Abstract
The use of dendritic cells (DCs) in therapeutic cancer vaccination requires their loading with tumor-specific antigen(s). DEC-205, a phagocytosis receptor mediating antigen uptake, is associated with CD8+ T-cell responses in mice. Here we fused an anti–DEC-205scFv to an HLA-DP4–restricted epitope from the tumor antigen MAGE-A3, and examined the suitability and efficacy of DEC-205 to deliver a helper epitope to human monocyte-derived DCs (moDCs). The construct specifically bound DEC-205 on human moDCs without negative impact on DC phenotype and function. We measured antigen presentation with specific autologous CD4+ T cells, generated by TCR-RNA transfection. DEC-205 targeting resulted in significant major histocompatibility complex class II–restricted antigen presentation, and was superior to loading DCs by electroporation of mRNA encoding endosome-targeted MAGE-A3-DCLAMP or by direct peptide pulsing. Anti–DEC-205scFv-MAGE-A3 was presented 100 times more efficiently than the control constructs. DC maturation before or during incubation with anti–DEC-205scFv-MAGE-A3 reduced the interleukin-10/interleukin-2 ratio. Moreover, we successfully applied the DEC-205 targeting strategy to moDCs from malignant melanoma patients. Again, DEC-205–targeted mature DCs (mDCs) presented the antigen more efficiently than peptide-pulsed DCs and maintained their stimulatory capacity after cryoconservation. Thus, DEC-205 targeting represents a feasible and effective method to deliver helper epitopes to DCs in anticancer vaccine strategies, which may also be suitable for DC targeting in vivo.
Introduction
Uptake and presentation of antigens (Ag) by dendritic cells (DCs) are a prerequisite for the induction of Ag-specific immune responses.1,2 In absence of danger signals, DCs remain immature (iDCs) and contribute to the maintenance of tolerance. In contrast, when danger signals are present, DCs mature and induce Ag-specific immunity.3 In the last decades, great efforts have been made to improve DC vaccination approaches for cancer immunotherapy. In several clinical trials, ex vivo–generated, most commonly, monocyte-derived DCs (moDCs) were administered. These DCs were loaded with tumor Ag by various methods, including direct pulsing with tumor Ag-derived peptides, and electroporation with RNA encoding tumor Ag.3-7 Although DC vaccines proved immunogenic and showed first promising clinical results, the overall clinical efficacy still remains unsatisfactory.8,9 To improve cancer vaccination, DC vaccines must be optimized in various ways, to induce effective T-cell responses, including CD4+ T helper cells, whose important role in the priming and boosting of long-lasting high-avidity effector and memory T cells becomes increasingly prominent.3
As Ag uptake is their natural function, DCs possess several receptors that mediate Ag uptake and presentation. These receptors can be used to target Ag of choice to DCs ex vivo and in vivo.4 DEC-205 (CD205) is an endocytosis-mediating receptor that belongs to the C-type lectin receptor family. In mice, DEC-205 is expressed on cortical thymic epithelium, thymic medullary DCs (CD11c+/CD8+), and subsets of peripheral DCs (CD11c+/CD8+ splenic/lymph node DCs, dermal/interstitial DCs, and Langerhans cells).10 Targeting DEC-205 with antibody (Ab)–Ag fusion proteins combined with maturation stimuli has led to Ag-specific immune responses in mice.11,12 Moreover, DEC-205–targeted delivery of the melanoma tumor Ag gp100 and tyrosinase-related protein-2 (TRP-2) cured 70% of tumor-bearing mice.13 Although DEC-205 contains a motive that entails efficient recycling through late endosomes, thus enhancing major histocompatibility complex (MHC) class II presentation,14 DEC-205+ DCs seem to be specialized in cross-presentation of Ag to CD8+ T cells in mice,15 although also presentation to CD4+ T cells after DEC-205 targeting of Langerhans cells was reported.16 In humans, DEC-205 is expressed at relatively high levels on myeloid blood DCs, but in contrast to mice, expression is also present on monocytes and B lymphocytes, and at low levels on NK cells, plasmacytoid blood DCs, and T lymphocytes.17 In vitro, Ab-mediated delivery of HIV gag p24 protein to DEC-205 on human moDCs resulted in the presentation of different MHC class I–restricted peptides.18 Furthermore, targeting the nuclear-Ag-1 of Epstein-Barr virus to human DEC-205 elicited a protective T-cell response in a mouse model with reconstituted components of the human immune system.19
The use of single-chain Fragments variable (scFv), instead of complete Abs, offers several advantages. Because of their smaller size, scFvs penetrate tissue better than whole Abs.20 Furthermore, scFvs can be produced more economically than whole Abs.21 Most importantly, scFvs lack an Fc domain and can therefore be administered repeatedly without inducing a deleterious host Ab response.22 Moreover, they do not bind to Fc receptors expressed on DCs and various other cell types, that may reduce unspecific uptake, improving DEC-205–specific Ag delivery. DNA vaccines consisting of vectors containing the sequence for the DEC-205 scFv and the mycobacterium tuberculosis-derived Ag85B gene22 or other Ag, such as ovalbumin, HIV gag, or HIV p24,23 have elicited Ag-specific immune responses in mice. In addition, liposomes containing ovalbumin protein and decorated with anti–DEC-205 scFvs,24 as well as an anti–DEC-205 scFv-gp100 fusion construct25 have been successful in DEC-205–targeted Ag-delivery to mouse DCs, resulting in Ag-specific activation of the immune system in mice.
Although some CD4+ T-cell reactions were observed after DEC-205 targeting with viral epitopes,19 the use of anti–DEC-205 scFvs to load human DCs with helper epitopes has not yet been thoroughly investigated. Therefore, we generated an scFv directed against DEC-205 fused to a MAGE-A3 peptide (AA 243-258, KKLLTQHFVQENYLEY), which is presented on HLA-DP4 molecules, to load human DCs. MAGE-A3 is a cancer-testis Ag that is highly expressed in malignant melanoma, multiple myeloma, head and neck squamous cell carcinoma, lung carcinoma, and breast carcinoma, but not in normal tissues, except for testis.26,27 To analyze the Ag-loading efficiency of our scFv-Ag fusion protein, we targeted ex vivo–generated moDCs. This strategy allowed us to quantify the presentation and to carefully validate the quality of the DEC-205–targeted DCs.
Methods
Cells and reagents
DCs were generated from monocytes of healthy donors and malignant melanoma patients (obtained after informed consent and approved by the institutional review board in accordance with the Declaration of Helsinki) and cultured as described.28,29 DC maturation was performed with 200 IU/mL interleukin-1β (IL-1β; Cellgenix), 1000 U/mL IL-6 (Cellgenix), 10 ng/mL tumor necrosis factor (TNF; Beromun, Boehringer Ingelheim Pharma), and 1 μg/mL prostaglandin E2 (PGE2; Pfizer). CD4+ T cells were isolated ex vivo from fresh or cryoconserved nonadherent fractions or cryoconserved elutriation fraction 3 using anti-CD4 MACS beads (Miltenyi Biotec). T cells were cultured in T-cell medium containing RPMI 1640, 10% human serum (Lonza), 2mM l-glutamine, 20 mg/mL gentamycin, 10mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (PAA), 1mM sodium pyruvate (PAA), 1% modified Eagle medium nonessential amino acids (100×, PAA), with 10 ng/mL IL-7 (PeproTech). Cells were cryopreserved as described.29,30 HLA-DP4-binding peptides in this study were MAGE-A3(243-258) KKLLTQHFVQENYLEY (Clinalfa Merck) and NY-ESO-1(157-170) SLLMWITQCFLPVF (Clinalfa Merck).
Cloning of the MAGE-A3/HLA-DP4–specific TCR
The sequences coding for the MAGE-A3/HLA-DP4–specific TCR-α-chain (Va22) and β-chain (Vb13; provided by E. Schultz, Nuremberg Hospital, Nuremberg, Germany)31 were cloned into an RNA-production vector32 (provided by K. Thielemans, VUB, Brussels, Belgium). The TCR was modified by introduction of one cysteine according to Cohen et al.33
Production of in vitro transcribed RNA
The pGEM4Z-5′UTR-sig-MAGE-A3-DC.LAMP-3′UTR vector was provided by K. Thielemans, and the pGEM4Z-Mage-A3-64A vector was provided by Argos Therapeutics. RNA production was performed as described before.29
Composition of scFv-MAGE-A3 fusion proteins
As depicted in Figure 2A, the anti–DEC-205scFv-MAGE-A3-KKL and the anti–MCSPscFv-MAGE-A3-KKL constructs consisted of an anti–human DEC-205scFv (based on the clone MG38-3, IgG2B; provided by Prof R. Steinman, Rockefeller University, New York, NY) or an scFv against human melanoma-associated chondroitin sulfate proteoglycan (MCSP) fused to the HLA-DP4–restricted MAGE-A3-KKL peptide (AA 241-258, dpKKLLTQHFVQENYLEY) as well as the 2 N-terminally situated amino acids (DP; Figure 2A). Lipopolysaccharide concentration was tested to be less than 5 pg/mL. For sequence and detailed expression protocol, see supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Flow cytometric analysis
Stainings were performed in phosphate-buffered saline with 1% fetal calf serum and 0.02% NaN3 (Roth), for 30 minutes on ice. For determination of the binding capacity of the scFv chimeras, cells were incubated with 1 μg/mL anti–DEC-205scFv-MAGE-A3-KKL or anti–MCSPscFv-MAGE-A3-KKL protein, and the bound proteins were detected using a murine anti–penta-His Ab (QIAGEN) and a phycoerythrin-conjugated goat-anti–mouse-IgG (GAM-PE) Ab (BD Biosciences). To determine the binding capacity of the parental Ab, cells were incubated with an anti–DEC-205-fluorescein isothiocyanate (FITC) Ab (eBioscience) or an anti–DEC-205-PE Ab (BD Biosciences), or with a solution containing 5 μg/mL of murine mAb 9.2.27 specific for MCSP. Bound primary Abs were detected with GAM-PE. The DC phenotype was determined with: anti–DEC-205-FITC or anti–DEC-205-PE, anti–CD14-PE, anti–CD83-PE, anti–CD25-PE, anti–CD70-PE Ab (all from BD Biosciences), and anti–CCR7-FITC (R&D Systems), and the corresponding isotype controls. Stained cells were analyzed using a FACScan flow cytometer equipped with CellQuest software Version 3.3 (BD Biosciences). Specific mean fluorescence intensity (MFI) and percentage positive cells were calculated by subtracting the MFI or percentage positive cells obtained by control staining. Competition assays were performed by preincubating the DCs with different amounts of the FITC-labeled parental anti–DEC-205 Ab in the indicated molar ratios for 10 minutes on ice. Then the anti–DEC-205scFv-MAGE-A3-KKL construct was added to a final concentration of 1 μg/mL for 30 minutes. The construct was detected with anti–Strep-tag-PE, and cells were analyzed by flow cytometry. Binding assays with freshly isolated peripheral blood mononuclear cells (PBMCs) were performed as follows: LiveDead aqua (Invitrogen) was used according to the manufacturer to exclude dead cells. Cells were incubated with 3 μg/mL of the anti–DEC-205scFv-MAGE-A3-KKL construct or the anti–MCSPscFv-MAGE-A3-KKL control construct on ice for 30 minutes, and the constructs were detected with anti–Penta-His and GAM-PE. Then V450- or Pacific Blue–coupled Abs against CD3, CD14, CD16, CD19, and CD56 (all from BD Biosciences) were used as non-DC lineage markers, and DCs were defined with anti–CD11c-FITC, CD33-APC, HLA-DR-APC-H7, and CD123-PerCP-Cy5.5 Abs (all from BD Biosciences). Myeloid DCs were defined as Lin−, HLA-DR+, CD11c+, CD33+, and CD123−. Plasmacytoid DCs were defined as Lin−, CD11c−/low, and CD123+. Non DC lymphocytes were defined as Lin+ cells in the lymphocyte gate.
Loading of DCs with MAGE-A3
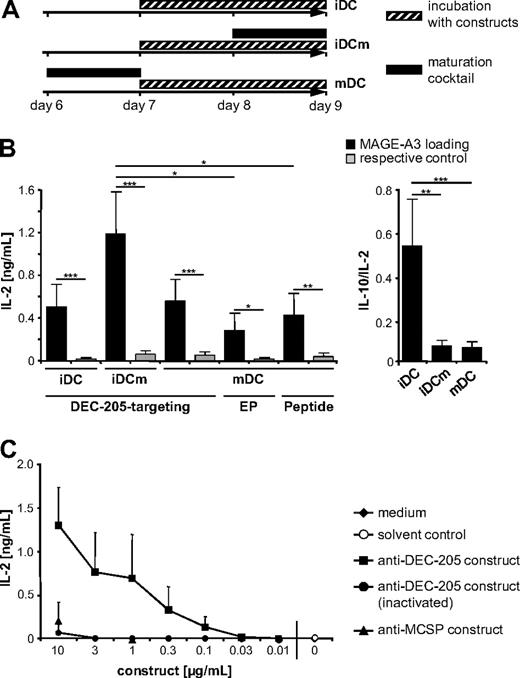
On day 6 of culture, DCs were matured (mDCs) with the maturation cocktail or were left immature (iDC; Figure 3A). DCs (1 × 106/mL) were incubated for 48 hours with the anti–DEC-205scFv-MAGE-A3-KKL construct at a concentration of 1 μg/mL or at indicated concentrations (Figure 3A). After 24 hours of incubation with the construct, a fraction of the iDCs was matured for 24 hours (iDCm; Figure 3A). As respective controls, 1 × 106/mL DCs were treated with the heat-inactivated anti–DEC-205scFv-MAGE-A3-KKL protein or with the anti–MCSPscFv-MAGE-A3-KKL protein at a concentration of 1 μg/mL or at indicated concentrations. Additional controls were performed using the same volume of solvent or medium alone. In addition, mDCs were electroporated with 30 μg MAGE-A3-DCLAMP RNA32 or with 30 μg control MAGE-A3 RNA as described.34 After electroporation, the cells were rested for 24 hours before they were used in stimulation assays. For direct peptide pulsing, 1 × 106 DCs per mL were incubated for 3 hours with 10 μg/mL of the MAGE-A3/HLA-DP4 peptide or the NY-ESO-1/HLA-DP4 control peptide.
Cytokine-secretion assays
MAGE-A3/HLA-DP4–specific CD4+ T cells were generated by RNA electroporation as described,29 rested for 4 hours, and then coincubated at a 1:1 ratio with DCs that had been Ag-loaded as described in the previous paragraph. Coincubation was performed for 16 to 18 hours in T-cell medium containing 10 ng/mL IL-7. The supernatants were analyzed for cytokines using a TH1/TH2 cytometric bead array (BD Biosciences) according to the manufacturer's protocol.
Results
DEC-205 is expressed on immature and mature human moDCs
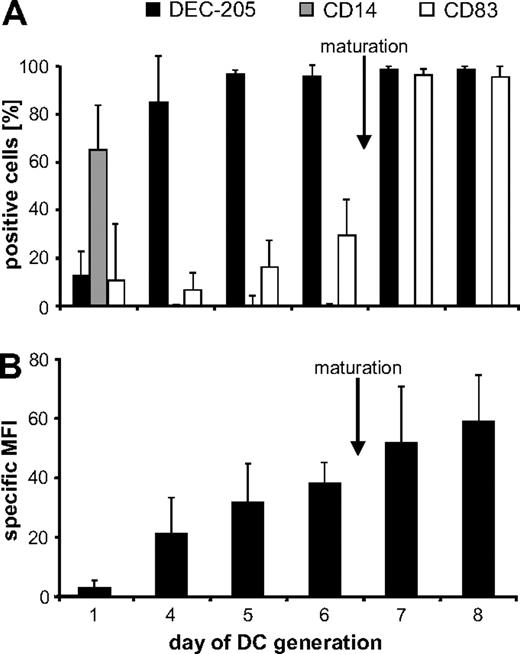
DEC-205 is expressed on different murine and human DC populations and can therefore be used to target Ag to these DCs. For DC vaccination, moDCs are most commonly used. Hence, we first followed the expression of DEC-205 during the development and the maturation of human moDCs (Figure 1; supplemental Figure 1). Successful maturation was monitored by staining for the maturation marker CD83. More than 95% of the cells were positive 24 hours after administration of the maturation cocktail (Figure 1A; supplemental Figure 1). The monocyte marker CD14 was no longer detected from day 4 onwards, indicating that the cells had already begun to differentiate into DCs at this time (Figure 1A). DEC-205 expression constantly increased during DC development, as indicated by the increasing specific MFI (Figure 1B; supplemental Figure 1). Virtually all immature (i)DCs were DEC-205 positive (Figure 1A day 6); however, the expression level further increased during maturation (Figure 1B; supplemental Figure 1).
DEC-205 expression kinetics during the development from monocytes to DCs. To generate moDCs, the plastic-adherent fraction of PBMCs from healthy donors was cultured in medium supplemented with granulocyte-macrophage colony-stimulating factor and IL-4. After 6 days of culture, cells were matured for an additional 48 hours with IL-1β, IL-6, PGE2, and TNF. Cells were harvested on days 1, 4, 5, 6, 7, and 8, and analyzed for DEC-205, CD14, and CD83 expression (A). Furthermore, the specific MFI (ie, MFI with DEC-205–specific Ab−; MFI of isotype control) of DEC-205 expression was determined (B). Data are mean values ± SD from 3 independent experiments.
DEC-205 expression kinetics during the development from monocytes to DCs. To generate moDCs, the plastic-adherent fraction of PBMCs from healthy donors was cultured in medium supplemented with granulocyte-macrophage colony-stimulating factor and IL-4. After 6 days of culture, cells were matured for an additional 48 hours with IL-1β, IL-6, PGE2, and TNF. Cells were harvested on days 1, 4, 5, 6, 7, and 8, and analyzed for DEC-205, CD14, and CD83 expression (A). Furthermore, the specific MFI (ie, MFI with DEC-205–specific Ab−; MFI of isotype control) of DEC-205 expression was determined (B). Data are mean values ± SD from 3 independent experiments.
As the majority of both iDCs and mature (m)DCs were DEC-205-positive in the human system, we investigated whether these DC populations could be loaded with tumor Ag by DEC-205 targeting.
Construction of an scFv-MAGE-A3-chimera targeting the endocytosis receptor DEC-205 on human DCs
To load human moDCs with tumor Ag by DEC-205 targeting, we designed a construct consisting of an scFv directed against DEC-205 fused to the HLA-DP4-resticted peptide epitope KKLLTQHFVQENYLEY31 of the cancer-testis Ag MAGE-A3 (Figure 2A). The construct carried an N-terminal Strep-tag and a C-terminal His-tag for purification and detection (Figure 2A). In addition, an scFv-Ag control construct was generated, which was similar in design and contained the same antigenic peptide but an scFv directed against the surface molecule MCSP, which is not expressed on DCs (Figure 2A). Both proteins were transiently expressed in 293T cells and purified via their His tags.
Design and specific binding of the anti–DEC-205scFv-MAGE-A3-KKL and the anti–MCSPscFv-MAGE-A3-KKL fusion proteins. (A) Schematic presentation of the anti–DEC-205scFv-MAGE-A3-KKL (termed DK hereafter) and the anti–MCSPscFv-MAGE-A3-KKL (termed MK hereafter) fusion proteins. Estimated molecular weights are indicated. S indicates N-terminal Strep-tag; VL and VH, variable region light and heavy chain, respectively, of the scFv; (G4S)3 or (G4S)4, 15 or 20 amino acid linker composed of 3 or 4 repeats of the Gly4Ser unit, respectively; G4SA3, 8 amino acid linker composed of one Gly4Ser unit followed by an Ala3 unit; MAGE-A3-KKL, nucleotide sequence of MAGE-A3, presented in HLA-DP4; and H, hexahistidine-tag. (B) iDCs, mDCs, and the MCSP-positive cell line A2058 were incubated with the anti–DEC-205scFv-MAGE-A3-KKL and with the anti–MCSPscFv-MAGE-A3-KKL control proteins, and stained with murine anti–His-tag Ab and GAM-PE Ab. As a negative control, the cells were stained only with anti–His-tag Ab and GAM-PE Ab. (Bottom panels) Staining of the same cells with the FITC-labeled anti–DEC-205 parental antibody or with the anti-MCSP parental Ab and GAM-PE. As a negative control, these cells were stained with an IgG2b-FITC isotype control or GAM-PE only. (C) Preincubation with different concentrations of the parental Ab. mDCs were preincubated with the FITC-labeled parental Ab in different molar ratios to the construct. Then the mDCs were incubated with 1 μg/mL of the anti–DEC-205scFv-MAGE-A3-KKL for 30 minutes, and the construct was detected with PE-labeled anti–Strep-tag Ab. Signals were normalized to stainings with the parental Ab or the construct in absence of each other. (D) Binding of the construct to DCs in human blood. PBMCs were incubated with the anti–DEC-205scFv-MAGE-A3-KKL (DK) or with the anti–MCSPscFv-MAGE-A3-KKL (MK) constructs or stained with the parental DEC-205–specific Ab (α-DEC-205 Ab). Plasmacytoid DCs and myeloid DCs were counterstained and analyzed by multicolor flow cytometry, and lymphocytes were gated as negative control.
Design and specific binding of the anti–DEC-205scFv-MAGE-A3-KKL and the anti–MCSPscFv-MAGE-A3-KKL fusion proteins. (A) Schematic presentation of the anti–DEC-205scFv-MAGE-A3-KKL (termed DK hereafter) and the anti–MCSPscFv-MAGE-A3-KKL (termed MK hereafter) fusion proteins. Estimated molecular weights are indicated. S indicates N-terminal Strep-tag; VL and VH, variable region light and heavy chain, respectively, of the scFv; (G4S)3 or (G4S)4, 15 or 20 amino acid linker composed of 3 or 4 repeats of the Gly4Ser unit, respectively; G4SA3, 8 amino acid linker composed of one Gly4Ser unit followed by an Ala3 unit; MAGE-A3-KKL, nucleotide sequence of MAGE-A3, presented in HLA-DP4; and H, hexahistidine-tag. (B) iDCs, mDCs, and the MCSP-positive cell line A2058 were incubated with the anti–DEC-205scFv-MAGE-A3-KKL and with the anti–MCSPscFv-MAGE-A3-KKL control proteins, and stained with murine anti–His-tag Ab and GAM-PE Ab. As a negative control, the cells were stained only with anti–His-tag Ab and GAM-PE Ab. (Bottom panels) Staining of the same cells with the FITC-labeled anti–DEC-205 parental antibody or with the anti-MCSP parental Ab and GAM-PE. As a negative control, these cells were stained with an IgG2b-FITC isotype control or GAM-PE only. (C) Preincubation with different concentrations of the parental Ab. mDCs were preincubated with the FITC-labeled parental Ab in different molar ratios to the construct. Then the mDCs were incubated with 1 μg/mL of the anti–DEC-205scFv-MAGE-A3-KKL for 30 minutes, and the construct was detected with PE-labeled anti–Strep-tag Ab. Signals were normalized to stainings with the parental Ab or the construct in absence of each other. (D) Binding of the construct to DCs in human blood. PBMCs were incubated with the anti–DEC-205scFv-MAGE-A3-KKL (DK) or with the anti–MCSPscFv-MAGE-A3-KKL (MK) constructs or stained with the parental DEC-205–specific Ab (α-DEC-205 Ab). Plasmacytoid DCs and myeloid DCs were counterstained and analyzed by multicolor flow cytometry, and lymphocytes were gated as negative control.
Specific binding of the anti–DEC-205scFv-MAGE-A3-KKL fusion protein and the anti–MCSPscFv-MAGE-A3-KKL control protein
The anti–DEC-205scFv-MAGE-A3-KKL fusion protein reacted with moDCs in an Ag-specific manner (Figure 2B). The protein bound to iDCs and to mDCs but not to the DEC-205-negative melanoma cell line A2058. In contrast, the control protein anti–MCSPscFv-MAGE-A3-KKL only bound to MCSP-expressing A2058 cells, but not to DCs (Figure 2B). Binding of the scFv-Ag fusion proteins was similar to binding of the corresponding parental Ab (Figure 2B bottom panels). The DEC-205 specificity of the binding was confirmed by preincubating mDCs with FITC-labeled parental Ab at different concentrations before adding the anti–DEC-205scFv-MAGE-A3-KKL construct (Figure 2C). A 10-fold molar excess of the Ab almost completely blocked the binding of the construct, whereas one-tenth of the molarity did not influence the binding of the construct. Staining DEC-205–transfected CHO cells and DEC-205-negative CHO 16-10 cells further confirmed the specificity (supplemental Figure 2). Incubation of PBMCs with the anti–DEC-205scFv-MAGE-A3-KKL fusion protein resulted in specific binding to plasmacytoid and myeloid DCs in a similar pattern to the parental Ab, whereas the anti–MCSPscFv-MAGE-A3-KKL control construct did not bind to these cell populations (Figure 2D). Hence, we were able to produce a DEC-205-binding Ab-Ag construct and a corresponding control construct.
DEC-205 targeting has no influence on DC phenotype, cytokine secretion, and migration capacity
We assured that DEC-205 targeting leaves phenotype and functionality of the DCs unaltered to be able to interpret the subsequent results, and to ascertain the immunotherapeutic potential of DCs treated in this manner in cancer vaccine applications. The incubation of DCs with the anti–DEC-205scFv-MAGE-A3-KKL chimera did not change the phenotype (supplemental Figure 3A,C). Furthermore, the cytokine profile as well as the migratory capacity of the DC to a CCL19 gradient (supplemental Figure 3B,D) were not altered by DEC-205 targeting. Thus, DEC-205–targeted DCs are suitable for the use in anticancer DC vaccine applications.
DEC-205–targeted DCs have a higher T cell–stimulatory capacity than RNA-electroporated or peptide-pulsed DCs
Next, we determined the HLA-DP4–restricted presentation of the MAGE-A3 epitope by DCs that were DEC-205–targeted or Ag-loaded by other methods. To this end, autologous Ag-specific CD4+ T cells were used, which were generated by electroporation of RNA encoding a MAGE-A3/HLA-DP4–specific TCR.29 DCs were incubated for 48 hours with the anti–DEC-205scFv-MAGE-A3-KKL fusion protein that binds to DEC-205 and contains the MAGE-A3 peptide or the anti–MCSPscFv-MAGE-A3-KKL control construct that contains the same peptide but binds MCSP. A fraction of the DCs was matured before the incubation (mDCs), some during the incubation (iDCm), and some were left immature (iDCs). A schematic timeline of loading and maturation is given in Figure 3A. The DCs were then cocultured with the MAGE-A3/HLA-DP4–specific CD4+ T cells for 16 to 18 hours, and activation of the CD4+ T cells was assayed by measuring IL-2 secretion. The DEC-205–targeted iDCs, iDCm, and mDCs induced a significantly higher activation of MAGE-A3/HLA-DP4–specific CD4+ T cells (Figure 3B black bars) than DCs incubated with the anti–MCSPscFv-Ag control fusion protein (Figure 3B gray bars). This indicates that DEC-205 targeting indeed resulted in class II presentation of the fused Ag and led, in turn, to Ag-specific T-cell stimulation. DEC-205 targeting of iDCm resulted in the highest capacity to stimulate Ag-specific T cells (Figure 3B). To evaluate the reason for these differences between the differently matured DCs, we examined the surface half-life of the anti–DEC-205scFv-Ag fusion protein but observed no difference (supplemental Figure 4).This indicates that different rates of internalization are not the reason for the observed differences.
IL-2 secretion by MAGE-A3/HLA-DP4–specific CD4+ T cells after stimulation with differently loaded DCs. (A) Ag loading and maturation of DCs during this study. Depicted is a scheme indicating at which time point and at which stage DCs were loaded with the constructs and at which time point the DCs were matured. The plastic-adherent fraction of PBMCs from healthy donors was cultured in medium supplemented with granulocyte-macrophage colony-stimulating factor and IL-4. After 6 days of culture, cells were matured (black bar) for 24 hours with IL-1β, IL-6, PGE2, and TNF (mDCs), or were left immature (iDCs). iDCs and mDCs were incubated for 48 hours with the anti–DEC-205scFv-MAGE-A3-KKL or anti–MCSPscFv-MAGE-A3-KKL constructs (▨). After 24 hours of incubation with the constructs, a fraction of the iDCs was matured with the maturation cocktail (■) for 24 hours (iDCm). This ultimately leads to 3 different Ag-loaded DC populations, which were used in subsequent experiments. (B) Comparison of the stimulatory capacity of DCs loaded with MAGE-A3 by DEC-205 targeting, RNA electroporation, or direct peptide pulsing. DCs were either matured (mDCs) or were left immature (iDCs) and were incubated with 1 μg/mL of the anti–DEC-205scFv-MAGE-A3-KKL or the anti–MCSPscFv-MAGE-A3-KKL construct (as respective control) for 48 hours. A fraction of the iDCs was matured during the incubation (iDCm). In addition, mDCs were electroporated with MAGE-A3-DCLAMP RNA or MAGE-A3 RNA (as respective control) and pulsed with MAGE-A3/HLA-DP4 or NY-ESO-1/HLA-DP4 (as respective control) peptides. All these differently loaded DC populations were used to stimulate MAGE-A3/HLA-DP4–specific autologous CD4+ T cells, which were generated by TCR-RNA electroporation (“Cytokine-secretion assays”). IL-2 secretion by specific T cells was analyzed after 18 hours of coculture. Furthermore, the ratio between IL-10 to IL-2 secretion after activation of specific T cells with DEC-205–targeted iDCs, iDCm, and mDCs was analyzed (right panel). P values were calculated with the Mann-Whitney U test. *P < .05. **P < .005. ***P < .001. (C) Dose-dependent IL-2 secretion by MAGE-A3/HLA-DP4–specific CD4+ T cells after stimulation with DEC-205–loaded iDCm, generated as described in panel A, except that they were loaded with different concentrations of anti–DEC-205scFv-MAGE-A3-KKL fusion protein (as indicated). As negative control, heat-inactivated anti–DEC-205scFv-MAGE-A3-KKL protein in the same concentrations, 10 μg/mL and 1 μg/mL of anti–MCSPscFv-MAGE-A3-KKL protein, medium, and solvent control (generated by similar protein-purification protocol from mock-transfected 293T cells) were used. These DCs were used to stimulate MAGE-A3/HLA-DP4–specific autologous CD4+ T cells; and after 18 hours of coincubation, IL-2 concentrations in the supernatants were measured. Data are mean values ± SD of 4 independent experiments. DK indicates anti–DEC-205scFv-MAGE-A3-KKL fusion protein; MK, anti–MCSPscFv-MAGE-A3-KKL fusion protein; and EP, electroporation.
IL-2 secretion by MAGE-A3/HLA-DP4–specific CD4+ T cells after stimulation with differently loaded DCs. (A) Ag loading and maturation of DCs during this study. Depicted is a scheme indicating at which time point and at which stage DCs were loaded with the constructs and at which time point the DCs were matured. The plastic-adherent fraction of PBMCs from healthy donors was cultured in medium supplemented with granulocyte-macrophage colony-stimulating factor and IL-4. After 6 days of culture, cells were matured (black bar) for 24 hours with IL-1β, IL-6, PGE2, and TNF (mDCs), or were left immature (iDCs). iDCs and mDCs were incubated for 48 hours with the anti–DEC-205scFv-MAGE-A3-KKL or anti–MCSPscFv-MAGE-A3-KKL constructs (▨). After 24 hours of incubation with the constructs, a fraction of the iDCs was matured with the maturation cocktail (■) for 24 hours (iDCm). This ultimately leads to 3 different Ag-loaded DC populations, which were used in subsequent experiments. (B) Comparison of the stimulatory capacity of DCs loaded with MAGE-A3 by DEC-205 targeting, RNA electroporation, or direct peptide pulsing. DCs were either matured (mDCs) or were left immature (iDCs) and were incubated with 1 μg/mL of the anti–DEC-205scFv-MAGE-A3-KKL or the anti–MCSPscFv-MAGE-A3-KKL construct (as respective control) for 48 hours. A fraction of the iDCs was matured during the incubation (iDCm). In addition, mDCs were electroporated with MAGE-A3-DCLAMP RNA or MAGE-A3 RNA (as respective control) and pulsed with MAGE-A3/HLA-DP4 or NY-ESO-1/HLA-DP4 (as respective control) peptides. All these differently loaded DC populations were used to stimulate MAGE-A3/HLA-DP4–specific autologous CD4+ T cells, which were generated by TCR-RNA electroporation (“Cytokine-secretion assays”). IL-2 secretion by specific T cells was analyzed after 18 hours of coculture. Furthermore, the ratio between IL-10 to IL-2 secretion after activation of specific T cells with DEC-205–targeted iDCs, iDCm, and mDCs was analyzed (right panel). P values were calculated with the Mann-Whitney U test. *P < .05. **P < .005. ***P < .001. (C) Dose-dependent IL-2 secretion by MAGE-A3/HLA-DP4–specific CD4+ T cells after stimulation with DEC-205–loaded iDCm, generated as described in panel A, except that they were loaded with different concentrations of anti–DEC-205scFv-MAGE-A3-KKL fusion protein (as indicated). As negative control, heat-inactivated anti–DEC-205scFv-MAGE-A3-KKL protein in the same concentrations, 10 μg/mL and 1 μg/mL of anti–MCSPscFv-MAGE-A3-KKL protein, medium, and solvent control (generated by similar protein-purification protocol from mock-transfected 293T cells) were used. These DCs were used to stimulate MAGE-A3/HLA-DP4–specific autologous CD4+ T cells; and after 18 hours of coincubation, IL-2 concentrations in the supernatants were measured. Data are mean values ± SD of 4 independent experiments. DK indicates anti–DEC-205scFv-MAGE-A3-KKL fusion protein; MK, anti–MCSPscFv-MAGE-A3-KKL fusion protein; and EP, electroporation.
Furthermore, we compared the DEC-205-loading strategy with the commonly used loading strategies, RNA electroporation and peptide pulsing. To facilitate MHC class II presentation after mRNA electroporation, a MAGE-A3-DC-LAMP (DC-specific lysosome-associated membrane glycoprotein) fusion protein was used.32 mDCs were electroporated with 30 μg MAGE-A3-DC-LAMP RNA or with MAGE-A3 wild-type RNA with an optimized protocol resulting in high protein expression.34 After 24 hours, the electroporated DCs were used to stimulate autologous MAGE-A3/HLA-DP4–specific CD4+ T cells. Peptide pulsing was performed for 3 hours with 10 μg/mL HLA-DP4-binding MAGE-A3 peptide and the NY-ESO-1/HLA-DP4 peptide as negative control, and the DCs were cocultured with the specific T cells immediately afterward. Both electroporation and peptide pulsing resulted in significant Ag presentation, but with a significantly lower efficiency than DEC-205 targeting of iDCm (Figure 3B), although the molarity of the peptide was more than 100-fold higher than that of the construct. In addition, a stronger Ag presentation was also observed for DEC-205–targeted iDCs and mDCs than for RNA-electroporated or peptide-pulsed DCs (Figure 3B).
Because iDCs can induce tolerance instead of immunity, we analyzed the production of IL-10 in relation to IL-2 on activation of the specific T cells with the DEC-205–targeted iDCs, iDCm, and mDCs (Figure 3B). Interestingly, iDCs induced a significantly higher secretion of IL-10 in relation to IL-2 than the iDCm and the mDCs. The use of iDCs is therefore unfavorable because of a potential induction of tolerance or immunosuppressive effects instead of immunity.
To evaluate the concentration of the fusion protein needed for efficient DEC-205 targeting of DCs, we incubated iDCm with different concentrations of anti–DEC-205scFv-MAGE-A3-KKL construct in the active or heat-inactivated state. As negative controls, cells were also incubated with 10 and 1 μg/mL control protein or with the same volume of solvent or medium. After 48 hours of incubation, the iDCm were used to stimulate MAGE-A3/HLA-DP4–specific CD4+ T cells, and IL-2 secretion was measured. Loading of DCs with the anti–DEC-205scFv-MAGE-A3-KKL fusion protein resulted in a far more efficient T-cell stimulation than the incubation with the control proteins, which required a 100-fold higher concentration for a similar degree of T-cell stimulation (Figure 3C). From 0.1 to 1 μg/mL, the stimulatory capacity of the DCs correlated with the concentration of the construct but remained almost constant for concentrations between 1 and 3 μg/mL. After loading with 10 μg/mL, the stimulatory capacity further increased, but at this concentration unspecific uptake of the control proteins was also observed (Figure 3C). Therefore, 1 μg/mL of fusion protein appears to be the most suitable concentration for DEC-205 targeting of DCs.
Taken together, as the DEC-205 targeting of iDCm led to a significantly increased stimulatory capacity of Ag-specific CD4+ T cells over RNA electroporation and direct peptide pulsing, this technique therefore presents an attractive method for Ag delivery to moDCs.
DEC-205 targeting of mDCs derived from malignant melanoma patients leads to MAGE-A3- Ag presentation and T-cell stimulation
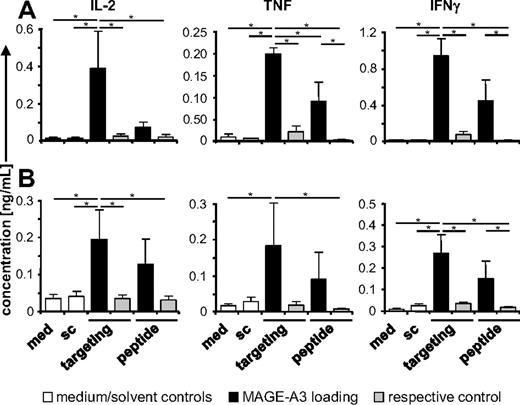
Immune cells from melanoma patients may behave differently than cells from healthy donors. Therefore, we investigated whether patient-derived DCs could also be targeted via DEC-205. mDCs from 5 malignant melanoma patients were used (patient information and disease status are summarized in Table 1). First, we confirmed the DEC-205 expression and the mature phenotype of these DCs, observing CD83 expression, as well as a high expression level of DEC-205 (Table 1). Next, mDCs were incubated for 48 hours with the anti–DEC-205scFv-MAGE-A3-KKL or the control fusion proteins. Alternatively, DCs were treated with the same volume of medium or solvent. The stimulatory capacity of these DCs was analyzed using autologous MAGE-A3/HLA-DP4–specific CD4+ T cells. Clearly, DEC-205 targeting of patient-derived mDCs led to activation of Ag-specific T cells indicated by significantly increased secretion of IL-2, TNF, and interferon-γ (IFN-γ) over the negative controls (Figure 4A), and by induced Ag-specific CD4+ T-cell proliferation (supplemental Figure 5A). MAGE-A3 peptide-loaded, but not control peptide-loaded, mDCs were also able to stimulate the Ag-specific T cells, albeit to a lower extent than the DEC-205–targeted mDCs (Figure 4A).
Maturation status of DCs derived from patients with malignant melanoma
| Patient no. . | Sex . | Age, y . | Stage* . | Distant metastasis* . | DC generation† . | DEC-205, %‡ . | MFI DEC-205§ . | CD83, %‡ . | MFI CD83§ . |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Female | 73 | IV | M1a | Adherence | 97 | 210 | 96 | 91 |
| 2 | Male | 52 | IV | M1c | Elutriation | 98 | 244 | 97 | 50 |
| 3 | Female | 65 | IIIc | — | Adherence | 96 | 238 | 93 | 59 |
| 4 | Male | 66 | IV | M1b | Adherence | 93 | 193 | 98 | 143 |
| 5 | Female | 46 | IV | — | Elutriation | 92 | 92 | 88 | 63 |
| Patient no. . | Sex . | Age, y . | Stage* . | Distant metastasis* . | DC generation† . | DEC-205, %‡ . | MFI DEC-205§ . | CD83, %‡ . | MFI CD83§ . |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Female | 73 | IV | M1a | Adherence | 97 | 210 | 96 | 91 |
| 2 | Male | 52 | IV | M1c | Elutriation | 98 | 244 | 97 | 50 |
| 3 | Female | 65 | IIIc | — | Adherence | 96 | 238 | 93 | 59 |
| 4 | Male | 66 | IV | M1b | Adherence | 93 | 193 | 98 | 143 |
| 5 | Female | 46 | IV | — | Elutriation | 92 | 92 | 88 | 63 |
DC indicates dendritic cell; MFI, mean fluorescence intensity; and —, no distant metastasis.
According to Balch et al.35
DC generation from monocytes enriched by the Elutra counterflow elutriation system or the standard gradient centrifugation/adherence technique.
Percentage of DEC-205-positive or CD83-positive DCs.
MFI above background.
DEC-205 targeting of mDCs from melanoma patients results in cytokine release by MAGE-A3/DP4–specific CD4+ T cells. (A) Stimulatory capacity of DEC-205–targeted mDCs from melanoma patients. mDCs, generated from melanoma patients, were loaded for 48 hours with MAGE-A3 by DEC-205 targeting (targeting), using 1 μg/mL anti–DEC-205scFv-MAGE-A3-KKL fusion protein, or for 3 hours by peptide pulsing using the MAGE-A3/HLA-DP4 peptide. As negative control, mDCs were incubated with 1 μg/mL of the anti–MCSPscFv-MAGE-A3-KKL protein (respective control), the NY-ESO-1/HLA-DP4 peptide (respective control), medium (med), or with solvent (sc). These differently treated DCs were used to stimulate autologous MAGE-A3/HLA-DP4–specific CD4+ T cells, generated by TCR-RNA electroporation (“Cytokine-secretion assay”). IL-2, TNF, and IFN-γ concentrations were analyzed in the supernatants after 18 hours of coculture. Data are mean values ± SEM of 4 experiments with cells from patients 1, 2, 3, and 4. (B) Stimulatory capacity of DEC-205–targeted DCs from melanoma patients after cryopreservation. The remaining mDCs from panel A were frozen after the different treatments. After thawing, the DCs were used to stimulate allogenic MAGE-A3/HLA-DP4–specific CD4+ T cells generated by electroporation with RNA encoding for a TCR. Supernatants were analyzed as described in panel A. Data are mean values ± SEM of 4 experiments with cells from patients 2, 3, 4, and 5. P values were calculated with the Mann-Whitney U test. *P < .05.
DEC-205 targeting of mDCs from melanoma patients results in cytokine release by MAGE-A3/DP4–specific CD4+ T cells. (A) Stimulatory capacity of DEC-205–targeted mDCs from melanoma patients. mDCs, generated from melanoma patients, were loaded for 48 hours with MAGE-A3 by DEC-205 targeting (targeting), using 1 μg/mL anti–DEC-205scFv-MAGE-A3-KKL fusion protein, or for 3 hours by peptide pulsing using the MAGE-A3/HLA-DP4 peptide. As negative control, mDCs were incubated with 1 μg/mL of the anti–MCSPscFv-MAGE-A3-KKL protein (respective control), the NY-ESO-1/HLA-DP4 peptide (respective control), medium (med), or with solvent (sc). These differently treated DCs were used to stimulate autologous MAGE-A3/HLA-DP4–specific CD4+ T cells, generated by TCR-RNA electroporation (“Cytokine-secretion assay”). IL-2, TNF, and IFN-γ concentrations were analyzed in the supernatants after 18 hours of coculture. Data are mean values ± SEM of 4 experiments with cells from patients 1, 2, 3, and 4. (B) Stimulatory capacity of DEC-205–targeted DCs from melanoma patients after cryopreservation. The remaining mDCs from panel A were frozen after the different treatments. After thawing, the DCs were used to stimulate allogenic MAGE-A3/HLA-DP4–specific CD4+ T cells generated by electroporation with RNA encoding for a TCR. Supernatants were analyzed as described in panel A. Data are mean values ± SEM of 4 experiments with cells from patients 2, 3, 4, and 5. P values were calculated with the Mann-Whitney U test. *P < .05.
For potential clinical applications, the DCs need to be cryoconserved. Therefore, patient-derived mDCs were frozen after Ag loading. After thawing, the DCs were still able to stimulate Ag-specific T cells (Figure 4B); albeit production of IL-2 and IFN-γ, but not of TNF, was reduced compared with freshly prepared DCs (Figure 4). Again, the DEC-205–targeted mDCs led to an increased cytokine release over peptide-pulsed DCs (Figure 4B). In addition, cryoconserved DCs were able to stimulate T-cell proliferation (supplemental Figure 5B). However, because of the limited access to autologous patient material, allogeneic T cells had to be used.
In conclusion, DEC-205 targeting of patient-derived mDCs led to Ag presentation, and these DCs could be cryopreserved without loss of the capacity to stimulate T cells. Thus, DEC-205 targeting represents an alternative to peptide-pulsing for the loading of DCs with helper epitopes for DC vaccination.
Discussion
Successful active immunotherapy of cancer requires the induction of both CD4+ and CD8+ T-cell responses against the tumor. Nevertheless, efforts have so far mainly focused on cytotoxic T cells.36 This study presents a first description of the use of an anti–DEC-205scFv to target a MHC class II–restricted tumor epitope to human moDCs for an intended immunotherapeutic application. We showed that immunologically relevant features of the DCs were not negatively influenced and that DEC-205-mediated Ag loading was more efficient than other standard methods commonly used to achieve presentation of helper epitopes. This new procedure was successful for moDCs from both healthy donors and patients with malignant melanoma.
DEC-205 targeting is superior to RNA electroporation and peptide pulsing to load MHC class II molecules on DCs with Ag
In mice, DEC-205-positive DC subtypes appear specialized in cross-presentation, thus eliciting predominantly CD8+ T-cell responses.11,15,37 However, CD4+ T-cell responses after DEC-205 targeting have also been observed12,38 and recently the DEC-205-positive splenic DC subset in mice has been shown to be specialized in inducing Fox-p3-positive regulatory T cells.39
Ag presentation on MHC class II after DEC-205 targeting is not unexpected as a lysosomal targeting motive in the cytoplasmic domain of DEC-205 should be providing access to the MHC class II presentation pathway.14 In the human system, only one study showed CD4+ T-cell responses after targeting of human DEC-205.19 Because of limited experience and the need for highly efficient procedures to load MHC class II on human DCs for immunotherapy, we fused a DEC-205–specific scFv to an HLA-DP4–restricted peptide, derived from the cancer testis Ag MAGE-A3. This Ag is highly expressed in many tumors26,27,40 but not in normal tissue except in testis and ovary, therefore representing a prime target for cancer immunotherapy. HLA-DP4 restriction was deliberately chosen because this is the most frequent MHC class II haplotype in the white population,41 making the construct widely applicable.
As DEC-205-expression was observed on both immature and mature moDCs (Figure 1; supplemental Figure 1; Table 1), in vitro DEC-205 targeting of DCs was carefully analyzed, and the impact of the fusion protein on DC phenotype and functionality was investigated. Three different DC populations were analyzed (Figure 3A): (1) DCs, loaded in the immature state, and left immature (iDCs), (2) DCs, loaded in the immature state, but matured during loading (iDCm), and (3) DCs, loaded in the mature state (mDCs). We observed no changes in surface expression of several maturation markers between DEC-205–targeted DCs and DCs treated with the control protein (supplemental Figure 3A,C). In addition, the migratory capacity was not altered after DEC-205 targeting (supplemental Figure 3D). Most importantly, the incubation with the construct did not result in any measurable IL-10 secretion by the DCs (supplemental Figure 3B), which is known to contribute to de novo induction of Treg and immunosuppression.42
To investigate Ag presentation after DEC-205 targeting of human moDCs, we used a novel read-out system developed by us. Ag-specific T cells were generated by electroporation with TCR-RNA as previously described.29,43 Bulk CD4+ T cells were electroporated with RNA encoding the α- and β-chain of a MAGE-A3/HLA-DP4–specific TCR. These T cells were then used to scan Ag-loaded DCs and the release of IL-2 was used as a measure for efficiency of Ag-presentation by DCs. Unlike T-cell clones, specific T cells generated by RNA electroporation can be produced with a constant quality and therefore represent a reliable read-out system. In addition to IL-2 as a measure for T-cell activation, other cytokines can be assayed in this system to provide an indication of the type of immune response initiated by the DCs.
In this study, targeting of human moDCs via DEC-205 in vitro led to Ag presentation as indicated by the high IL-2 secretion of the Ag-specific CD4+ T cells (Figure 3B black bars), whereas incubation with the control protein did not result in cytokine secretion (Figure 3B gray bars). Interestingly, DCs matured during loading (ie, iDCm) were the most potent stimulators of Ag-specific T cells. This may be explained by the fact that this loading strategy mimics the in vivo situation, where immature DCs encounter Ag and maturation stimuli simultaneously. However, we did not observe differences in the internalization rate of the DEC-205scFv complex (supplemental Figure 4). In contrast, Butler et al clearly showed a loss of internalization of DEC-205 on DC maturation but also saw a much faster internalization on immature DCs.44 This could be because they used lipopolysaccharide for maturation and used a cross-linking anti–DEC-205 Ab, not a monovalent scFv. A possible conclusion would be that, apart from the internalization rate, influenced by some maturation stimuli, other factors, such as intracellular processing, influence the DC's capacity to present Ags targeted to DEC-205. In addition, targeted iDCs induced significantly more IL-10 in relation to IL-2, which may lead to the induction of tolerance instead of immunity. This is in line with the observations of several other researchers, who reported that DEC-205 targeting of murine DCs with Ab-Ag constructs in vivo led to the induction of tolerance instead of immunity, when no additional maturation stimuli were added.4,39 Thus, DEC-205–targeted iDCm and mDCs should be more suitable for vaccination approaches because these DCs are matured ex vivo, resulting in a more favorable IL-2/IL-10 ratio (Figure 3B).
Strikingly, DEC-205 targeting of Ag, especially in the iDCm condition, resulted in stronger MHC class II presentation than RNA electroporation of an endosome-targeted Ag-DCLAMP construct,32 although an optimized electroporation protocol34 and a high RNA concentration were used. In addition, direct pulsing with peptide in a high concentration of 10 μg/mL was less effective than DEC-205 targeting, even though this peptide concentration represents a 156-fold higher molarity because of the lower molecular weight of the peptide compared with the anti–DEC-205scFv-MAGE-A3-KKL construct. This indicates the superiority of DEC-205-mediated active Ag uptake over passive peptide pulsing.
In addition, we showed that 1 μg/mL of the anti–DEC-205scFv-MAGE-A3-KKL construct is sufficient to elicit efficient Ag presentation (Figure 3C). The increase in IL-2 secretion by the stimulated Ag-specific T cells after DEC-205 targeting with 10 μg/mL of the fusion protein may represent an unspecific uptake by endocytosis because incubation with 10 μg/mL of the control fusion protein also led to a detectable stimulation of Ag-specific T cells (Figure 3C).
Possible clinical application of DEC-205 targeting for the treatment of melanoma
To our knowledge, it had not yet been investigated whether DCs generated from late-stage cancer patients can be targeted via DEC-205. In our study, not only DCs from healthy donors, but also DCs from malignant melanoma patients were efficiently loaded by DEC-205 targeting (Figure 4A), which is a critical prerequisite for the application of this strategy in cancer immunotherapy. For these experiments, we targeted mDCs; albeit targeting iDCm of healthy donors resulted in a higher stimulation capacity than targeting of mDCs. This strategy was chosen because of the scarcity of patient material and for practical reasons. Especially under Good Manufacturing Practice conditions, harvesting and loading of mDCs are more economical and require less construct. Moreover, DEC-205–targeted patient-derived mDCs maintained their stimulatory capacity after cryoconservation, which will be necessary for repetitive application of the DCs in a vaccination study (Figure 4B). Furthermore, DEC-205–targeted DCs induced proliferation of Ag-specific T cells (supplemental Figure 5).
Although in several vaccination trials Ag was formulated with adjuvant, which directly or indirectly mediates uptake by DCs and their maturation,45 receptor targeting of DCs with specific constructs was not yet performed in humans. Experiments in mice and in vitro experiments with human DCs showed first promising results, indicating that in vivo targeting of DCs may be applicable as a therapeutic modality in cancer immunotherapy. It will, however, be necessary, before translation into the clinic, to better define the DC subsets in patients to avoid unexpected results, including tolerance induction by specialized DC subsets.4,39 Another logical prerequisite for targeting DCs in vivo are in vitro studies, as we have performed here, to systematically test and optimize Ag loading via targeting receptors, such as DEC-205. With respect to a later in vivo targeting of DCs, it is noteworthy that loading occurred irrespective of the maturation status of DC.
Ex vivo loading of DC allows the production of a highly controlled and patient-specific DC vaccine with a clearly defined and controllable mature phenotype. Vaccinating patients by adoptively transferring such ex vivo loaded DCs will provide valuable insight if accompanied by immunomonitoring to define both quantity and quality of induced Ag-specific T cells (eg, effector vs regulatory T cells). Then it will be possible to advance to in vivo targeting to determine whether this will indeed be superior.
In conclusion, we showed that DEC-205 targeting of moDCs using an anti–DEC-205scFv-MAGE-A3-KKL construct led to effective presentation of the MAGE-A3-derived MHC class II epitope and had no negative effect on DC phenotype, cytokine secretion profile, and migratory capacity. Importantly, moDCs derived from patients with malignant melanoma can be loaded with tumor Ag by DEC-205 targeting and retain their stimulatory capacity after cryoconservation. DEC-205 targeting appears to be superior to peptide pulsing and even mRNA transfection for the loading of DCs with helper epitopes for vaccination purposes. As we have shown that strong MHC class II presentation can occur on DEC-205-mediated Ag targeting to human DCs, it will be attractive to explore the induction of effector or regulatory CD4+ T cells, first in preclinical models and finally in clinical trials.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Ralph Steinman for the plasmids encoding the anti–DEC-205 Ab; Diana Dudziak for the CHO DEC-205 cell line and for fruitful discussions; Ingo Schubert for cloning the DEC-205scFv; Erwin Schultz for the MAGE-A3/HLA-DP4–specific T-cell clone; Kris Thielemans for the pGEM4Z-5′UTR-MAGE-A3.DCLAMP-3′-UTR vector; Argos Therapeutics for the pGEM4Z-MAGE-A3-64A vector; Stefanie Baumann and Tanja Schunder for technical assistance; Michael Erdmann and Manuel Wiesinger for helping with patient material; Tina Schmid, Hans Simon, Stina Rosenheinrich, Sandra Schiemann, and Doris Schuster for collection of blood; and Stefanie Hoyer, Christian Hofmann, Isabell Pfeiffer, and Ilka Knippertz for fruitful discussions.
This work was supported by Deutsche Forschungsgemeinschaft (SFB643 and DFG-SCHA 1247/1-1), DC-THERA (contract LSHB-CT-2004-512074 of the EU Sixth Framework Program), and CIMT (CancerImmunoTherapy) EU Integrated Project.
Authorship
Contribution: K.B., S.G., and J.D. performed research; M.S. and C.K. designed constructs and established protein expression techniques; K.B., N.S., S.G., G.F., G.S., and J.D. designed research; B.S.-T. provided patient material; and K.B., G.S., N.S., J.D. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Niels Schaft, Department of Dermatology, University Hospital Erlangen, Hartmannstrasse 14, 91052 Erlangen, Germany; e-mail: niels.schaft@uk-erlangen.de.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal