically different from those in Mock-injected animals of both genotypes, *P < .05, **P < .01; statistically different values from WT_Ad5-GFP mice, #P < .05, ##P < .01. IL-12p40 indicates p40 subunit of interleukin-12; GCSF indicates granulocyte colony-stimulating factor; RANTES, regulated on activation normal T cell expressed and secreted; and MIP-1β, macrophage-inflammatory protein 1β.
Systemic administration of Ad5 vectors capsid-displaying retro-DAF complement inhibitor significantly reduces Ad-mediated cytokine and chemokine release in C57BL/6 mice. C57BL/6 wild-type (WT) and C3-KO mice were intravenously injected with 0.75 × 1011 vp/mouse (medium dose) or 2 × 1011 vp/mouse (high dose) of Ad5-based control and experimental vectors. Plasma samples, collected at 6 hours after injection (n = 6 for virus-treated groups, N = 4 for Mock-injected groups) were analyzed with the use of a multiplexed bead array–based quantitative system. Statistical analysis was completed with the use of 1-way analysis of variance with a Student-Newman-Keuls post hoc test. The bars represent mean ± SD; plasma cytokine values that are statistically different from those in Mock-injected animals of both genotypes, *P < .05, **P < .01; statistically different values from WT_Ad5-GFP mice, #P < .05, ##P < .01. IL-12p40 indicates p40 subunit of interleukin-12; GCSF indicates granulocyte colony-stimulating factor; RANTES, regulated on activation normal T cell expressed and secreted; and MIP-1β, macrophage-inflammatory protein 1β.
We have previously shown that systemic injections of Ad5 vectors can cause a generalized transcriptome dysregulation in the murine liver subsequent to gene transduction by the vector platform, inclusive of genes regulating cytokine and chemokine production.2-5,15,26,33 Therefore, we have quantified a number of liver gene transcripts, several of which are known to be induced by Ads in a complement-dependent manner.2-5,15,26 Interestingly, this analysis also showed a significant advantage to the use of a retro-DAF-displaying Ad5 vector, because we found that relative to the injection of conventional Ad5 vectors, injection of retro-DAF-displaying Ads resulted in significantly reduced transcription levels of numerous proinflammatory genes compared with both conventional Ad5 vectors, as well as the non–reoriented DAF-displaying Ad (Table 1).
Intravenous injection of conventional Ad5 vectors at different dosages also causes significant thrombocytopenia, a complement-dependent, Ad-induced disorder that was also avoided after injection of the retro-DAF-displaying Ad vector (Figure 5A). Importantly, this observation paralleled results noted when conventional Ad5 vectors are injected into complement-deficient, C3-KO mice. We also investigated Ad5-induced liver damage at 6, 24, and 72 hours after injection, by determining plasma ALT levels.34 At 6 and 24 hours after injection, Ad vectors did not trigger any increase in ALT levels; however, plasma ALT levels at 72 hours after systemic injection of conventional Ad5 vectors (2 × 1011 vp/mouse) were elevated in 2 of 4 mice, in contrast to the marginal ALT elevations noted after identical injections of the retro-DAF-displaying Ads, the latter paralleled results achieved after injections of conventional Ads into C3-KO mice (Figure 5B). These data also appeared to correlate with slightly decreased levels of leukocyte infiltration in the livers of mice injected with the DAF-displaying Ads; however these differences were not statistically significant (Figure 5C).
Ad5 vectors capsid-displaying retro-DAF complement inhibitor do not suffer from significant thrombocytopenia, liver damage, and proinflammatory leukocytes infiltration to the livers, toxicities typically triggered by conventional Ad vectors in C57BL/6 mice. (A) C57BL/6 WT and C3-KO mice (n = 4 for all groups) were intravenously injected with 0.75 × 1011 vp/mouse or 2 × 1011 vp/mouse of Ad5-based control and experimental vectors. Platelets enumerations were performed at 24 and 72 hours after injection as described in “Platelet enumeration.” The bars represent mean ± SD. Statistical analysis was completed with the use of 1-way analysis of variance with a Student-Newman-Keuls post hoc test, P < .05 was deemed a statistically significant difference. Values statistically different from those in WT_Mock–injected animals, *P < .05, **P < .001; statistically different values in WT_Ad5-GFP-IX-dDAF_REO and C3-KO_Ad5-GFP groups compared with WT_Ad5-GFP and WT_Ad5-IX-dGFP groups at the same time point, #P < .05, ##P < .001. Note that the normal range levels were adapted from studies at The Jackson Laboratories on C57BL/6 mice (http://phenome.jax.org/db/qp?rtn = views/measplot&brieflook = 6219). (B) ALT activity was determined at 72 hours after injection from C57BL/6 WT and C3-KO mice, injected with PBS (Mock) or different Ad5 vectors; Mock-injected animals (n = 3), for Ad5-injected mice (n = 4). Statistical analysis was completed with the use of 1-way analysis of variance with a Student-Newman-Keuls post hoc test; P < .05 was deemed a statistically significant difference. No significant differences were detected. (C) Wild-type (WT) and C3-KO mice injections (n = 4 for all groups) and morphometric evaluation of liver sections were performed as described in “Hemotoxylin and eosin staining.” Representative sections from each treated animal were analyzed, scored, and averaged for the levels of portal, periportal, and lobular inflammation, as described in “Hemotoxylin and eosin staining.” The sum of averages for each category was computed to obtain a total inflammation index score. The error bars represent ± SD. Statistical analysis was completed with the use of 1-way analysis of variance with a Student-Newman-Keuls post hoc test; P < .05 was deemed a statistically significant difference. No significant differences were detected between virus-injected groups.
Ad5 vectors capsid-displaying retro-DAF complement inhibitor do not suffer from significant thrombocytopenia, liver damage, and proinflammatory leukocytes infiltration to the livers, toxicities typically triggered by conventional Ad vectors in C57BL/6 mice. (A) C57BL/6 WT and C3-KO mice (n = 4 for all groups) were intravenously injected with 0.75 × 1011 vp/mouse or 2 × 1011 vp/mouse of Ad5-based control and experimental vectors. Platelets enumerations were performed at 24 and 72 hours after injection as described in “Platelet enumeration.” The bars represent mean ± SD. Statistical analysis was completed with the use of 1-way analysis of variance with a Student-Newman-Keuls post hoc test, P < .05 was deemed a statistically significant difference. Values statistically different from those in WT_Mock–injected animals, *P < .05, **P < .001; statistically different values in WT_Ad5-GFP-IX-dDAF_REO and C3-KO_Ad5-GFP groups compared with WT_Ad5-GFP and WT_Ad5-IX-dGFP groups at the same time point, #P < .05, ##P < .001. Note that the normal range levels were adapted from studies at The Jackson Laboratories on C57BL/6 mice (http://phenome.jax.org/db/qp?rtn = views/measplot&brieflook = 6219). (B) ALT activity was determined at 72 hours after injection from C57BL/6 WT and C3-KO mice, injected with PBS (Mock) or different Ad5 vectors; Mock-injected animals (n = 3), for Ad5-injected mice (n = 4). Statistical analysis was completed with the use of 1-way analysis of variance with a Student-Newman-Keuls post hoc test; P < .05 was deemed a statistically significant difference. No significant differences were detected. (C) Wild-type (WT) and C3-KO mice injections (n = 4 for all groups) and morphometric evaluation of liver sections were performed as described in “Hemotoxylin and eosin staining.” Representative sections from each treated animal were analyzed, scored, and averaged for the levels of portal, periportal, and lobular inflammation, as described in “Hemotoxylin and eosin staining.” The sum of averages for each category was computed to obtain a total inflammation index score. The error bars represent ± SD. Statistical analysis was completed with the use of 1-way analysis of variance with a Student-Newman-Keuls post hoc test; P < .05 was deemed a statistically significant difference. No significant differences were detected between virus-injected groups.
We also confirmed that Ad5 capsid modifications did not reduce the in vivo transductional capability of the retro-DAF-displaying Ad vectors, because equal numbers of DAF-Ad5 genomes were present in these tissues at 6, 24, and 72 hours after injection (supplemental Figure 1A-B). However, retro-DAF display did alter the kinetics of transgene expression with the liver and spleen (supplemental Figure 2A-B), a result probably attributable to the altered cytokine responses elicited by these vectors, an observation previously described by others.35 Importantly, retro-DAF-displaying Ads yielded levels of transgene expression in the liver, identical to unmodified Ads at 28 days after systemic Ad injection (supplemental Figure 3), further confirming that DAF-display does not compromise transduction efficiency of Ads. Moreover, transduction efficiency of the retro-DAF-displaying Ad was not significantly different from Ad5-GFP, as tested by multiple HEK293-based assays, as detailed in supplemental Methods.
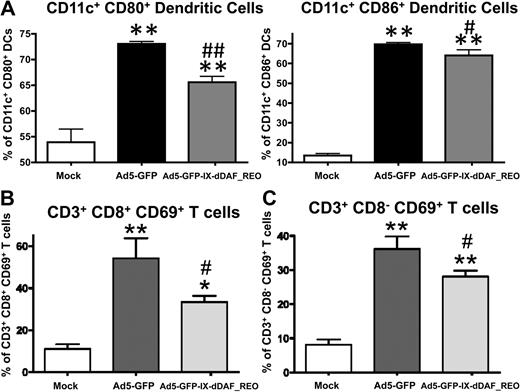
The lack of induction of several Ad-triggered innate inflammatory responses in mice triggered by the retro-DAF-displaying Ads prompted us to evaluate how specific subpopulations of immune effector cells (NK cells, CD8+ T cells, and CD3+CD8− T cells) as well as dendritic cells responded after Ad5 injections.36 Although systemic injection of conventional Ad5 vectors resulted in activation of each of these cell types, the retro-DAF-displaying Ad5 vector triggered significantly lower levels of NK-cell activation, as measured by both interferon-γ expression and CD69 surface expression at 48 hours after injection (Figure 6). Furthermore, we observed significantly reduced levels of activation of CD11c+ dendritic cells (as measured by surface expression of CD80 and CD86 activation/maturation markers) as well as CD3+CD8+ and CD3+CD8− T cells after injection of the retro-DAF-displaying Ad5 vector (Figure 7A-C).
Ad5 vectors capsid-displaying retro-DAF complement inhibitor significantly reduce Ad-triggered activation of NK cells, including reduced expression of interferon-γ by NK cells. Early activation of NK cells was studied by flow cytometric-based methods: 0.75 × 1011 vp/mouse of Ad5-GFP or Ad5-GFP-IX-dDAF_REO were injected intravenously into C57BL/6 mice. Splenocytes were harvested and processed at 48 hours after injection as described in “Cell staining and flow cytometry.” CD69-phycoerythrin (PE), CD3–allophycocyanin (APC)–cyanine 7 (Cy7), CD19–peridinin chlorophyll protein-cyanine 5.5, NK1.1-PECy7, CD8a-Alexa Fluor 700 antibody cocktail was used for surface staining. Interferon γ (IFNγ)–-APC was used for intracellular staining. Samples were analyzed on BD LSR II instrument and analyzed with the use of FlowJo software. The bars represent mean ± SD. Statistical analysis was completed with the use of 1-way analysis of variance with a Student-Newman-Keuls post hoc test, P < .05 was deemed a statistically significant difference. Values statistically different from those in WT_Mock-injected animals, *P < .05, **P < .001; statistically different values from WT_Ad5-GFP mice, #P < .05. n = 3 for Mock-injected animals, n = 4 for virus-injected mice.
Ad5 vectors capsid-displaying retro-DAF complement inhibitor significantly reduce Ad-triggered activation of NK cells, including reduced expression of interferon-γ by NK cells. Early activation of NK cells was studied by flow cytometric-based methods: 0.75 × 1011 vp/mouse of Ad5-GFP or Ad5-GFP-IX-dDAF_REO were injected intravenously into C57BL/6 mice. Splenocytes were harvested and processed at 48 hours after injection as described in “Cell staining and flow cytometry.” CD69-phycoerythrin (PE), CD3–allophycocyanin (APC)–cyanine 7 (Cy7), CD19–peridinin chlorophyll protein-cyanine 5.5, NK1.1-PECy7, CD8a-Alexa Fluor 700 antibody cocktail was used for surface staining. Interferon γ (IFNγ)–-APC was used for intracellular staining. Samples were analyzed on BD LSR II instrument and analyzed with the use of FlowJo software. The bars represent mean ± SD. Statistical analysis was completed with the use of 1-way analysis of variance with a Student-Newman-Keuls post hoc test, P < .05 was deemed a statistically significant difference. Values statistically different from those in WT_Mock-injected animals, *P < .05, **P < .001; statistically different values from WT_Ad5-GFP mice, #P < .05. n = 3 for Mock-injected animals, n = 4 for virus-injected mice.
Ad5 vectors capsid-displaying retro-DAF complement inhibitor significantly reduce Ad-triggered activation of dendritic cells as well as CD8+CD3+ and CD8−CD3+ T cells. Early activation of dendritic cells and T cells was studied by flow cytometric–based methods: 0.75 × 1011 vp/mouse of Ad5-GFP or Ad5-GFP-IX-dDAF_REO were injected intravenously into C57BL/6 mice. Splenocytes were harvested and processed at 6 or 48 hours after injection as described in “Cell staining and flow cytometry”; n = 3 for Mock-injected animals, n = 4 for virus-injected mice. (A) For dendritic cell activation, splenocytes were harvested at 6 hours after injection and stained with the following antibodies: CD11c–phycoerythrin-cyanine 7 (PECy7), CD86–allophycocyanin (APC), CD80-PE. The bars represent mean ± SD. Statistical analysis was completed using 1-way analysis of variance with a Student-Newman-Keuls post hoc test, P < .05 was deemed a statistically significant difference. Values statistically different from those in WT_Mock-injected animals, **P < .001; statistically different values from WT_Ad5-GFP mice, #P < .01, ##P < .001. For CD8+CD3+ (B) and CD8−CD3+ (C) T cell activation, splenocytes were harvested at 48 hours after injection and stained with the following antibodies: CD69-PE, CD3-APCCy7, CD19–peridinin chlorophyll protein-cyanine 5.5 (PerCpCy5.5), NK1.1-PECy7, CD8a–Alexa Fluor 700. The bars represent mean ± SD. Statistical analysis was completed with the use of 1-way analysis of variance with a Student-Newman-Keuls post hoc test; P < .05 was deemed a statistically significant difference. Values statistically different from those in WT_Mock-injected animals, *P < .05, **P < .001; statistically different values from WT_Ad5-GFP mice, #P < .05.
Ad5 vectors capsid-displaying retro-DAF complement inhibitor significantly reduce Ad-triggered activation of dendritic cells as well as CD8+CD3+ and CD8−CD3+ T cells. Early activation of dendritic cells and T cells was studied by flow cytometric–based methods: 0.75 × 1011 vp/mouse of Ad5-GFP or Ad5-GFP-IX-dDAF_REO were injected intravenously into C57BL/6 mice. Splenocytes were harvested and processed at 6 or 48 hours after injection as described in “Cell staining and flow cytometry”; n = 3 for Mock-injected animals, n = 4 for virus-injected mice. (A) For dendritic cell activation, splenocytes were harvested at 6 hours after injection and stained with the following antibodies: CD11c–phycoerythrin-cyanine 7 (PECy7), CD86–allophycocyanin (APC), CD80-PE. The bars represent mean ± SD. Statistical analysis was completed using 1-way analysis of variance with a Student-Newman-Keuls post hoc test, P < .05 was deemed a statistically significant difference. Values statistically different from those in WT_Mock-injected animals, **P < .001; statistically different values from WT_Ad5-GFP mice, #P < .01, ##P < .001. For CD8+CD3+ (B) and CD8−CD3+ (C) T cell activation, splenocytes were harvested at 48 hours after injection and stained with the following antibodies: CD69-PE, CD3-APCCy7, CD19–peridinin chlorophyll protein-cyanine 5.5 (PerCpCy5.5), NK1.1-PECy7, CD8a–Alexa Fluor 700. The bars represent mean ± SD. Statistical analysis was completed with the use of 1-way analysis of variance with a Student-Newman-Keuls post hoc test; P < .05 was deemed a statistically significant difference. Values statistically different from those in WT_Mock-injected animals, *P < .05, **P < .001; statistically different values from WT_Ad5-GFP mice, #P < .05.
Discussion
The complement system is an important part of the host's innate immune response against newly encountered pathogens. During the past decade, it has become increasingly recognized that complement-mediated immune responses also facilitate the robust induction of adaptive immune responses to complement-activating pathogens.9 Normal activation of the complement system accomplishes a number of beneficial tasks; however, complement activation can also be excessive and can cause damage, if not tightly regulated. Humans genetically encode a variety of proteins (including CR1, DAF, MCP1, CD59), each having inherent ability to down-regulate the complement system so as to prevent damage or lysis of host cells.30,37 One of these complement regulatory proteins, DAF, is an integral membrane protein that decreases complement system activation by increasing the rate of decay of both the classical and alternative C3 convertases (C4b2a and C3bBb) generated during pathogen-mediated complement activation.
Specifically, human DAF contains 4 CCPRs.37 When inserted as a fusion protein with various viral capsid/envelope proteins, CCPR1 to CCPR4 of DAF has been shown to retain its functional complement inhibitory activity. Specifically, baculovirus vectors were shown to evade complement-mediated lysis when DAF-derived sequences were embedded within their envelope proteins.38 Retrovirus- and lentivirus-based vectors, derived from growth in packaging cells that overexpressed DAF (but not MCP or CD59), generate DAF-enriched envelopes that protected the viruses from complement-mediated lysis.39-41 It has been confirmed that proteins displayed at the C-terminus of Ad5 protein IX can retain their functionality (GFP, TK).20,24 Our studies now strongly suggest that DAF protein can also be directly displayed from the non–enveloped Ad capsid as a fusion protein. In this manner, each virus particle displays an exact amount of DAF per particle (240 pIX molecules per virion), a situation amenable to current good manufacturing practice–compliant production. One important advantage of retro-DAF-display from the Ad capsid is that it is inherently safer, relative to other strategies directed to mitigate complement activation, such as repeated injections of soluble complement inhibitors (CR1, DAF, and modified analogs30 ), because the latter might predispose the host to viral and/or bacterial infections because of systemic complement blockade for prolonged periods of time.42
To better mimic the natural DAF orientation from human cells, we created a novel cDNA encoding a retro-form of the human DAF protein for display on the Ad capsid. Reversed or retro-forms of proteins are created by this maneuver, allowing for ribosomal translation of reversed copies of a respective parent protein. Obvious concerns about proper foldability and functionality of retro proteins being translated in a reverse fashion have been confirmed.43 However, it has been confirmed that proper folding and function of specific retro proteins can also occur.44 Specifically, the retro-form of the 110 amino acid long, α-crystallin–like small heat-shock protein, HSP12.6, was able to fold and generate a secondary structure identical to the original protein.21,45 Moreover, the retro-form of the human metallothionein-2 α domain retained its ultraviolet absorption spectrum, pH dependence, and its foldability and was also shown to bind Cd in identical stoichiometries, compared with the parent peptide.22,23 Our study is the first (to our knowledge) to strongly suggest that retro-orientation of an enzymatically active protein (such as human DAF) can be used to facilitate the ultimate functionality of the enzymatically active protein as a novel fusion protein.2-4,25
We found that in vitro, retro-display of DAF from the Ad capsid was able to minimize complement activation to levels noted in human blood not exposed to any virus. As an important rationale for our animal model selection, it has been previously shown that human DAF can down-regulate complement activation in the serum of a variety of species, including rodents.46 Mice injected with the optimized retro-DAF-displaying Ad5 vector showed a dramatically improved safety profile compared with traditional Ad5 vectors. This was evidenced by significant reductions in EC activation and cytokine release, avoidance of thrombocytopenia, minimized activation of proinflammatory gene expression, and reduced plasma ALT levels. These results positively correlated with a significantly decreased activation of dendritic cells, NK cells, CD3+CD8+ T cells, and CD3+CD8− T cells.7,8,25,30,36 The latter results suggest that these vectors not only facilitate an improved safety capacity but also may improve the efficacy of this platform for use in gene therapy applications.
Our studies suggest that capsid display of DAF can prevent evidence of complement activation, although the complement system must first be activated, before DAF can act to “decay” C3 convertases. It has been shown that intravenous injection of a mutant Ad, which is unable to escape from endosomes (and therefore incapable of gene transduction), also did not activate the complement in mice.6 That study suggested that Ad-mediated activation of the complement system possibly occurs at the cellular membrane and/or endosomal compartments, where activated complement components are known to accumulate.6,10,47 Therefore, DAF-displaying Ads may prevent the amplification of the complement cascade by directly interacting with high concentrations of membrane-deposited complement components in the near vicinity of the endocytosed Ad, as well as in the fluid compartment of the vascular space.30,47
In summary, results obtained in this study confirm that the human DAF protein cannot only be displayed from the Ad virion but can also retain anticomplement activity when displayed from the surface of the Ad capsid in a retro-oriented fashion. This is the first report confirming that capsid-display of a protein from an Ad vector can dramatically reduce Ad capsid–triggered immune responses in vivo, while retaining the transduction capability of the native Ad capsid. Therefore, these novel, DAF-displaying Ads may become indispensable tools for future gene therapy applications.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Amy S. Porter from Michigan State University (MSU) Investigative Histopathology Laboratory for assistance in performing H&E staining and IHC experiments, MSU animal support facility for their assistance in the humane care and maintenance of the animals, MSU Proteomics facility for their assistance in performing mass spectrometry of Ad-GFP-IX-dDAF_REO, and MSU Electron Microscopy facility for performing electron microscopy of purified Ad vectors.
This work was supported by American Heart Association Midwest Affiliate Fellowship (0815660G; S.S.S.), the National Institutes of Health (grants RO1DK-069884 and P01 CA078673; A.A.), the MSU Foundation (A.A.), and the Osteopathic Heritage Foundation (A.A.).
National Institutes of Health
Authorship
Contribution: S.S.S. designed and performed research, analyzed data, and wrote the paper; Y.A.A. and Z.C.H. designed and performed research; D.M.A. designed research and analyzed data; N.J.S., J.S., S.G., and H.J. performed research; M.M.F. designed research, provided unique reagents, and analyzed data; and A.A. designed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Andrea Amalfitano, 4194 Biomedical and Physical Sciences Bldg, Michigan State University, East Lansing, MI 48823; e-mail: amalfit1@msu.edu.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal