Abstract
Natural killer (NK) cells are innate effector lymphocytes that control the growth of major histocompatibility complex class I negative tumors. We show here that γδ T lymphocytes, expanded in vitro in the presence isopentenylpyrophosphate (IPP), induce NK cell–mediated killing of tumors that are usually resistant to NK cytolysis. The induction of cytotoxicity toward these resistant tumors requires priming of NK cells by immobilized human immunoglobulin G1 and costimulation through CD137L expressed on activated γδ T lymphocytes. This costimulation increases NKG2D expression on the NK-cell surface, which is directly responsible for tumor cell lysis. Moreover, culturing peripheral blood mononuclear cells with zoledronic acid, a γδ T lymphocyte activating agent, enhances NK-cell direct cytotoxicity and antibody-dependent cellular cytotoxicity against hematopoietic and nonhematopoietic tumors. Our data reveal a novel function of human γδ T lymphocytes in the regulation of NK cell–mediated cytotoxicity and provide rationale for the use of strategies to manipulate the CD137 pathway to augment innate antitumor immunity.
Introduction
Natural killer (NK) cells contribute to innate immune responses against virally infected and neoplastic cells.1 NK cells usually recognize and attack tumor cells that lack major histocompatibility complex (MHC) class I.2 Our previous studies in murine tumor models clearly demonstrated that gamma delta (γδ) T lymphocytes play an important role in the regulation of antitumor NK-cell function.3 Specifically, we have shown that γδ T lymphocytes are required for the antitumor activity of NK cells in vivo. More recently, we have demonstrated that culturing human peripheral blood mononuclear cells (PBMCs) with agents that activate γδ T lymphocytes induce NK cell–mediated cytotoxicity against tumors that normally resist NK killing.4 These findings are concordant with other studies that show that γδ T lymphocytes regulate the early phase of NK cell–mediated antibacterial responses in mice.5 Taken in concert these data strongly suggest that γδ T lymphocytes are important in the regulation of NK-cell functions.
γδ T cells are characterized by the expression of a T-cell receptor (TCR) consisting of both gamma and delta chains,6 and account for 1% to 10% of CD3+ cells in the peripheral blood of healthy adults.7 Approximately 70% of γδ T lymphocytes express the Vγ2Vδ2 TCR and can be expanded and activated by phosphoantigens such as the cholesterol biosynthesis intermediate, isopentenylpyrophosphate (IPP), or synthetic bisphosphonates (eg, pamidronate disodium and zoledronic acid).8-10 Upon stimulation, γδ T lymphocytes acquire the capacity to destroy solid tumors of diverse origins such as squamous cell carcinoma of the head and neck (SCCHN), melanoma, colon cancer, and breast carcinoma,4,11-13 suggesting that γδ T lymphocytes are important antitumor effector cells. The validity of this antitumor function is further supported by mouse models demonstrating that mice deficient in γδ T cells have increased sensitivity to the development of methylcholanthrene (MCA)–induced tumors.14 In addition, a recent pilot clinical study showed that γδ T lymphocyte adoptive therapy for patients with advanced renal cell carcinoma was well tolerated and induced antitumor immune responses.15
The antitumor effects of γδ T lymphocytes are recognized to result from both direct killing of tumor targets and trans-activation of adaptive immune responses. For example, recent data demonstrate that activated γδ T lymphocytes cause the maturation of dendritic cells that promote development of acquired immunity.16 In addition, γδ T cells are known to cross-present tumor antigens (Ags) to CD8+ cytolytic T lymphocytes.17,18 Despite their well- characterized role in mediating adaptive immune responses, the mechanisms by which γδ T cells regulate cells of the innate immune system, such as NK cells, are unclear.
In this report we demonstrate that γδ T lymphocytes provide a costimulatory function for NK cells stimulated with suboptimal doses of immobilized human immuglobulin G1 (hIgG1). Costimulated NK cells display up-regulation of the activation markers CD25, CD54 and CD69 and effectively kill solid tumors that are traditionally resistant to NK-mediated lysis. These costimulatory effects are partially regulated by the interaction of CD137L, expressed on activated γδ T lymphocytes, with CD137, present on activated NK cells. CD137/CD137L engagement increases NKG2D expression on NK cells that augmented tumor killing. In addition, ex vivo culture of PBMCs with zoledronic acid induces γδ T lymphocyte activation, resulting in enhanced NK cell–mediated tumor cytotoxicity. Our data define a novel mechanism through which γδ T lymphocytes enhance the cytolytic function of NK cells and provide a clear opportunity to enhance existing cancer treatment strategies combining antibody-dependent cellular cytotoxicity (ADCC) and killing of nonopsonized tumor targets.
Methods
Tumor cell lines
Squamous cell carcinoma head and neck tumor cell lines TU167, TU159, and MDA1986 were graciously provided by Dr Gary Clayman (M. D. Anderson Cancer Center). 012SCC was provided by Dr Bert O'Malley (University of Pennsylvania). The K562 cell line was purchased from ATCC (CCL-213). CD137L transfected and mock transfected P815 cell lines were established in our laboratory as previously described.19 All tumor cell lines and PBMCs were cultured in complete RPMI 1640 media (Gibco) supplemented with 10% fetal bovine serum (Atlanta Biologicals), 2mM l-glutamine, penicillin (100 U/mL), streptomycin (100 μg/mL), and 10mM HEPES (all purchased from Gibco). To ensure the purity of original and cultured tumor cell lines we performed haplotyping using a polymerase chain reaction (PCR) kit (One Lambda Inc) and/or flow cytometric staining with antibodies against human leukocyte antigen (HLA) class I (BD Biosciences).
Antibodies and fusion proteins
Fluorochrome-conjugated monoclonal antibodies (mAbs) against the following Ags were purchased from the vendors indicated and used according to the manufacturer's instructions: γδ TCR, CD56, CD3, CD69, CD54, CD40L, CD80, CD86, CD28, CD94, CD161, CD16, CD152, CD278, CD279, CD134, CD137, CD252, CD137L, interferon-γ, tumor necrosis factor-α (TNF-α; BD Biosciences); CD44, CD46, NKG2D (Biolegend). Blocking experiments with NKG2D and CD54 were purchased from R&D Systems USA. Human IgG1 was obtained from Sigma-Aldrich. Human soluble recombinant CD137Ig, CD134Ig, CD152Ig fusion proteins were purchased from R&D Systems USA.
Flow cytometry
All Ab staining for cell surface markers was performed according to the following protocol. The cells were washed once in phosphate-buffered saline (PBS) containing 1% fetal bovine serum and 0.05% NaN3, incubated with appropriate amounts of mAbs at 4°C for 30 minutes and rewashed in PBS. For intracellular cytokine analysis, cells were cultured with various stimuli and 3μM monensin (Golgi stop) was added during the last 4 hours of culture. The cells were stained with mAbs against cell surface molecules (eg, γδTCR, CD3, CD56), fixed and permeabilized using the BD Cytofix/Cytoperm Kit as described by the manufacturer (BD Biosciences). After permeabilization, the cells were stained with phycoerythrin-conjugated mAbs specific to interferon-γ and TNF-α. To determine granzyme A and B expression, permeabilized cells were stained with phycoerythrin-conjugated anti–human Granzyme A and B or the appropriate isotype control (BD Biosciences).
In most flow cytometry samples, at least 3 × 104 gated NK cells or γδ T lymphocytes (defined as CD3−CD56+ and CD3+ and γδ TCR+, respectively) were acquired using a BD LSRII flow cytometer (Becton Dickinson). All samples were analyzed using FACS Diva software (Becton Dickinson).
γδ T-cell expansion and activation
Buffy coats from healthy donors were purchased through Biologic Specialty Corp as approved under the University of Maryland Institutional Review Board exemption. For expansion of γδ T cells, whole PBMCs were separated on a Ficoll gradient (Amersham Biosciences) and 1 × 106 cells/mL were cultured in complete media with 15μM isopentyl pyrophosphate (IPP; Sigma-Aldrich) and 100 U/mL human recombinant interleukin-2 (IL-2; Tecin; Biological Resources Branch, National Institutes of Health, Bethesda, MD). Fresh complete medium and IL-2 supplement at 100 U/mL were added every 3 days. After 14 days of culture, cells were harvested and the percentage of γδ T cells was measured by flow cytometry. The percentage of γδ T lymphocytes in IPP expanded cultures varied from 60% to 90%, and the range for individual experiments is reported in the figure legends. γδ T lymphocytes were not purified prior coculture with NK cells.
Alternatively, PBMCs isolated from buffy coats (3 × 106 cells/mL) were cultured with 15μM Zometa (Novartis) alone or in the presence of 10 μg/mL rituximab (Genentech). Activation of NK cells was verified by fluorescence-activated cell sorting or NK cells were purified from the cultures using magnetic beads and used in cytotoxicity assay.
Immunomagnetic bead purification of NK cells
NK cells were isolated from fresh PBMCs by negative selection using MACS NK-cell isolation kit (Miltenyi Biotec) according to the manufacturer's instructions. The purity of the resulting cell populations was checked routinely by flow cytometry. NK-cell purity generally exceeded 97%.
NK-cell and γδ T-lymphocyte coculture
hIgG1 was immobilized on plastic culture plates by incubating hIgG1 (2.5 μg/mL) in PBS at 4°C overnight, a condition that provides stable attachment of Igs on neutral plastic substrates. Purified NK cells (2 × 106 cells/well) and IPP-expanded γδ T lymphocytes (1 × 106 cells/well) were cocultured in 1 mL of RPMI in 24-well cell plates (Falcon) precoated with 10 μg/mL hIgG1. After 48 hours of culture, NK cells were assessed by flow cytometry and/or purified using MACS negative isolation kits for analysis of cytolytic activity. In some experiments purified NK cells (2 × 106 cells/well) were cocultured with mock- or CD137L-transfected P815 cells (1 × 106 cells/well). In blocking experiments, human soluble recombinant Ig fusion proteins or mAbs (ie, CD137Ig, CD152Ig, CD134Ig) at 10 μg/mL were included at the onset of the NK- and γδ T-cell cocultures. After 48 hours, NK cells were purified and tested for cytotoxicity against SCCHN targets.
In some experiments purified NK cells (1 × 106 cells/mL) were cultured with live TU167 (0.5 × 106 cells/mL) alone, in the presence of 10 μg/mL hIgG1 (isotype control), or cetuximab (Bristol-Myers). Activation of NK cells was confirmed by fluorescence-activated cell sorting.
Transwell coculture
Purified NK cells (2 × 106 cells/well) were resuspended in 1 mL of RPMI and placed in 24-well plates precoated with 10 μg/mL hIgG1. IPP-expanded γδ T lymphocytes were resuspended at 0.5 × 106 cells/mL and 0.5 mL of cells were added into the Transwell (Costar) with a polycarbonated membrane (pore diameter 0.4μM) permeable for soluble factors. Cells, separated by a transwell, were cultured for 48 hours as described in the previous paragraph and expression of activation markers was analyzed by flow cytometry.
Cytotoxicity assay
NK-cell cytotoxicity was measured using a standard 51Cr-release assay, as described previously.20 Briefly, target cells (2 × 106 in 0.3 mL of complete media) were incubated for 90 minutes at 37°C in 5% CO2 with 150 μCi of 51Cr (GE Healthcare). The labeled cells were then washed twice with media and incubated for an additional 30 minutes to reduce background radioactivity. Cells were then washed 2 more times and adjusted to a concentration of 5 × 104 cells/mL in complete media. Labeled targets cells were cultured for 30 minutes with 4 μg/mL rituximab or cetuximab. Effector NK cells were purified from γδ T lymphocyte coculture or from cultured PBMCs by immunomagnetic MACS NK negative selection kit (Miltenyi Biotec). Serial dilutions of effector cells (100 μL/well) were added into each well of 96-well V-bottomed plates (Corning). Aliquots of 51Cr-labeled target cells (100 μl/well) were dispensed into wells containing effector cells. The plates were centrifuged at 20g for 2 minutes and incubated at 37°C in 5% CO2. After 4 hours of incubation, the plates were centrifuged again at 300g for 5 minutes and 100-μL aliquots of the supernatants from each well were transferred to a new plate containing 100 μL/well of Optiphase Supermix scintillation fluid (Perkin Elmer). Radioactivity was measured using 1450 Microbeta counter (Wallac). In some experiments anti-NKG2D or isotype control mouse IgGs were added at 10 μg/mL, 15 minutes before the addition of labeled target cells. The percentage of specific cytotoxicity was calculated as (experimental release − spontaneous release)/(maximum release − spontaneous release) × 100. Spontaneous release was determined by incubating the targets with 100 μL of complete media. Maximum release was determined by incubating the target cells with 100 μL of 0.5% Triton-X.
Statistical analysis
All cytotoxicity data were analyzed using the Student test, whereby P less than .05 indicated that the value of the test sample was significantly different from the relevant control.
Results
γδ T lymphocytes activate hIgG1-primed NK cells
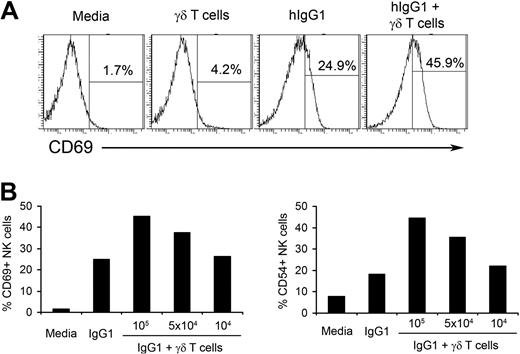
We have previously shown that long-term (14-day) culture of PBMCs with IPP+IL-2 results in the induction of NK cell–mediated cytotoxicity against tumors that are normally resistant to NK-cell killing.4 We also observed that IPP did not stimulate NK cells directly and γδ T lymphocytes were critical for IPP-induced NK-cell cytotoxicity. Usually NK cells do not survive for a long period of time without cytokine support.21 Therefore, to understand the mechanisms of γδ T lymphocyte–mediated NK-cell activation in a more physiologic model, in this study we used a short-term culture of fresh NK cells purified from PBMCs of healthy donors and γδ T lymphocytes expanded in vitro. As shown in Figure 1A, fresh NK cells cultured with media or γδ T lymphocytes alone do not express CD69, a molecule associated with NK-cell activation. In contrast, consistent with our previously published data,22 after incubating in wells precoated with hIgG1, 25% of NK cells express CD69 on their surface. Adding γδ T lymphocytes to hIgG1-coated wells further increases the expression of CD69 on NK cells to 45.9% (Figure 1A). A similar pattern of expression was seen for CD54, another marker of NK-cell activation (supplemental Figure 2, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Neither immobilized hIgG1 alone nor in combination with NK cells induced activation of γδ T lymphocytes, as measured by the expression of activation markers (supplemental Figure 3). Because short-term (48-hour) culture of γδ T lymphocytes without hIgG1 did not result in the activation of NK cells, plastic immobilized hIgG1 was included in all subsequent experiments as a putative initial signal for NK-cell priming.
γδ T lymphocytes increase hIgG1-induced activation of NK cells. (A) Purified natural killer (NK) cells and isopentenylpyrophosphate plus interleukin-2 (IPP+IL-2) expanded γδ T lymphocytes were cocultured at a 4:1 ratio in the presence or absence of plate-immobilized human immunoglobulin G1 (hIgG1; 2.5 μg/mL) for 48 hours. The expression of CD69 was analyzed by flow cytometry. Histograms represent gated CD3−CD56+ NK cells. An example of gating strategy is shown in supplemental Figure 1. (B) Purified NK cells (2 × 105 cells/well) were cultured with indicated numbers of IPP+IL-2 expanded γδ T lymphocytes in the presence of immobilized hIgG1 (2.5 μg/mL) for 48 hours. Expression of activation markers CD69 and CD54 were assessed by flow cytometry and plotted as a percentage of CD69 and CD54 positive gated NK cells. Representative data from 1 of 10 independent experiments is shown.
γδ T lymphocytes increase hIgG1-induced activation of NK cells. (A) Purified natural killer (NK) cells and isopentenylpyrophosphate plus interleukin-2 (IPP+IL-2) expanded γδ T lymphocytes were cocultured at a 4:1 ratio in the presence or absence of plate-immobilized human immunoglobulin G1 (hIgG1; 2.5 μg/mL) for 48 hours. The expression of CD69 was analyzed by flow cytometry. Histograms represent gated CD3−CD56+ NK cells. An example of gating strategy is shown in supplemental Figure 1. (B) Purified NK cells (2 × 105 cells/well) were cultured with indicated numbers of IPP+IL-2 expanded γδ T lymphocytes in the presence of immobilized hIgG1 (2.5 μg/mL) for 48 hours. Expression of activation markers CD69 and CD54 were assessed by flow cytometry and plotted as a percentage of CD69 and CD54 positive gated NK cells. Representative data from 1 of 10 independent experiments is shown.
We also determined an optimal ratio for NK-cell activation by γδ T lymphocytes. As shown in Figure 1B, culturing 2 × 105 NK cells with 105 or 5 × 104 T lymphocytes (2:1 and 4:1 ratios, respectively) induced significant increases in CD69 and CD54 expression on NK cells primed with hIgG1, while ratios of 20:1 (104 IPP-expanded γδ T lymphocytes) failed to increase the expression of activation markers on NK cells. Therefore, in subsequent experiments we used a ratio of 4:1. These results indicate that in vitro expanded γδ T lymphocytes stimulate the activation of hIgG1-primed human NK cells.
γδ T lymphocytes enhance NK cell–mediated antitumor cytotoxicity
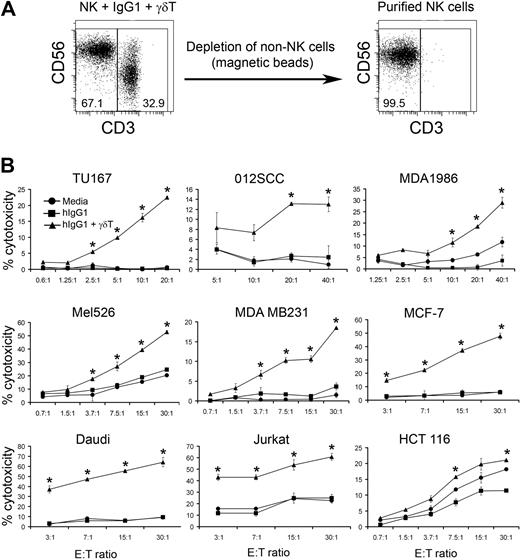
To determine whether γδ T-lymphocyte stimulation of hIgG1-primed NK cells enhances their cytolytic activity, we stimulated purified NK cells with media, hIgG1, or IPP-expanded γδ T lymphocytes in the presence of immobilized hIgG1. After 48 hours, NK cells were repurified by negative selection, enabling us to obtain a highly pure population of “untouched” NK cells (> 99%) for functional analysis (Figure 2A). Use of these NK cells as effectors against various tumor cell lines revealed that cells cultured with media or immobilized hIgG1 alone did not kill SCCHN (TU167, 012SCC, MDA1986), melanoma (Mel526), breast cancer (MDA MB231 and MCF-7), B-cell lymphoma (Daudi), or T-cell lymphoma (Jurkat) tumor cell lines. However, γδ T lymphocytes significantly increased the lytic activity of hIgG1-primed NK cells against the above cell lines (Figure 2B). The killing of colon cancer lines (HCT 116), that appears to be sensitive to NK cell–mediated cytotoxicity, was also significantly increased by γδ T lymphocytes. The activation of NK cells was independent of donor HLA-type, because both autologous and allogeneic γδ T lymphocytes enhanced cytolytic activity. This lack of HLA restriction in NK-cell activation by γδ T lymphocytes was very reproducible and observed in more than 20 independent experiments. Based on these findings, in subsequent studies we used NK cells and γδ T lymphocytes derived from the PBMCs of different donors, enabling us to obtain sufficient numbers of NK cells for functional and phenotypic analysis. Overall these data suggest that IPP-activated γδ T lymphocytes enhance direct NK cell–mediated cytolytic activity against hematopoietic and nonhematopoietic tumors.
γδ T lymphocytes induce NK cell–mediated cytotoxicity against various tumor cell lines. (A) Purified NK cells were cultured with bulk IPP-expanded peripheral blood mononuclear cells (PBMCs) at a 2:1 ratio for 48 hours (ie, 2 NK cells/1 IPP-expanded PBMC). IPP-expanded PBMCs in these experiments contained 70% to 80% γδ T lymphocytes, so the actual ratio of NK cells to IPP-expanded γδ T lymphocytes was approximately 2:0.8. NK cells were repurified from the cultures by immunomagnetic depletion of non-NK cells. Representative dot plots of NK + γδ T cells (right) and NK cells purified after 48 hours of culture (left) are shown. (B) Cytolytic activity of NK cells purified after coculture with expanded γδ T cells and immobilized hIgG1 (2.5 μg/mL) for 48 hours was analyzed in a standard 4-hour 51Cr-release assay against indicated tumor targets. Data are presented as mean ± SD of triplicate samples and are representative of 7 independent experiments. *P < .05 compared with NK cells cultured with hIgG1 alone.
γδ T lymphocytes induce NK cell–mediated cytotoxicity against various tumor cell lines. (A) Purified NK cells were cultured with bulk IPP-expanded peripheral blood mononuclear cells (PBMCs) at a 2:1 ratio for 48 hours (ie, 2 NK cells/1 IPP-expanded PBMC). IPP-expanded PBMCs in these experiments contained 70% to 80% γδ T lymphocytes, so the actual ratio of NK cells to IPP-expanded γδ T lymphocytes was approximately 2:0.8. NK cells were repurified from the cultures by immunomagnetic depletion of non-NK cells. Representative dot plots of NK + γδ T cells (right) and NK cells purified after 48 hours of culture (left) are shown. (B) Cytolytic activity of NK cells purified after coculture with expanded γδ T cells and immobilized hIgG1 (2.5 μg/mL) for 48 hours was analyzed in a standard 4-hour 51Cr-release assay against indicated tumor targets. Data are presented as mean ± SD of triplicate samples and are representative of 7 independent experiments. *P < .05 compared with NK cells cultured with hIgG1 alone.
Cell-to-cell contact is essential for NK-cell activation by γδ T lymphocytes
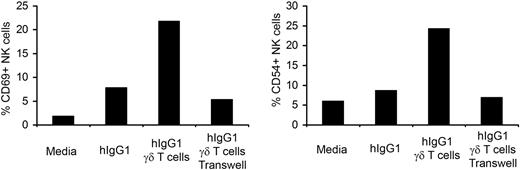
We have previously shown that soluble factors produced by γδ T lymphocytes are responsible for activation of NK cells in long-term culture.4 To ascertain whether the enhanced NK-cell activation by γδ T lymphocytes in short-term culture is mediated by cell-to-cell contact or soluble factors, we used a transwell system. Purified NK cells were placed in lower wells coated with hIgG1 and IPP-expanded γδ T lymphocytes were added to either the lower or the upper wells. As expected, NK cells cocultured with immobilized hIgG1 and γδ T lymphocytes, placed in lower wells, showed increased expression of the CD69 and CD54 activation markers (Figure 3). When NK cells were separated from γδ T lymphocytes by a membrane with 0.4-μm pore size there was no increase in the expression of activation markers. These data indicate that, in contrast to our long-term exposure data, cell-to-cell contact is required for NK-cell activation by γδ T lymphocytes during short term interaction.
Cell-to-cell contact is required for the activation of NK cells by γδ T lymphocytes. Trans-well experiments were performed by culturing purified NK cells in lower wells coated with hIgG1 (2.5 μg/mL). Expanded γδ T lymphocytes were added either to the lower wells (cell-to-cell contact) or to the upper wells (soluble factors). The ratio of NK to γδ T cells was 4:1. After 48 hours of culture, the expression of CD69 and CD54 was analyzed by flow cytometry. The bar diagrams depict the percentage of CD69 and CD54 expressing cells in gated NK populations. Representative data from 1 of 3 independent experiments is shown.
Cell-to-cell contact is required for the activation of NK cells by γδ T lymphocytes. Trans-well experiments were performed by culturing purified NK cells in lower wells coated with hIgG1 (2.5 μg/mL). Expanded γδ T lymphocytes were added either to the lower wells (cell-to-cell contact) or to the upper wells (soluble factors). The ratio of NK to γδ T cells was 4:1. After 48 hours of culture, the expression of CD69 and CD54 was analyzed by flow cytometry. The bar diagrams depict the percentage of CD69 and CD54 expressing cells in gated NK populations. Representative data from 1 of 3 independent experiments is shown.
Expression of costimulatory molecules on activated γδ T lymphocytes and NK cells
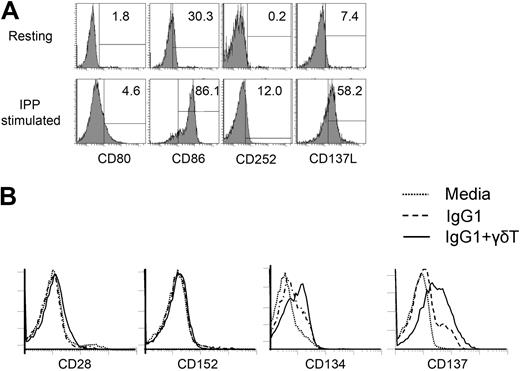
It is known that ligands belonging to the B7 and TNF super families are essential for costimulation of immune cells.23,24 Having demonstrated the activation of hIgG1-primed NK cells by γδ T lymphocytes requires cell-to-cell contact, we characterized the expression of costimulatory molecules on these cells. First, we analyzed the expression of known costimulatory ligands on γδ T lymphocytes. As shown in Figure 4A, γδ T lymphocytes in unstimulated PBMCs did not express CD80, CD86, CD252 (OX40L), or CD137L (4-1BBL) on their surface. However, stimulation of PBMCs with IPP and IL-2 for 14 days induced the expression of CD86 (86%), CD252 (12%), and CD137L (58%). Moreover, CD134 (OX40) and CD137 (4-1BB) expression were enhanced on the surface of NK cells cultured with immobilized hIgG1 and this expression was further augmented by the addition of γδ T lymphocytes (Figure 4B). No expression of CD28, CD152 (CTLA-4), CD278 (inducible co-stimulator [ICOS]), and CD279 (PD-1) were observed on NK cells even after coculture with γδ T lymphocytes in the presence of immobilized hIgG1 (Figure 4B and supplemental Figure 4).
Expression of costimulatory ligands and receptors on γδ T lymphocytes and NK cells. (A) Fresh γδT lymphocytes from healthy donors (top histograms) or γδT lymphocytes expanded in the presence of IPP+IL-2 (bottom histograms) were stained with mAbs specific for CD80, CD86, CD252 (OX40L) and CD137L (41BBL). The expression of indicated costimulatory ligands on gated CD3+γδTCR+ cells is shown. (B) NK cells cultured with media alone or immobilized hIgG1 with or without in vitro expanded γδ T lymphocytes for 48 hours were stained with mAbs specific for CD28, CD152 (CTLA-4), CD134 (OX40), and CD137 (4-1BB). Overlays of histograms representing gated CD3−CD56+ NK cells are shown. Depicted data represent 1 of 5 independent experiments.
Expression of costimulatory ligands and receptors on γδ T lymphocytes and NK cells. (A) Fresh γδT lymphocytes from healthy donors (top histograms) or γδT lymphocytes expanded in the presence of IPP+IL-2 (bottom histograms) were stained with mAbs specific for CD80, CD86, CD252 (OX40L) and CD137L (41BBL). The expression of indicated costimulatory ligands on gated CD3+γδTCR+ cells is shown. (B) NK cells cultured with media alone or immobilized hIgG1 with or without in vitro expanded γδ T lymphocytes for 48 hours were stained with mAbs specific for CD28, CD152 (CTLA-4), CD134 (OX40), and CD137 (4-1BB). Overlays of histograms representing gated CD3−CD56+ NK cells are shown. Depicted data represent 1 of 5 independent experiments.
Activation of NK cells by γδ T lymphocytes is partially mediated by CD137/CD137L interactions
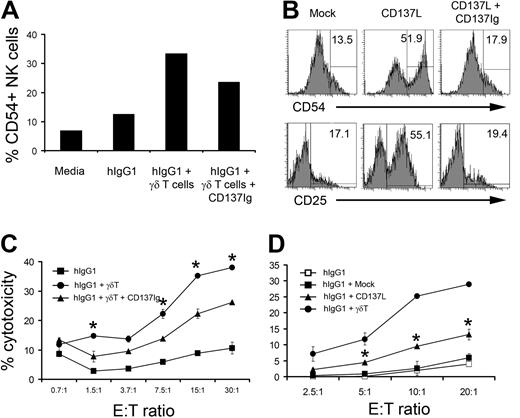
The fact that NK-cell costimulation with γδ T lymphocytes and IgG1 induces CD137 and CD134 suggested that some of the observed antitumor effects might be mediated by TNF superfamily members. To determine whether CD134 and/or CD137 are involved in the activation of NK cells by γδ T lymphocytes, we used fusion proteins to block engagement of CD134 and CD137 with their cognate ligands. Addition of CD152Ig (used as negative control) or CD134Ig fusion proteins into the culture did not inhibit the activation of NK cells by γδ T lymphocytes (supplemental Figure 5A). However, CD137Ig partially inhibited CD54 expression on NK cells (Figure 5A). These results suggest that stimulation of hIgG1-primed NK cells by γδ T lymphocytes involves CD137.
Blocking of CD137L partially inhibits γδ T lymphocyte–induced cytolytic activity of NK cells. (A) Purified NK cells were cocultured with IPP+IL-2 expanded γδT lymphocytes at a 4:1 ratio in the presence of immobilized hIgG1 (2.5 μg/mL) for 48 hours. In some groups, soluble CD137Ig fusion protein at 10 μg/mL was added to block CD137 receptor and ligand interactions. The bar diagram represents the percentage of cells expressing CD54 in gated CD3−CD56+ NK-cell population. Representative data from 1 of 4 independent experiments is shown. (B) Purified NK cells were cocultured with either mock (left histograms) or CD137L-transfected P815 (middle histograms) at a 4:1 ratio in the presence of immobilized hIgG1 (2.5 μg/mL). In some wells containing NK cells and CD137L-transfected P815 tumors, soluble CD137Ig fusion protein (10 μg/mL) was added (right histograms). After 48 hours of culture, cells were stained for CD54 and CD25. Histograms represent cells gated on CD56+CD3− NK population. (C) Soluble CD137Ig fusion protein (10 μg/mL) was included during the culture of purified NK cells and γδ T lymphocytes (4:1 ratio) in hIgG1-precoated plates for 48 hours. Cytotoxicity of NK cells repurified after culture was analyzed in a standard 4-hour 51Cr-release assay against the TU167 SCCHN cell line. Data are presented as mean ± SD of triplicate samples and are representative of 4 independent experiments. *P < .05 compared with NK cells cultured in the presence of CD137Ig blocking. (D) NK cells were purified from PBMCs of healthy donors and cocultured with irradiated mock or CD137L-transfected P815 cells at a 4:1 ratio. Expanded γδ T lymphocytes were used as a positive control for NK-cell activation. After 48 hours of culture, NK cells were repurified and used as effectors against TU167 SSCHN target cells. Data are presented as mean ± SD of triplicate samples and representative of 3 independent experiments. *P < .05 compared with NK cells cultured with mock transfected P815.
Blocking of CD137L partially inhibits γδ T lymphocyte–induced cytolytic activity of NK cells. (A) Purified NK cells were cocultured with IPP+IL-2 expanded γδT lymphocytes at a 4:1 ratio in the presence of immobilized hIgG1 (2.5 μg/mL) for 48 hours. In some groups, soluble CD137Ig fusion protein at 10 μg/mL was added to block CD137 receptor and ligand interactions. The bar diagram represents the percentage of cells expressing CD54 in gated CD3−CD56+ NK-cell population. Representative data from 1 of 4 independent experiments is shown. (B) Purified NK cells were cocultured with either mock (left histograms) or CD137L-transfected P815 (middle histograms) at a 4:1 ratio in the presence of immobilized hIgG1 (2.5 μg/mL). In some wells containing NK cells and CD137L-transfected P815 tumors, soluble CD137Ig fusion protein (10 μg/mL) was added (right histograms). After 48 hours of culture, cells were stained for CD54 and CD25. Histograms represent cells gated on CD56+CD3− NK population. (C) Soluble CD137Ig fusion protein (10 μg/mL) was included during the culture of purified NK cells and γδ T lymphocytes (4:1 ratio) in hIgG1-precoated plates for 48 hours. Cytotoxicity of NK cells repurified after culture was analyzed in a standard 4-hour 51Cr-release assay against the TU167 SCCHN cell line. Data are presented as mean ± SD of triplicate samples and are representative of 4 independent experiments. *P < .05 compared with NK cells cultured in the presence of CD137Ig blocking. (D) NK cells were purified from PBMCs of healthy donors and cocultured with irradiated mock or CD137L-transfected P815 cells at a 4:1 ratio. Expanded γδ T lymphocytes were used as a positive control for NK-cell activation. After 48 hours of culture, NK cells were repurified and used as effectors against TU167 SSCHN target cells. Data are presented as mean ± SD of triplicate samples and representative of 3 independent experiments. *P < .05 compared with NK cells cultured with mock transfected P815.
To confirm the role of CD137 in the activation of NK cells, purified NK cells were cultured with irradiated P815 cells expressing CD137L (supplemental Figure 6). Culturing purified NK cells with mock transfected P815 cells in the presence of immobilized hIgG1 did not induce the expression of CD54 or CD25 (Figure 5B). In contrast, CD137L-expressing P815 tumors significantly increased the expression of activation markers on NK cells. The inclusion of the CD137Ig fusion protein to cultures containing NK cells and CD137L-transfected P815 tumors completely abrogated the expression of CD54 and CD25 (Figure 5B), indicating that CD137Ig fusion protein blocks CD137/CD137L engagement. Overall, these results demonstrate that CD137/CD137L interactions are at least partially involved in the activation of NK cells by γδ T lymphocytes.
CD137 mediates the induction of NK-cell cytotoxicity by γδ T lymphocytes
We next investigated whether CD137 engagement enhances the cytolytic potential of NK cells cultured with γδ T lymphocytes. Reproducibly, and consistent with our previous findings,22 hIgG1 alone did not induce cytolytic function of NK cells while addition of γδ T lymphocytes significantly increased killing of SCCHN targets (Figure 5C). The addition of soluble CD137Ig fusion protein decreased the cytolytic activity of NK cells cultured in the presence of immobilized hIgG1 and γδ T lymphocytes by 40%, suggesting that CD137 engagement is important for the regulation of NK-cell cytolytic function. CD152Ig fusion protein containing the same Fc portion did not inhibit the induction of NK-cell cytotoxicity (supplemental Figure 5B). To confirm the role of CD137 signaling in the activation of NK cell–mediated cytotoxicity, purified NK cells were cultured with CD137L-transfected P815 cells (supplemental Figure 6). Data presented in Figure 5D indicate that NK cells cultured with hIgG1 and γδ T lymphocytes induced 29% cytotoxicity against SCCHN cells at a 20:1 effector:target ratio. Thirteen percent cytotoxicity was observed in NK cells cultured with CD137L-transfected P815 while only 6% cytotoxicity was mediated by NK cells cultured with mock P815. These data suggest that CD137 is at least partially involved in the regulation of NK-cell cytolytic activity costimulated by γδ T lymphocytes.
hIgG1-primed, CD137-costimulated NK cells use NKG2D for tumor cytolysis
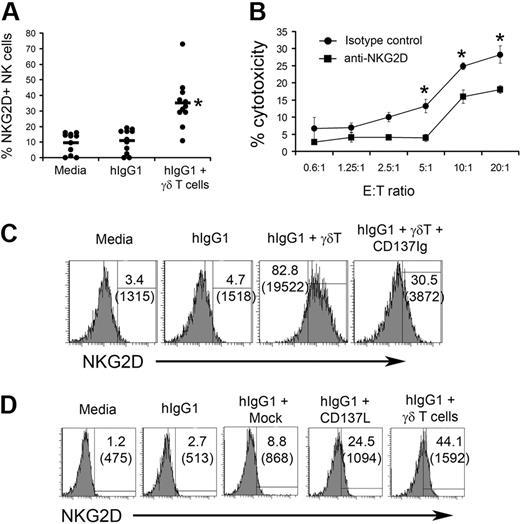
We next sought to understand the mechanism of tumor killing by NK cells cultured in the presence of γδ T lymphocytes. It is well known that NKG2D regulates NK-cell cytotoxicity against many tumors.25 Resting NK cells express considerable amount of NKG2D on their surface (supplemental Figure 7). We observed reproducible increases in the expression of NKG2D on the surface of NK cells from 11 different donors cultured with immobilized hIgG1 and γδ T lymphocytes (Figure 6A). Furthermore, the cytolytic activity of NK cells cultured with γδ T lymphocytes correlated with the levels of NKG2D expression (supplemental Figure 8). In contrast, we found no expression of other well-characterized NK-cell receptors (ie, CD16, NKp30, NKp44, NKp46, CD94 CD161) on stimulated NK cells (supplemental Figure 9).
CD137 ligation on NK cells results in enhanced NKG2D expression that is involved in tumor cell killing. (A) NK cells purified from PBMCs of 11 individual donors were cocultured in the presence of expanded γδ T lymphocytes (4:1 ratio) on plates precoated with hIgG1. After 48 hours of culture, the expression of NKG2D was analyzed on NK cells. Dots represent individual values of NKG2D expression on gated NK cells. Horizontal lines represent average values of NKG2D expression in indicated groups. (B) Cytotoxic activity of NK cells purified after 48 hours of culture with in vitro expanded γδ T lymphocytes was measured in a standard 4-hour 51Cr-release assay against TU167 squamous cell carcinoma of the head and neck (SCCHN). Blocking anti-NKG2D antibodies or isotype control IgG were added into the wells containing purified NK cells and TU167 targets for the duration of the cytotoxicity test. Data are presented as mean ± SD of triplicate samples and are representative of 2 independent experiments. *P < .05 compared with isotype control. (C) CD137Ig (10 μg/mL) was added to wells containing NK cells and IPP+IL-2 expanded γδ T lymphocytes. After 48 hours of culture, cells were stained with anti-NKG2D mAb. The histograms depict NKG2D expression on gated CD56+CD3− NK cells. Numbers in brackets indicate mean fluorescent intensity of NKG2D expression. (D) Purified NK cells were cultured with irradiated mock or CD137L-transfected P815 cells (4:1) on plates precoated with hIgG1. After 48 hours the expression of NKG2D was analyzed by fluorescence-activated cell sorting (FACS). Expanded γδ T lymphocytes were used as a positive control for NK-cell activation. Numbers in brackets indicate mean fluorescent intensity of NKG2D expression.
CD137 ligation on NK cells results in enhanced NKG2D expression that is involved in tumor cell killing. (A) NK cells purified from PBMCs of 11 individual donors were cocultured in the presence of expanded γδ T lymphocytes (4:1 ratio) on plates precoated with hIgG1. After 48 hours of culture, the expression of NKG2D was analyzed on NK cells. Dots represent individual values of NKG2D expression on gated NK cells. Horizontal lines represent average values of NKG2D expression in indicated groups. (B) Cytotoxic activity of NK cells purified after 48 hours of culture with in vitro expanded γδ T lymphocytes was measured in a standard 4-hour 51Cr-release assay against TU167 squamous cell carcinoma of the head and neck (SCCHN). Blocking anti-NKG2D antibodies or isotype control IgG were added into the wells containing purified NK cells and TU167 targets for the duration of the cytotoxicity test. Data are presented as mean ± SD of triplicate samples and are representative of 2 independent experiments. *P < .05 compared with isotype control. (C) CD137Ig (10 μg/mL) was added to wells containing NK cells and IPP+IL-2 expanded γδ T lymphocytes. After 48 hours of culture, cells were stained with anti-NKG2D mAb. The histograms depict NKG2D expression on gated CD56+CD3− NK cells. Numbers in brackets indicate mean fluorescent intensity of NKG2D expression. (D) Purified NK cells were cultured with irradiated mock or CD137L-transfected P815 cells (4:1) on plates precoated with hIgG1. After 48 hours the expression of NKG2D was analyzed by fluorescence-activated cell sorting (FACS). Expanded γδ T lymphocytes were used as a positive control for NK-cell activation. Numbers in brackets indicate mean fluorescent intensity of NKG2D expression.
We have found that many SCCHN tumors express ULBP-2 and ULBP-3, NKG2D ligands (supplemental Figure 10), suggesting a role of NKG2D in SCCHN killing by NK cells. Results presented in Figure 6B indicate that anti-NKG2D mAb blockade reduced the cytolysis of TU167 tumors by NK cells cultured with γδ T lymphocytes from 27% to 17% at a 20:1 effector/target ratio. These observations indicate that NK cells cocultured with γδ T lymphocytes may kill SCCHN tumors by recognizing NKG2D ligands.
To verify the role of CD137 engagement in the induction of NKG2D expression on NK cells cultured with γδ T lymphocytes, we used CD137Ig fusion protein to block CD137/CD137L interaction. The addition of CD137Ig decreased γδ T lymphocyte–induced expression of NKG2D on NK cells from 82.8% (mean fluorescent intensity 19 522) to 30.5% (mean fluorescent intensity 3872; Figure 6C), indicating that CD137 engagement is important for the induction of NKG2D expression. Experiments using CD137L-transfected P815 cells further confirmed the involvement of CD137 signaling in the NKG2D expression (Figure 6dD. Overall, these data indicate that CD137 engagement plays a significant role in the control of NKG2D expression that is important for tumor killing by NK cells cultured with expanded γδ T lymphocytes.
Zoledronic acid enhances both direct NK cytotoxicity and ADCC against SCCHN and lymphoma
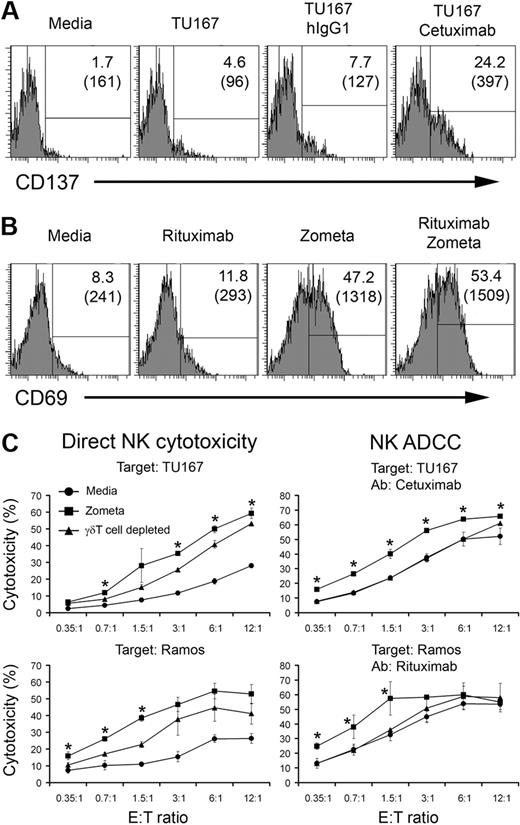
Our data suggest that priming of NK cells by immobilized hIgG1 induces CD137 expression that is important for γδ T lymphocyte–induced activation. To provide a platform for translating these observations, we next sought to develop a clinically applicable system for immobilizing human IgG in vivo and providing simulatanoeus γδ T-lymphocyte activation. First, we evaluated if opsonized tumor could serve as a platform for IgG immobilization. The results shown in Figure 7A indicate that the EGFR positive SCCHN cell line, TU167, when opsonized with the anti-EGFR mAb (cetuximab), used clinically for the treatment of patients with SCCHN, induces expression of CD137 on NK cells. These data suggest that mAbs that target tumor-associated antigens, when composed of the appropriate IgG isotype, can also provide a first signal for NK activation.
Zoledronate, a γδ T lymphocyte–activating agent, enhances NK-cell activation and cytotoxicity. (A) Purified NK cells were cultured for 96 hours in the presence of media, TU167 cells alone, TU167 + 10 μg/mL hIgG1 (isotype control), or TU167 + 10 μg/mL cetuximab. CD137 expression on CD56+ NK cells was analyzed by FACS. Two representative experiments are shown. (B) Whole PBMCs were incubated in the presence of media, 10 μg/mL rituximab, 15μM zoledronate, or a combination of rituximab with zoledronate. The expression of CD69 on gated CD3−CD56+ NK cells was analyzed by FACS 96 hours after initiation of the cultures. A representative of 3 independent experiments is depicted. (C) Whole PBMCs were cultured with media (circles) or zoledronate (squares). Alternatively, γδ T lymphocyte–depleted PBMCs were cultured with zoledronate (triangles) for 96 hours. NK cells were purified from the groups described. NK-cell direct cytotoxicity (left plots) or antibody-dependent cellular cytotoxicity (right plots) was measured in a standard 4-hour 51Cr-release assay against TU167 SCCHN or Ramos B-cell lymphoma targets. Data are presented as mean ± SD of triplicate samples and are representative of 2 independent experiments. *P < .05 compared with NK cells purified from γδ T lymphocyte–depleted cultures.
Zoledronate, a γδ T lymphocyte–activating agent, enhances NK-cell activation and cytotoxicity. (A) Purified NK cells were cultured for 96 hours in the presence of media, TU167 cells alone, TU167 + 10 μg/mL hIgG1 (isotype control), or TU167 + 10 μg/mL cetuximab. CD137 expression on CD56+ NK cells was analyzed by FACS. Two representative experiments are shown. (B) Whole PBMCs were incubated in the presence of media, 10 μg/mL rituximab, 15μM zoledronate, or a combination of rituximab with zoledronate. The expression of CD69 on gated CD3−CD56+ NK cells was analyzed by FACS 96 hours after initiation of the cultures. A representative of 3 independent experiments is depicted. (C) Whole PBMCs were cultured with media (circles) or zoledronate (squares). Alternatively, γδ T lymphocyte–depleted PBMCs were cultured with zoledronate (triangles) for 96 hours. NK cells were purified from the groups described. NK-cell direct cytotoxicity (left plots) or antibody-dependent cellular cytotoxicity (right plots) was measured in a standard 4-hour 51Cr-release assay against TU167 SCCHN or Ramos B-cell lymphoma targets. Data are presented as mean ± SD of triplicate samples and are representative of 2 independent experiments. *P < .05 compared with NK cells purified from γδ T lymphocyte–depleted cultures.
To build on this finding, we next evaluated whether clinically relevant agents that activate γδ T lymphocytes can be used in combination with mAb opsonized tumors to enhance tumor killing through direct cytolysis and ADCC. Coculture of PBMCs with rituximab (a clinical-grade mAb recognizing CD20 on B cells) and Zometa (zoledronic acid), a bisphosphonate approved for clinical use that induces activation of γδ T lymphocytes, results in notable up-regulation of CD69 on NK cells (Figure 7B). Interestingly, culturing PBMCs with zoledronic acid alone also induces NK-cell activation (Figure 7B).
To verify the effects of γδ T lymphocyte activation on NK-cell cytotoxicity in physiologic conditions, we cultured whole PBMCs with Zometa. NK cells were purified from stimulated cultures and their direct cytotoxicity and ADCC was evaluated in standard 4-hour Cr-release assays. As shown in Figure 7, incubation of PBMCs with Zometa significantly increases direct cytolytic activity of NK cells against SCCHN (TU167) and B-cell lymphoma (Ramos) targets. Interestingly, depletion of γδ T lymphocytes before culturing PBMCs with Zometa significantly reduced but did not completely abrogate the effects of Zometa on direct NK cytotoxicity.
To determine whether the interplay of Zometa and opsonized tumor can enhance killing of SCCHN, we employed a combinatorial approach. As expected, we observed higher levels of NK-mediated cytotoxicity against TU167 and Ramos targets in the presence of appropriate Abs (cetuximab and rituximab, respectively). Nevertheless, culturing PBMCs with Zometa significantly enhanced NK-mediated ADCC. Moreover, depletion of γδ T lymphocytes from PBMCs before the addition of Zometa reduced NK killing to the level of cytotoxicity observed in NK cells purified from PBMCs cultured without Zometa (Figure 7). This observation indicates that γδ T lymphocytes are critical for the Zometa-induced increase of NK killing of SCCHN and B-cell lymphoma. Furthermore, these results define the physiologic and clinical relevance of the interaction between γδ T lymphocytes and NK cells for the regulation of direct and antibody-dependent NK cytotoxicity.
Discussion
Although the role of CD137 signaling in the activation of cytolytic αβ T lymphocytes is well established,26 our results provide the first evidence that CD137 engagement costimulates antitumor function of hIgG1-primed NK cells. These findings suggest that 2 signals are required for the optimal activation of NK cells in a manner similar to the 2-signal model established for αβ T-lymphocyte activation. This idea is relevant to the improvement of mAb-based cancer immunotherapy where a combination of mAbs specific to tumor Ag(s) with CD137 cross-linking induces direct NK cell–mediated tumor lysis. Specifically, our findings suggest that combination of Ab-based cancer immunotherapies with either adoptive transfer of in vitro expanded γδ T lymphocytes or systemic injection of γδ T-lymphocyte stimulating agents (zoledronic acid)8-10 may improve clinical outcome through the enhancement of direct and ADDC tumor killing by NK cells.
While CD137/CD137L interactions partially account for the activation potential of γδ T lymphocytes on hIgG1-stimulated NK cells, other costimulatory molecules are likely to also play a role. Although it is reported that NK-cell activation can be triggered by CD80 and CD86,27,28 our study did not reveal known receptors for these ligands on the surface of stimulated NK cells. Similarly, CD152Ig did not block the activation of NK cells by γδ T lymphocytes, suggesting that CD80 and CD86 are not involved in the activation of NK cells. Furthermore, in contrast to previous reports describing the presence of functional ICOS on murine NK cells, we found no expression of ICOS on human NK cells.29 Taken in concert, our data suggest that other costimulatory and/or adhesion molecules expressed on the surface of activated γδ T lymphocytes are involved in the activation of NK cells' antitumor properties. The identification of these costimulatory molecules may improve Ab- and NK cell–based cancer immunotherapy.
Activated NK and CD8+ αβ T cells express the NKG2D receptor that recognizes specific ligands (ULBPs and MIC A/B) presented on tumors.30 It has been shown that CD137 regulates the expression of NKG2D in human cord blood CD8 T lymphocytes.26 Our data indicate that CD137 engagement is important for the induction of NKG2D receptor expression on NK cells by γδ T lymphocytes. Moreover, anti-NKG2D mAb significantly inhibits the cytolytic potential of γδ T lymphocyte–stimulated NK cells against tumor cell lines, suggesting that the increased NKG2D expression mediated by CD137 augments the cytolytic potential of NK cells. However, it is conceivable that other molecules expressed on γδ T lymphocyte–activated NK cells are also involved in the killing of tumors, because anti-NKG2D mAb blocking did not completely abrogate cytolytic activity.
Our previous data indicate that long-term culture (14 days) of PBMCs with agents specifically activating γδ T lymphocytes (ie, IPP+IL-2) increases the cytolytic activity of NK cells.4 This NK-cell activation was mediated by soluble factors released by γδ T lymphocytes during the culture. In contrast, our current results indicate that activation of NK cells in short-term culture (48 hours) with expanded γδ T lymphocytes requires cell-to-cell contact and priming with immobilized hIgG1. These findings suggest pleiotropic mechanisms of NK-cell function regulation by activated γδ T lymphocytes. The direct physiologic role of NK- and γδ T-cell interaction in diseases and the maintenance of immune responses remain to be determined.
The clinical significance of γδ T lymphocytes and NK-cell interaction were confirmed by experiments using PBMCs cultured with clinically applicable reagents for the treatment of patients with SCCHN and lymphoma. Our data indicate that culturing PBMCs with Zometa increases direct and Ab-dependent NK cytotoxicity against SCCHN and lymphoma targets. It is conceivable that γδ T lymphocytes are more important for the regulation of ADCC because γδ T-cell depletion had only partial impact on zoledronic acid–induced direct NK-cell cytotoxicity. However, other molecular and cellular targets of zoledronic acid that are involved in the regulation of direct NK-cell cytotoxicity remain to be determined. Overall, our data suggest that administration of γδ T-cell activating agents may improve antitumor effects of cetuximab and rituximab used for the treatment of patients with SCCHN and B-cell lymphoma, respectively.
In summary, it has been shown that in vitro expanded γδ T lymphocytes can improve adaptive immune responses against tumor Ags, by effectively presenting tumor Ags to conventional αβ T lymphocytes.17,18 Our data indicate that in vitro culture with γδ T-lymphocyte activating agents (IPP or Zometa) can also improve antitumor innate function, as determined by increased NK-cell cytotoxicity. Thus, activation of γδ T lymphocytes in vivo or adoptive transfer of in vitro expanded γδ T lymphocytes has the potential to improve existing strategies for cancer immunotherapy. In particular, a combination of tumor-specific mAbs that engage Fc receptors on NK cells (cetuximab or rituximab)31 and γδ T-lymphocyte activating agents approved for clinical use (eg, Zometa)32,33 may improve existing cancer immunotherapy by stimulating both the adaptive and innate antitumor immunity. Of perhaps greater import, this activation strategy may overcome pre-existing defects in NK-cell function recognized to exist in patients with large tumor burdens, further augmenting the clinical utility of this strategy.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work is partially supported by grants from the Milheim Foundation for Cancer Research and the American Cancer Society and funds from the Paul Calabresi Clinical Oncology Training Program to A.I.C. Part of this work was supported by a generous grant from the Orakawa Foundation.
Authorship
Contribution: A.M. performed the experiments, analyzed the data, and wrote the manuscript; X.Z. and W.L. helped perform individual experiments; B.R.G. and C.D.P. designed the study and analyzed data; and S.E.S. and A.I.C. designed the study, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: S.E.S. is a cofounder and major stockholder in Gliknik, a biotechnology company. He also receives royalties from the Mayo Clinic College of Medicine for IP related to CD137 that is licensed to third parties. The remaining authors declare no competing financial interests.
Correspondence: Andrei Chapoval, PhD, HSF-1, Rm 334, Office 325C, 685 W Baltimore St, Baltimore, MD 21201; e-mail: achapoval@smail.umaryland.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal