Abstract
Patients with HIV-1 immune-related thrombocytopenia have a unique antibody (Ab) against integrin GPIIIa49-66 capable of inducing oxidative platelet fragmentation via Ab activation of platelet nicotinamide adenine dinucleotide phosphate oxidase and 12-lipoxygenase releasing reactive oxygen species. Using a phage display single-chain antibody (scFv) library, we developed a novel human monoclonal scFv Ab against GPIIIa49-66 (named A11) capable of inducing fragmentation of activated platelets. In this study, we investigated the in vivo use of A11. We show that A11 does not induce significant thrombocytopenia or inhibit platelet function. A11 can prevent the cessation of carotid artery flow produced by induced artery injury and dissolve the induced thrombus 2 hours after cessation of blood flow. In addition, A11 can prevent, as well as ameliorate, murine middle cerebral artery stroke, without thrombocytopenia or brain hemorrhage. To further optimize the antithrombotic activity of A11, we produced a bifunctional A11-plasminogen first kringle agent (SLK), which homes to newly deposited fibrin strands within and surrounding the platelet thrombus, reducing effects on nonactivated circulating platelets. Indeed, SLK is able to completely reopen occluded carotid vessels 4 hours after cessation of blood flow, whereas A11 had no effect at 4 hours. Thus, a new antithrombotic agent was developed for platelet thrombus clearance.
Introduction
We have discovered a unique antiplatelet integrin GPIIIa49-66 antibody (Ab) derived from patients with HIV or hepatitis C-related immunologic thrombocytopenia (HIV-1-ITP), which induces complement-independent platelet oxidative fragmentation and death by generation of platelet peroxide after nicotinamide adenine dinucleotide phosphate oxidase activation.1-3 The development of this Ab in HIV-1-ITP patients is the result of molecular mimicry of epitopes on the polymorphic regions of HIV or HCV protein.4,5 By screening a human single-chain fragment variable region (scFv) library with the GPIIIa49-66 peptide, we identified A11, which acts similarly to the antiplatelet integrin GPIIIa49-66 Ab, and we have shown it to be capable of destroying arterial platelet thrombi in vitro.6 In our current studies, we sought to determine whether the A11 would be associated with any significant thrombocytopenia or inhibition of platelet function in vivo using mice, as well as to assess its effectiveness and safety in 2 murine stroke models.
Animal stroke experiments with antiplatelet GPIIb-IIIa agents have successfully diminished brain infarct formation as well as permanent neurologic damage.7,8 However, this has been associated with cerebral hemorrhage and death because Abs against GPIIb-IIIa inhibit platelet function and induce thrombocytopenia. A recent double-blind clinical study on the role of Abciximab (anti–GPIIb-IIIa) in stroke was discontinued because of its high rate of hemorrhage, as well as ineffectiveness.9,10
Current treatment of acute occlusive stroke is with tissue plasminogen activator (tPA), an agent that is most effective when given within 3 hours of occlusion with recent recommendations extending this therapeutic window in a subset of patients to 4.5 hours.11-13 This is feasible only in a minority of patients, with hemorrhage being a significant complication in a minority of patients. Our data with tPA in a murine cerebral stroke model revealed that tPA protects from infarction at 2 hours, but not at 4 hours. In addition, 4 of 12 mice died as a result of intracranial bleeding.14 Hence, there is a clear need to develop agents with a longer therapeutic window and a lower risk of associated cerebral hemorrhage.
In addition to testing A11 in vivo, we sought to increase its safety and efficacy. This was done by coupling it to the first kringle of plasminogen (single-chain linked first kringle) SLK, which homes selectively to terminal lysines of partially degraded fibrin within the platelet thrombus by forming a covalent bond.15 In this report, we tested and compared the ability of both A11 and SLK to prevent and dissolve arterial platelet thrombi within an experimentally induced carotid artery thrombus as well as postischemic cerebral stroke in mice.
Methods
Mice
GPIIIa−/− mice with C57/BL6 background and BALB/C mice were purchased from The Jackson Laboratory. Swiss Webster mice were obtained from The Jackson Laboratory. All mice experiments were approved by the Institutional Animal Care and Use Committee of the New York University School of Medicine.
Cloning of bifunctional scFv-A11-plasminogen first Kringle reagent
The Tomlinson J phage Library was used to screen against a biotin-conjugated GPIIIa49-66 peptide. Specific clones enriched for anti–GPIIIa49-66 Abs were screened and A11 selected for highest binding avidity, as described.6
The nucleotide sequence of the cassette ScFv-Linker-Kringle 1 was generated by 3-step polymerase chain reaction (PCR). The first PCR fragment transcribed from the start codon of ScFv to the middle half of the linker sequence.
First step.
The sequence was generated by PCR using pET29a-ScFv-A11 as template. The primers used for this amplification were (single-chain forward N-terminal or SCFN) 5′-GGAATTCCATATGG CCGAGGTGCAGCTGTT-3′; and (single-chain forward C-terminal or SCFC) 5′- ACTAGTAGATCCACCACTTGTCGACCCACCAGAGTACTTCCCCGTTT GATTTCCACCTTGGTCC-3′. The resulting product was a 768-bp fragment with an NdeI site at the 5′ end. The second half of the expression cassette carried a sequence encoding the C-terminal half of the linker and the Kringle 1 domain. This sequence was generated by PCR using pET29a-Kringle 1 as template.
Second step.
The forward linker primer (kringle 1 N-terminal or KRN) 5′-ACAAGTGGTGGATCTACTAGTGGCTCTGGATCCGGAATTTGCAAGACTGGGAATGGAAAG-3′ has 3 components: the first 20-bp component is the reverse complement sequence of the linker attached to the SCFC primer; the residual 2 sequences encode the C-terminal half of the linker and the beginning of the Kringle 1 domain. The reverse primer coding for kringle 1 C-terminal domain (KRC) is 5′-TAGGATCCGCGGCCGCCTCAAGAAT GTCGCAGTAGT-3′. The resulting product has a 270-bp fragment with a Not I site at the 3′ end.
Third step.
The full-length ScFv-A11-Linker-Kringle 1 cassette was generated by the third PCR using the primers for SCFN and KRC. The resulting 1038-bp Nde I–Not I fragment was digested by Nde I and Not I and inserted into pET-29a to generate pET29a-ScFv A11-Linker-Kringle 1 (SLK; supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Expression, purification, and refolding of ScFv-A11 and SLK bifunctional reagent grown in E coli
Escherichia coli Rosetta cells transformed with the expression vector pET29a-ScFv-A11 and pET29a-SLK were cultured in 1 L 2YT medium containing carbenicillin (50 μg/mL) and chloramphenicol (34 μg/mL) with shaking at 37°C until the optical density (600 nm) was 0.4 to 0.6. Production was induced by the addition of 1mM isopropyl-β-D-thiogalactopyranoside and the cells incubated at 37°C for 4 hours with shaking. Refolding and purification of the recombinant Ab were performed at 4°C as described.16
Enzyme-linked immunosorbent assay
Plastic microtiter plates (Corning) were coated with GPIIIa49-66 peptide (20 μg/mL) in 50mM sodium bicarbonate buffer (pH 9.6) or washed platelets (1 × 106/well) in phosphate-buffered saline (PBS) at 4°C overnight. The plate was then blocked with blocking buffer (3% bovine serum albumin [BSA] in PBS with 0.1% Tween-20) for 2 hours at room temperature. Soluble A11 or SLK with histidine tag was added to the plates and incubated for 1 hour at room temperature. A11 or SLK was detected with primary mouse anti-His tag antibodies followed by secondary goat anti–mouse horseradish peroxidase-conjugated IgG. After washing thoroughly, ABTS (Pierce Chemical) was added for color development, and the absorbance was determined at 405 nm.
For fibrin binding, human fibrinogen (5 μg/mL) was coated onto the wells of a plastic microtiter plate and left overnight at 4°C. After blocking the exposed sites with blocking buffer, the wells were incubated with a solution of phosphate-buffered saline containing human thrombin (1 NIH unit/mL) and CaCl2 (20mM) for 2 hours at 37°C. Cross-linked fibrin clots on the wells were partially digested with plasmin (1pM/mL) for different time periods. Purified A11 and SLK in PBS were added to the wells at a final concentration of 500nM (50 pmol/well). Binding of A11 or SLK to the partially digested fibrin was monitored as in the previous paragraph.
Assay of platelet particle formation
Gel-filtered murine or human platelets were isolated from ethylenediaminetetraacetic acid-anticoagulated blood and labeled with anti-CD61–fluorescein isothiocyanate (FITC, human) or anti-CD41–FITC (murine) monoclonal antibody (mAb) as previously described.1 Fluorescent-labeled platelets/particles were measured by flow cytometry using a FACScan (BD Biosciences). Gates were adjusted for platelets by exclusion of other blood cells.
Assay of platelet oxidation
Gel-filtered platelets were loaded with 10μM 2-dichlorodihydrofluorescein diacetate (DCFH-DA) for 30 minutes at 37°C as described1 and challenged with A11 or SLK. Intracellular DCFH is converted to a fluorescent form by hydrogen peroxide generated in this reaction. Oxidation was quantified by measuring the increase in mean fluorescence by flow cytometry.
Assay of LDH
Lactate dehydrogenase (LDH) release from platelets was assayed using the commercial LDH Kit supplied by Biotrin Diagnostics.
Assay of platelet aggregation
Platelet aggregation was performed using citrated human or mouse platelet-rich plasma. After incubation at 37°C for 5 minutes with various concentrations of A11 or SLK, aggregation was initiated by adding 10μM adenosine diphosphate (ADP) or 2 μg/mL collagen. The maximal platelet aggregation within 5 minutes was recorded using a 4-channel platelet aggregometer (Helena Laboratories).
Assay of platelet aggregation and adhesion
Platelet adhesion to immobilized ligand was done as described previously.17
Ex vivo assay of the effect of A11 and SLK on platelet function
To assay the ex vivo effect of A11 and SLK on platelet function, 6-week-old female BALB/C mice were randomly divided into 3 groups (n = 3 per group). Purified control scFv 13CG2 (20 μg/mouse), A11 (25 μg/mouse), or SLK (37.5 μg/mouse) in 100 μL PBS was injected into mice through tail vein. Anticoagulated blood samples were collected 4 hours after injection. Platelet-rich plasma from different treatment groups was stimulated with various platelet agonists, including 10μM ADP (Helena Lab), 2 μg/mL collagen (Helena Lab), or 50nM thrombin (Sigma-Aldrich), and then stained with anti-CD62P–PE (BD Biosciences) at room temperature in the dark for 20 minutes, respectively. Fluorescent-labeled platelets were analyzed by flow cytometry, using a FACScan (BD Biosciences). A total of 10 000 platelets were counted per sample. To measure platelet-monocyte aggregate formation, aliquots (300 μL) of anticoagulated blood samples from treated mice were stimulated with the aforementioned agonists, then stained with anti-CD14–FITC (BD Biosciences), and anti-CD41a–PE (BD Biosciences) and incubated for 20 minutes in the dark. The samples were then processed immediately for flow cytometry. Monocytes were identified by anti-CD14–FITC fluorescence and differentiated from other cells based on cell size and granularity using the forward (a size characteristic) and side scatter. The 2-color analysis (CD14-FITC vs CD41a-PE) enabled discrimination of platelet-coupled and platelet-free monocytes. The percentages of platelet-monocyte conjugates were measured in single parameter histograms of anti-CD41a–PE fluorescence displaying events from the monocyte gate.
Disaggregation and destruction of ex vivo human platelet aggregates
For collagen-induced aggregates, 1 μg/mL collagen (Helena Lab) was incubated with platelets for 1 hour at 37°C with intermittent shaking, followed by gravity sedimentation at room temperature for 30 minutes. The top 50% volume was removed, and the remainder of the platelet aggregate suspension was added directly into Tyrode buffer, pH 7.4 with testing reagents. For ADP-induced aggregates, 107 gel-filtered platelets were incubated with 100 mg/mL fibrinogen and ADP (10μM) for 30 minutes at 37°C to create platelet aggregates. Excess reagents were removed by washing in PBS. A total of 0.85μM A11 or SLK was added for various time intervals, and the remaining platelet/aggregate was enumerated as previously reported.5
Determination of mouse platelet count
To determine the effect of A11 and SLK on platelet count, BALB/C mice were injected with the same molar amount of Abs (∼ 0.9 nmol): 25 μg/mouse with A11 (molecular weight: 29 000), 37.5 μg/mouse SLK (molecular weight: 40 000) or 20 μg/mouse control scFv 13CG2 (molecular weight: 25 000; anti-BSA, MRC Gene Service). A total of 20 μL of blood was drawn into Unopettes (no. 365855, BD Biosciences) for platelet count determination by phase-contrast microscopy.
Bleeding time
To determine the bleeding time, the mouse tail vein was severed 2 mm from its tip and blotted every 30 seconds on a circular sheet of filter paper to obtain an objective measurement. Termination of the bleeding time was recorded after absence of blood on the filter paper. Bleeding time differences were recorded by an unbiased observer and confirmed by 2 other observers.
Carotid artery thrombus formation
FeCl3-induced arterial injury was performed in 6- to 8-week-old Swiss Webster mice. A 1 × 2-mm2 strip of no. 1 Whatman filter paper (Fisher Scientific) soaked in 10% FeCl3 was dried and prepared for use. The right common carotid artery was exposed by blunt dissection, and a 4 × 8-mm paraffin strip was placed around the artery to isolate it from surrounding tissue. A Doppler flow probe (Model BPM2 Blood Perfusion Monitor; Vasamedics) was positioned around the artery, 1 mm proximal to the bifurcation of the external carotid artery. The strip of filter paper was applied to the adventitial surface of the artery, 5 mm from probe for 20 minutes. The filter paper was removed, the field was flushed with saline, and the blood flow continuously monitored and recorded at baseline, start injury, and each minute for the duration of the experiment.
Postischemic stroke model
A 3 × 0.2-mm polyethylene thread attached to a 9-mm 7/0 suture was inserted into the right internal carotid artery and advanced to the bifurcation of the middle cerebral artery. The obstruction was removed at 60 to 90 minutes. Mice were killed at 48 hours or 8 days. Mouse brains were dissected into 8 coronal planes at 1-mm intervals. Each section was stained red with triphenyltetrazolium chloride, fixed in 10% formalin, scanned into a commercial photo-image-editing program, and the data stored on a disk. The unstained area of infarct was computer calculated using an automated image analysis program (Image J, Version 1.32, National Institutes of Health).
Neurologic test battery
Animals were evaluated on the postural reflex, rotor rod, and traverse beam behavioral tests, 8 days after stroke onset, by methods previously described in detail.5
GFAP and CD31 immunohistochemistry staining
Two or 8 days after ischemia, the mice were anesthetized with sodium pentobarbital (150 mg/kg intraperitoneally) and perfused transaortically with 0.1M PBS, pH 7.4. After fixation, 40-μm serial coronal brain sections were cut on a microtome and incubated with a polyclonal anti–mouse glial fibrillary acidic protein (GFAP; rabbit polyclonal, 1:1000, Dako) or with rabbit anti-CD31 (1:200, Abbiotec) in a diluent of 0.3% Triton X-100, 0.1% sodium azide, 1% BSA, and 10% normal goat serum, at 4°C for 12 hours, as previously described.18 For the CD31 immunolabeled sections, 10-minute heat-induced epitope retrieval in boiling 10mM citrate buffer, pH 6.0, was used. The CD31 immunostaining was done on sections from mice killed 2 days after ischemia, whereas the GFAP immunostaining was performed on sections from mice killed 8 days after ischemia. GFAP-immunostained sections were quantified with a Bioquant stereology semiautomated image analysis system (R&M Biometrics), using a random unbiased hierarchical sampling scheme of the non-necrotic cortex on the infarction side as previously published.18,19 The total GFAP burden (defined as the percentage of the test area occupied by GFAP immunoreactivity) was quantified for the cortex, on 8 sections per animal.
Intravital microscopy
Intravital microscopy was performed on similarly sized 8-week-old Swiss Webster mice. Platelets from donor mouse were prepared as previously described1 and labeled with rhodamine 6G (0.5 mg/mL; Sigma-Aldrich). Acceptor mice were anesthetized by inhalation of isoflurane-N2O, and the left carotid artery and left femoral vein were exposed. A total of 1 × 108 labeled platelets were used as bolus and injected to acceptor mouse by femoral vein, and thrombosis was initiated with 10% FeCl3 paper patch applied to the carotid artery. Clot formation was observed in real-time under a Leica LZM510 fluorescent microscope (Leica). A total of 37.5 μg SLK (containing His-tag), 25 μg A11, or 20 μg control scFv Ab was infused by tail vein and traced by anti-Histag–FITC polyclonal Ab (AnaSpec) after platelet thrombus fluorescence is stable. The binding of A11 or SLK on platelet thrombus was recorded using fluorescent microscope.
Statistical analysis
Data are analyzed using GraphPad Prism, Version 5.0, by 2-way or 1-way analysis of variance (ANOVA) followed by a Bonferroni or Neuman-Keuls post hoc test, respectively, except for comparisons of platelet count reduction, infarction size, and behavioral testing, which were done by 2-tailed, unpaired Student t test.
Results
A11 inhibits carotid artery platelet thrombus formation
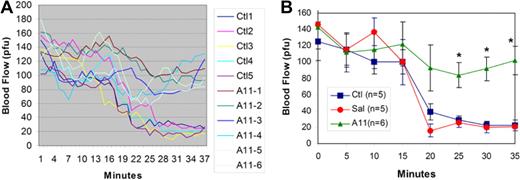
Initial experiments revealed that A11 could prevent the cessation of carotid artery flow in a FeCI3-induced carotid thrombus in which the FeCI3 patch was applied 2 hours after the intravenous injection of A11. Figure 1A shows the reduced blood flow in all control Swiss-Webster mice, which were injected with scFv Ab 13CG2 (anti-BSA). At 20 to 37 minutes after application of the FeCl3 patch, blood flow is 30% to 15% of normal flow resulting from platelet occlusion. In contrast, all A11-treated mice kept blood flow at 85% to 75% of initial flow. Figure 1B is a 5-minute interval plot of the continuous flow diagram with statistically significant differences at 25 to 35 minutes between A11-treated mice versus both saline controls and scFv Ab 13CG2-injected mice [2-way ANOVA P < .001 for treatment effect, post hoc Bonferroni testing scFv A11 P < .01 versus both saline control (Sal) and control scFv Ab (Ctl) at 25, 30, and 35 minutes].
Effect of A11 on inhibition of FeCI3 induced carotid artery platelet thrombus formation. Swiss-Webster mice were injected intravenously with 25 μg of scFv A11 or irrelevant control scFv Ab (13CG2). The right carotid artery was exposed and a FeCI3 filter paper patch applied 2 hours after intravenously injection and removed at 20 minutes. Blood flow (recording in arbitrary perfusion units, pfu) was measured with a Doppler flow meter. (A) Mean continuous blood flow measured in 5 controls and 6 experimental A11 mice over a period of 38 to 43 minutes, respectively. (B) The same experiment with SEM given every 5 minutes as well as a single saline control experiment (SAL). *Statistically significant differences (P < .01) by post hoc Bonferroni testing scFv A11 versus either saline control (Sal) or control scFv Ab 13CG2 (Ctl) at 25, 30, and 35 minutes; n = 5 or 6 per group (SEM).
Effect of A11 on inhibition of FeCI3 induced carotid artery platelet thrombus formation. Swiss-Webster mice were injected intravenously with 25 μg of scFv A11 or irrelevant control scFv Ab (13CG2). The right carotid artery was exposed and a FeCI3 filter paper patch applied 2 hours after intravenously injection and removed at 20 minutes. Blood flow (recording in arbitrary perfusion units, pfu) was measured with a Doppler flow meter. (A) Mean continuous blood flow measured in 5 controls and 6 experimental A11 mice over a period of 38 to 43 minutes, respectively. (B) The same experiment with SEM given every 5 minutes as well as a single saline control experiment (SAL). *Statistically significant differences (P < .01) by post hoc Bonferroni testing scFv A11 versus either saline control (Sal) or control scFv Ab 13CG2 (Ctl) at 25, 30, and 35 minutes; n = 5 or 6 per group (SEM).
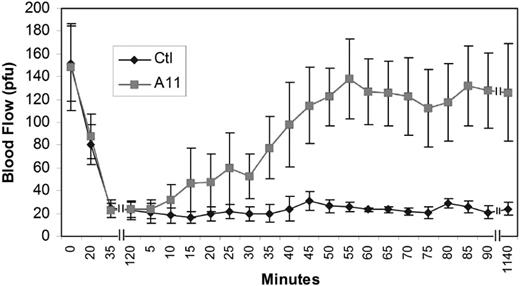
A11 reopens a platelet-thrombus induced occluded carotid artery 2 hours after cessation of blood flow
Figure 2 demonstrates that A11 reopens the occluded carotid artery after 2 hours of reduced blood flow, 10 minutes after intravenous infusion. The blood flow was curtailed by 87% 35 minutes after FeCI3 treatment. At the 2-hour time point, mice were either injected with A11 (n = 4) or control scFv Ab 13CG2 (n = 5). As shown in Figure 2, A11 injection resulted in reopening of blood flow to approximately 95% of original flow at 55 to 1140 minutes (P < .001 by 2-way ANOVA for treatment effect; by post hoc Bonferroni testing at 35 to 45 minutes, P < .01 A11 vs control scFv Ab, 13CG2). For time points between 50 and 1140 minutes, P < .001 A11 versus control. Noteworthy, no blood flow reocclusion was observed after A11 treatment within the extended monitoring time.
Ligation of GPIIIa49-66 with A11 reconstitutes blocked carotid artery thrombus blood flow 2 hours after cessation of blood flow. The carotid artery of mouse was dissected free of surrounding tissue and a FeCI3 patch applied for 20 minutes, followed by rinsing. Blood flow was monitored with a Doppler flow apparatus. Cessation of blood flow was generally at 35 minutes. Two hours after cessation of blood flow (vertical lines), the mice were infused intravenously with 25 μg of A11 or 20 μg of control scFv Ab (Ctl). Note the rapid reonset of blood flow, 10 to 15 minutes after infusion. P < .001 by 2-way ANOVA for treatment effect. By post hoc Bonferroni testing at 35 to 45 minutes, P < .01, A11 (n = 4) versus control scFv Ab (n = 5). For time points between 50 and 1140 minutes, P < .001, A11 (n = 4) versus control scFv Ab (n = 5).
Ligation of GPIIIa49-66 with A11 reconstitutes blocked carotid artery thrombus blood flow 2 hours after cessation of blood flow. The carotid artery of mouse was dissected free of surrounding tissue and a FeCI3 patch applied for 20 minutes, followed by rinsing. Blood flow was monitored with a Doppler flow apparatus. Cessation of blood flow was generally at 35 minutes. Two hours after cessation of blood flow (vertical lines), the mice were infused intravenously with 25 μg of A11 or 20 μg of control scFv Ab (Ctl). Note the rapid reonset of blood flow, 10 to 15 minutes after infusion. P < .001 by 2-way ANOVA for treatment effect. By post hoc Bonferroni testing at 35 to 45 minutes, P < .01, A11 (n = 4) versus control scFv Ab (n = 5). For time points between 50 and 1140 minutes, P < .001, A11 (n = 4) versus control scFv Ab (n = 5).
Effect of A11 on postischemic middle cerebral artery stroke
Figure 3A shows coronal sections of mice brains 48 hours after removal of obstruction of the middle cerebral artery. Note the white infarcted areas in each of the 6 control mice brains, whereas there was a clear protective effect on infarction in A11-treated mice. No microscopic hemorrhage was observed in A11-treated mice. Figure 3B shows the statistically significant differences in mean infarction size expressed as a percentage of the hemisphere, which is infarcted [P < .003 by 2-tailed t test, A11 (n = 10) vs vehicle group (n = 6)]. Thus, A11 provided a clearly protective effect when given 2 hours before obstruction. Representative immunohistochemistry staining for CD31 demonstrated the endothelium to be smooth and intact in A11-treated mice, whereas the endothelium appeared injured, with platelet thrombus adhering to the denuded surface in the control group (data not shown).
Coronal sections of mouse brains showing the infarcted areas in mice given scFv A11 or saline vehicle 2 hours before carotid obstruction. (A) Mouse brains were dissected into 8 coronal planes for each mouse at 1-mm intervals. Each section was stained red with triphenyltetrazolium chloride, fixed in 10% formalin, scanned into a commercial photo-image-editing program, and the data stored on a disk. (Left) A11-treated mice. (Right) Control mice injected with saline vehicle. The unstained area of infarct was computer calculated using an automated image analysis program (Image J 1.32, National Institutes of Health). (B) Quantitation of the protective effect of A11 given 2 hours before obstruction. *Statistically significant differences by Student t test (P < .003), A11 (n = 10) versus saline control (n = 6; SEM).
Coronal sections of mouse brains showing the infarcted areas in mice given scFv A11 or saline vehicle 2 hours before carotid obstruction. (A) Mouse brains were dissected into 8 coronal planes for each mouse at 1-mm intervals. Each section was stained red with triphenyltetrazolium chloride, fixed in 10% formalin, scanned into a commercial photo-image-editing program, and the data stored on a disk. (Left) A11-treated mice. (Right) Control mice injected with saline vehicle. The unstained area of infarct was computer calculated using an automated image analysis program (Image J 1.32, National Institutes of Health). (B) Quantitation of the protective effect of A11 given 2 hours before obstruction. *Statistically significant differences by Student t test (P < .003), A11 (n = 10) versus saline control (n = 6; SEM).
In a similar experimental cerebral stroke model, animals were examined 8 days after cessation of middle cerebral artery blood flow. Table 1 demonstrates that A11 both protected and ameliorated the histopathologic as well as functional neurologic damage (similar to its effect on carotid artery thrombosis). Recent infarction was prevented or ameliorated by approximately 42% (P = .002, by 2-tailed t test), cavitation by approximately 85% (P = .01), postural reflex improvement by approximately 82% (P = .002), rotor rod improvement by approximately 56% (P = .003), and traverse beam improvement by approximately 54% (P = .002).
Anti-GPIIIa49–66 scFv (A11) 8 days after removal of obstruction
| . | N . | Infarction, percentage . | P* . | Cavitation† . | P* . | Postural reflex‡ . | P* . | Rotor rod§ . | P* . | Traverse beam§ . | P* . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Post A11 | 9 | 24.8 ± 5.5 | .002 | 1.52 ± 1.0 | .01 | 0.41 ± 0.18 | < .001 | 4.98 ± 0.28 | .003 | 6.96 ± 0.037 | < .001 |
| Pre A11 | 9 | 25.5 ± 6.0 | < .001 | 4.64 ± 1.7 | < .001 | 0.82 ± 0.25 | < .001 | 3.94 ± 0.36 | .038 | 6.33 ± 0.39 | .049 |
| Saline | 14 | 43.0 ± 4.8 | — | 9.98 ± 1.9 | — | 2.31 ± 0.25 | — | 3.19 ± 0.34 | — | 4.52 ± 0.46 | — |
| Control scFv | 5 | 37.2 ± 6.2 | — | 8.80 ± 2.1 | — | 2.00 ± 0.39 | — | 3.33 ± 0.48 | — | 4.27 ± 0.61 | — |
| . | N . | Infarction, percentage . | P* . | Cavitation† . | P* . | Postural reflex‡ . | P* . | Rotor rod§ . | P* . | Traverse beam§ . | P* . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Post A11 | 9 | 24.8 ± 5.5 | .002 | 1.52 ± 1.0 | .01 | 0.41 ± 0.18 | < .001 | 4.98 ± 0.28 | .003 | 6.96 ± 0.037 | < .001 |
| Pre A11 | 9 | 25.5 ± 6.0 | < .001 | 4.64 ± 1.7 | < .001 | 0.82 ± 0.25 | < .001 | 3.94 ± 0.36 | .038 | 6.33 ± 0.39 | .049 |
| Saline | 14 | 43.0 ± 4.8 | — | 9.98 ± 1.9 | — | 2.31 ± 0.25 | — | 3.19 ± 0.34 | — | 4.52 ± 0.46 | — |
| Control scFv | 5 | 37.2 ± 6.2 | — | 8.80 ± 2.1 | — | 2.00 ± 0.39 | — | 3.33 ± 0.48 | — | 4.27 ± 0.61 | — |
Data are mean ± SEM.
— indicates not applicable.
Two-tailed t test comparison of saline vehicle versus intravenous injection of 25 μg of A11 Ab or control scFv given 2 hours before (Pre) or 2 hours after (Post) obstruction and release of right middle cerebral artery.
Cavitation refers to the formation of cavities in brain; a higher number reflects greater neurologic impairment.
With postural reflex, a higher number reflects a higher number of errors and greater neurologic impairment.
With rotor rod and traverse beam score, a higher number reflects greater neurologic impairment. No microscopic hemorrhage was seen.
Production of a bifunctional A11 capable of homing to fibrin within the platelet thrombus
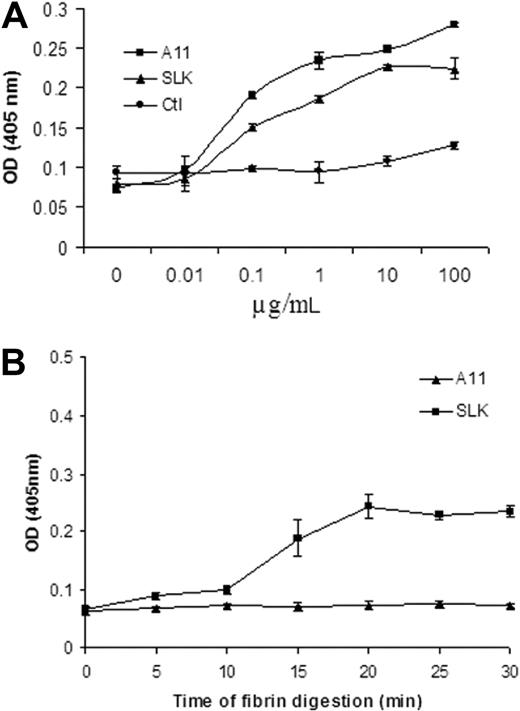
To enhance the protective effect of A11, we next devised an approach to home A11 to partially degraded fibrin using the first kringle of plasminogen (single-chain linked first kringle) named SLK.15 Supplemental Figure 1 demonstrates the rationale of the approach. We first showed that SLK bound to platelets in the same manner as A11, whereas the control scFv Ab (Ctl) had no effect (Figure 4A). Both A11 and SLK did not bind to β3−/− intact mouse platelets, indicating specificity of binding to intact β3 platelets (data not shown). We then demonstrated that SLK has a specific binding to partially degraded fibrin for various time intervals compared with A11 (Figure 4B), whereas SLK had no binding effect on intact fibrinogen, fibronectin, or collagen (data not shown).
Comparison of scFv-A11 and bifunctional fusion Ab (SLK) on platelet and fibrin binding. (A) Binding ability of A11 and SLK to platelets. Platelets were coated onto 96-well plates (1 × 106/well) at 4°C overnight. Various concentrations of scFv A11 or SLK were diluted in PBS, respectively (n = 3); 13CG2 was used as a control scFv Ab. (B) Binding activity of SLK to partially digested fibrin for various time intervals. Binding of SLK to the partially digested fibrin was monitored as described in “Enzyme-linked immunosorbent assay.” The amount of SLK Ab retained on the wells was estimated by measuring the activity of bound horseradish peroxidase. Data represent the summary of 3 independent experiment results (each time point represent 3 measurements; SEM).
Comparison of scFv-A11 and bifunctional fusion Ab (SLK) on platelet and fibrin binding. (A) Binding ability of A11 and SLK to platelets. Platelets were coated onto 96-well plates (1 × 106/well) at 4°C overnight. Various concentrations of scFv A11 or SLK were diluted in PBS, respectively (n = 3); 13CG2 was used as a control scFv Ab. (B) Binding activity of SLK to partially digested fibrin for various time intervals. Binding of SLK to the partially digested fibrin was monitored as described in “Enzyme-linked immunosorbent assay.” The amount of SLK Ab retained on the wells was estimated by measuring the activity of bound horseradish peroxidase. Data represent the summary of 3 independent experiment results (each time point represent 3 measurements; SEM).
Effect of SLK on disaggregation and destruction of ex vivo platelet aggregates
Because SLK demonstrated the same binding specificity to platelet β3 as A11, we reasoned it could induce platelet oxidative fragmentation as A11. Figure 5A-B demonstrates SLK-induced human platelet fragmentation (Figure 5A) and human platelet oxidation (Figure 5B) in the same manner as A11 and anti–GPIIIa49-66 Ab, whereas control Ab had no effect. Both fragmentation and oxidation induced by A11 or SLK were inhibited by peroxide inhibitors diphenylene iodonium and catalase (data not shown), as previously reported for anti–GPIIIa49-66 Ab.1,20 A similar mode of action was demonstrated with mouse platelets (supplemental Figure 2). Because A11 has a preferential effect on TFLLRN-activated platelets,5 we reasoned that SLK might preferentially bind with platelets pretreated with the thrombin receptor agonist TFLLRN. Figure 5C demonstrates this to be the case: increased sensitivity of TFLLRN-activated platelets to SLK, particularly at low SLK concentration (∼ 2-fold greater sensitivity). Figure 5D demonstrates that both A11 and SLK disaggregate collagen-induced platelet clumps to a similar extent, with a nadir at 7 hours, whereas control Ab was without effect. To determine whether decreased aggregate size was a function of disaggregation or platelet destruction or both, we assayed LDH release into the media. As could be predicted from previous experiments, which described Ab-induced oxidative platelet fragmentation in vitro with LDH release, similar results were obtained with A11 or SLK measurements of LDH release (∼ 11.5-fold at 1 hour above background; data not shown). Thus, A11 and SLK induce a combination of platelet clump disaggregation and destruction.
Effect of SLK on platelet oxidative fragmentation and dissolution of ex vivo collagen-induced platelet aggregates. (A) Effect of SLK on platelet fragmentation and (B) platelet DCFH oxidation. C indicates control IgG; and Pt, patient IgG. Black, gray, horizontal, vertical, and white bars represent serial dilutions of Ab, starting at 12.5 μg /mL. Data are representative of 2 different experiments. (C) Effect of prestimulated platelet PAR-1 on induction of platelet fragmentation with SLK. Gel-filtered platelets were treated with the thrombin PAR-1 agonist TFLLRN (200μM) for 30 minutes at 37°C and then followed with various SLK concentrations (5-40 μg/mL) for 4 hours. Gray and black bars represent without and with TFLLRN (T) prestimulation, respectively. Note increased sensitivity of activated platelets to SLK at low SLK concentration. (D) Collagen platelet aggregate. Platelet aggregates were prepared by incubating gel-filtered platelets with 1 μg/mL collagen at 37°C for 1 hour in Tyrode buffer with intermittent shaking. A11 or SLK or Ctl scFv (13CG2) was incubated with aggregates at different time points at 37°C. The numbers of platelet/aggregates were then counted. Data and SEM are given for 3 separate experiments at 0.85μM reagent in which each time point represents 5 measurements.
Effect of SLK on platelet oxidative fragmentation and dissolution of ex vivo collagen-induced platelet aggregates. (A) Effect of SLK on platelet fragmentation and (B) platelet DCFH oxidation. C indicates control IgG; and Pt, patient IgG. Black, gray, horizontal, vertical, and white bars represent serial dilutions of Ab, starting at 12.5 μg /mL. Data are representative of 2 different experiments. (C) Effect of prestimulated platelet PAR-1 on induction of platelet fragmentation with SLK. Gel-filtered platelets were treated with the thrombin PAR-1 agonist TFLLRN (200μM) for 30 minutes at 37°C and then followed with various SLK concentrations (5-40 μg/mL) for 4 hours. Gray and black bars represent without and with TFLLRN (T) prestimulation, respectively. Note increased sensitivity of activated platelets to SLK at low SLK concentration. (D) Collagen platelet aggregate. Platelet aggregates were prepared by incubating gel-filtered platelets with 1 μg/mL collagen at 37°C for 1 hour in Tyrode buffer with intermittent shaking. A11 or SLK or Ctl scFv (13CG2) was incubated with aggregates at different time points at 37°C. The numbers of platelet/aggregates were then counted. Data and SEM are given for 3 separate experiments at 0.85μM reagent in which each time point represents 5 measurements.
Effect of A11 and SLK on platelet count and bleeding time in vivo and platelet function in vitro
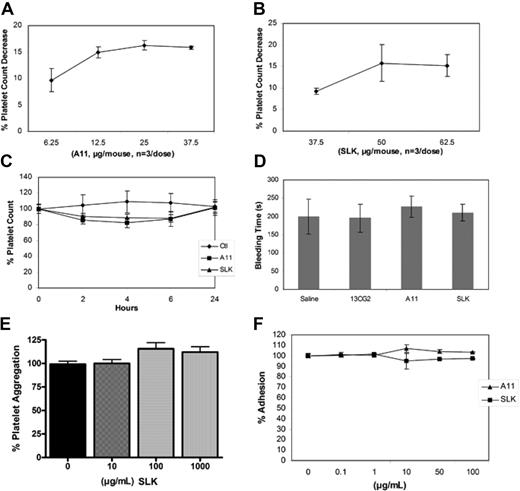
Because previous studies have revealed that a nadir of platelet drop induced by anti–GPIIIa49-66 Ab or its mimic Abs was at 4 hours with recovery to normal at 24 hours,1,4,5,20 we first tested A11 and SLK on induction of maximum platelet drop 4 hours after injection. Injection of various doses of A11 and SLK resulted in an approximately 10% to 17% (Figure 6A) and an approximately 9% to 15% (Figure 6B) platelet drop in mice, respectively. We next tested the effect of the optimal dose of A11 and SLK over 24 hours. Figure 6C demonstrated that injection of an optimal dose of A11 (25 μg/mouse) resulted in an average 17.8% drop in the platelet count at the 4-hour nadir postinjection time point (P < .006 t = 4 hour vs t = 0, by 2-tailed t test), whereas injection of the optimal dose of SLK (37 μg/mouse) produced an average 11% drop at the 4-hour nadir postinjection time point (P = .018 t = 4 hour vs t = 0, by 2-tailed t test, P < .012 A11 t = 4 hour vs SLK t = 4 hour). Injection of control 13CG2 (20 μg/mouse) did not significantly affect the platelet count (P = .41). This result confirmed our previous reports. Figure 6D demonstrated that both A11 and SLK had no significant effect on the mouse tail vein bleeding time 4 hours (nadir time point) after intravenous injection of Abs. Because both A11 and SLK preferentially bind to activated platelets, we reasoned that A11 and SLK will have little effect on resting platelet function. Figure 6E demonstrated that ADP-induced mouse platelet aggregation in vitro was unaffected by different doses of SLK. A similar result was obtained with A11 (data not shown). Supplemental Figure 3 demonstrates representative mouse and human platelet aggregation traces initiated by different platelet agonists. Different doses of A11 and SLK have no effect on platelet adhesion to immobilized fibrinogen (Figure 6F).
Effect of A11 and SLK on mouse platelet function in vivo and in vitro. (A) Effect of various doses of A11 or SLK (B) on platelet count decrease at the 4-hour nadir time point after injection in BALB/C mice. (C) Effect of Ab on induction of platelet count drop at different time points. Purified control scFv 13CG2, A11, and SLK were injected intravenously into BALB/C mice, and platelet counts followed for 24 hours; n = 4 (SEM). (D) Effect of Ab on mouse bleeding time. BALB/C mice were intravenously injected with control scFv 13CG2, A11, or SLK, and their bleeding time was monitored 4 hours later; n = 10 (SEM). (E) Effect of A11 and SLK on ADP-induced mouse platelet aggregation (%). Platelet aggregation was induced as the method described. Data are mean ± SD of at least 3 different determinations. (F) Effect of A11 and SLK on mouse platelet adhesion to immobilized ligands. Platelet adhesion was performed as described in “Assay of platelet aggregation and adhesion.” The extent of adhesion was expressed as the percentage of control platelets adhered without preincubation with A11 or SLK. Data are mean ± SD of at least 3 different determinations.
Effect of A11 and SLK on mouse platelet function in vivo and in vitro. (A) Effect of various doses of A11 or SLK (B) on platelet count decrease at the 4-hour nadir time point after injection in BALB/C mice. (C) Effect of Ab on induction of platelet count drop at different time points. Purified control scFv 13CG2, A11, and SLK were injected intravenously into BALB/C mice, and platelet counts followed for 24 hours; n = 4 (SEM). (D) Effect of Ab on mouse bleeding time. BALB/C mice were intravenously injected with control scFv 13CG2, A11, or SLK, and their bleeding time was monitored 4 hours later; n = 10 (SEM). (E) Effect of A11 and SLK on ADP-induced mouse platelet aggregation (%). Platelet aggregation was induced as the method described. Data are mean ± SD of at least 3 different determinations. (F) Effect of A11 and SLK on mouse platelet adhesion to immobilized ligands. Platelet adhesion was performed as described in “Assay of platelet aggregation and adhesion.” The extent of adhesion was expressed as the percentage of control platelets adhered without preincubation with A11 or SLK. Data are mean ± SD of at least 3 different determinations.
Ex vivo assay of the effect of A11 and SLK on platelet function
To further investigate a long-term in vivo effect of A11 and SLK on platelet function, platelets from mice treated for 4 hours with different agents were activated by a range of platelet agonists. Supplemental Figure 4A summarizes the mean fluorescence intensity of P selectin expression on activated platelets. For thrombin-stimulated platelets, the mean fluorescence intensity was 6.86 plus or minus 0.09 (13CG2) versus 6.57 plus or minus 0.21 (A11) versus 7.05 plus or minus 0.10 (SLK) compared with resting platelets 2.63 plus or minus 0.13 (P < .01). For collagen-stimulated platelets, the MFI was 5.91 plus or minus 0.09 (13CG2) versus 6.48 plus or minus 0.50 (A11) versus 6.31 plus or minus 0.32 (SLK). Supplemental Figure 4B demonstrates that thrombin-stimulated platelet-monocyte aggregates were 63% plus or minus 7.8% (13CG2) versus 61% plus or minus 5.4% (A11) versus 65% plus or minus 14% (SLK) compared with resting platelets 10.1% plus or minus 4.3% (P < .01). Collagen-stimulated platelet-monocyte aggregates were 70.5% plus or minus 21% (13CG2) versus 72% plus or minus 14% (A11) versus 65% plus or minus 11% (SLK). These results further confirm that the therapeutic results of A11 and SLK are not the result of altered platelet function.
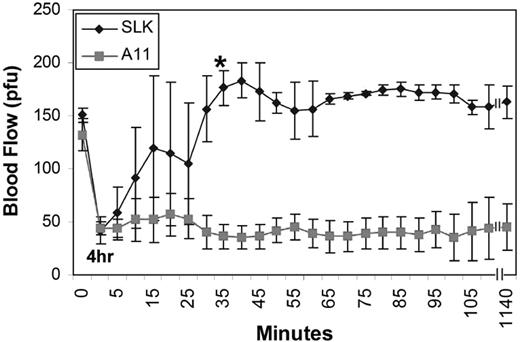
SLK prevents cerebral stroke formation and reopens a platelet-thrombus induced occluded carotid artery 4 hours after cessation of blood flow
Because A11 prevents and ameliorates cerebral stroke formation, we reasoned that SLK might have the same effect. Supplemental Figure 5 demonstrates this to be the case. There was a clear protective effect on infarction in SLK-pretreated mice compared with control [23.28 ± 5.91 vs 49.32 ± 1.51, P < .001 by 2-tailed t test, SLK (n = 9) vs vehicle group (n = 20)]. To further examine whether enhanced fibrin targeting ability of SLK will make it more potent than A11, SLK was then tested in a more rigorous carotid artery thrombus model in which the carotid blood flow was occluded for 4 hours to 15% of normal blood flow. Figure 7 demonstrates that, although 4 of 4 A11-treated mice had no effect at 4 hours, 3 of 3 SLK-treated mice resumed normal blood flow by 5 to 40 minutes after injection. P < .001 by 2-way ANOVA for treatment effect. Post hoc Bonferroni testing indicated P < .01, at 35 minutes, P < .01 at 40 to 50 minutes, and P < .001 from 55 minutes to 19 hours. Note that no blood flow reocclusion was observed after SLK treatment within the extended monitoring time of 19 hours.
Effect of bifunctional A11-plasminogen Kringle (SLK) on reinitiation of carotid artery flow after 4 hours of thrombus inhibition of blood flow. Intravenous infusion of A11 (25 μg/mouse) had no effect; n = 4 (SEM). SLK (37.5 μg/mouse) restored blood flow after 4 hours of cessation of blood flow; n = 3 (SEM). P < .001 for treatment effect, by 2-way ANOVA. Post hoc Bonferroni testing indicated P < .05 at 35 minutes, P < .01 at 40 to 50 minutes, and P < .001 from 55 minutes to 19 hours. * indicates the time point from which the SLK-treated mice were statistically different from controls by post-hoc testing.
Effect of bifunctional A11-plasminogen Kringle (SLK) on reinitiation of carotid artery flow after 4 hours of thrombus inhibition of blood flow. Intravenous infusion of A11 (25 μg/mouse) had no effect; n = 4 (SEM). SLK (37.5 μg/mouse) restored blood flow after 4 hours of cessation of blood flow; n = 3 (SEM). P < .001 for treatment effect, by 2-way ANOVA. Post hoc Bonferroni testing indicated P < .05 at 35 minutes, P < .01 at 40 to 50 minutes, and P < .001 from 55 minutes to 19 hours. * indicates the time point from which the SLK-treated mice were statistically different from controls by post-hoc testing.
Effect of SLK on postischemic stroke
Because SLK is more potent than A11 in the carotid artery thrombus model, we designed an experiment to compare their effect in postischemic stroke on cerebral infarction and neurologic function, 4 hours after removal of the occlusion. Table 2 demonstrates that, although A11 had no effect, SLK protected against infarct by approximately 34% (P = .037) and significantly preserved neurologic function (postural reflex by ∼ 60%) without cerebral bleeding. Representative immunohistochemistry staining of reactive astrocytes using anti-GFAP show that SLK given 4 hours and A11 given 2 hours after ischemic stroke dramatically decreased gliosis, as assessed by GFAP immunostaining density, compared with saline control (supplemental Figure 6). No microscopic hemorrhage was observed.
Comparison of A11 vs SLK on day 8, 4 hours after removal of obstruction
| . | N . | Infarction, % . | Postural reflex* . | Rotor rod† . | Traverse beam† . |
|---|---|---|---|---|---|
| A11 | 11 | 42.16 ± 13.47 (n = 8) | 2.73 ± 0.55 | 3.42 ± 1.61 | 4.33 ± 1.81 |
| SLK | 12 | 27.88 ± 12.26 (n = 9) | 1.08 ± 0.72 | 4.93 ± 1.71 | 5.95 ± 1.15 |
| P | .037 | < .001 | .05 | .018 |
| . | N . | Infarction, % . | Postural reflex* . | Rotor rod† . | Traverse beam† . |
|---|---|---|---|---|---|
| A11 | 11 | 42.16 ± 13.47 (n = 8) | 2.73 ± 0.55 | 3.42 ± 1.61 | 4.33 ± 1.81 |
| SLK | 12 | 27.88 ± 12.26 (n = 9) | 1.08 ± 0.72 | 4.93 ± 1.71 | 5.95 ± 1.15 |
| P | .037 | < .001 | .05 | .018 |
With postural reflex, a higher number reflects a higher number of errors and greater neurologic impairment.
With rotor rod and traverse beam score, a higher number reflects greater neurologic impairment. No microscopic hemorrhage was seen.
Confocal fluorescence imaging of binding activity of different agents to rhodamine-labeled platelet thrombus in vivo
Because SLK provided the best stroke protection, we reasoned that it is because SLK is enriched on platelet-rich clots resulting from its additional binding to fibrin, which is also an important constituent of platelet-rich clots. We therefore compared the binding activity of control 13CG2, A11, and SLK using confocal fluorescent microscopy. Supplemental Figure 7 demonstrates that SLK (green dye) could bind with rhodamine-labeled platelet thrombus (red dye) in vivo more strongly compared with A11 and control scFv (13CG2) Ab. The integrated fluorescence intensity associated with SLK was significantly higher than that associated with A11 or control antibodies (SLK: 1.79 × 107 ± 1.8 × 106; vs A11: 6.95 × 106 ± 0.35 × 106; and 13CG2: 2.47 × 106 ± 0.52 × 106, average of 3 independent experiments; P = .001).
Discussion
Integrin αIIbβ3 (platelet glycoprotein GPIIb/IIIa) is the fibrinogen receptor of platelets. It plays a key role in platelet aggregation and adhesion. When stimulated by platelet agonists, such as ADP or thrombin, integrin αIIbβ3 undergoes rapid conformational changes and leads to platelet binding of circulating adhesive macromolecules, particularly fibrinogen and von Willebrand factor resulting in the formation of platelet clots.10 Currently, there are 3 integrin αIIbβ3 antagonists that have been tested clinically for acute ischemic stroke, with current results being disappointing.10 Abciximab is a chimeric Fab created based on a murine mAb 7E3. Its mechanism of action is thought to be spatial hindrance of the receptor. Eptifibatide is a synthetic heptapeptide and tirofiban is a nonpeptide antagonist, both mimicking the RGD sequence of the receptor binding site. All exhibit high-affinity binding to integrin αIIbβ3, resulting in inhibition of ex vivo platelet aggregation and induction of thrombocytopenia.10,21 We show that A11 and SLK have a different mechanism of action and have distinct properties that may offer advantages over these existing agents.
We show that A11 is unique in that it has no effect on platelet function and minimal effect on platelet count (∼ 18% decrease). A further advantage is that it does not present foreign Ag to the human host with a potential for immunologic hypersensitivity.22 One reason A11 produces little thrombocytopenia is the lack of an Fc domain. We have previously shown that injection of HIV-1-ITP patient's anti–GPIIIa49-66 IgG into C57BL/6 mice induces a 80% drop in platelet count, versus only a 25% drop when anti–GPIIIa49-66 F(ab′)2 is injected.1 In addition, A11 preferentially binds to activated versus resting platelets.6 Clustering of the αIIbβ3 integrin occurs with platelet activation and enhances A11 binding.6 Increased binding to TFLLRN-activated platelets has also been demonstrated for ADAMTS-18, a disintegrin metalloproteinase with thrombospondin motifs produced in endothelial cells, which induces oxidative platelet fragmentation in an identical manner to anti–GPIIIa49-66 Ab. Hence A11 may be safer and more efficient than conventional anti-GPIIIa Abs, which block platelet function and induce thrombocytopenia and mortality, possibly because of GPIIb-IIIa activation.21,23-26
Our studies clearly demonstrate the efficacy of A11 in preventing the development of FeCl3-induced carotid artery thrombi as well as postischemic middle cerebral artery infarction. Furthermore, A11 corrects carotid artery thrombus-induced cessation of blood flow at 2 hours and ameliorates postischemic stroke at 2 hours.
We next reasoned that we could enhance the protective effect of A11 by producing a recombinant bifunctional reagent in which A11 is coupled to the first kringle of plasminogen, a known human product that homes to terminal lysines of partially digested fibrin within the arterial thrombus.12 This approach was highly successful in producing a bifunctional SLK, which we show to be associated with an even more modest platelet count drop compared with A11 (11% vs 18%) and to preferentially bind platelets activated with the thrombin PAR-1 receptor agonist TFLLRN (Figure 5C). We also show that both A11 and SLK have little effect on platelet aggregation, adhesion, and bleeding time. This is probably related to the fact that A11 and SLK do not contain RGD sequences or mimics of the RGD sequence, in contrast to other anti-αIIbβ3 targeting agents.10,27 The lack of an effect on tail bleeding time by A11 and SLK is probably linked to the marked differences in platelet content in arterial versus venous thrombi. Venous thrombi have a much higher red cell content compared with arterial thrombi; hence, A11/SLK can dissolve arterial platelet thrombi with no significant effect on tail bleeding time. In addition, we show that SLK is more potent than A11. Although A11 was protective 2 hours after cessation of carotid artery blood flow or postischemic stroke (Figure 3), it was not protective 4 hours after cessation of blood flow or induction of stroke. In contrast, SLK is effective at 4 hours after both cessation of blood flow or induction of stroke (Figure 7). Some variation was noted in the degree of protection from infarction with A11, with 4 of the 10 mice having less protection. This degree of variation is similar to our previous studies using this ischemia model and is probably related to variability in the cerebral circulation of individual experimental animals.5,14 The enhanced activity of SLK compared with A11 in vivo is related to its increased local concentration at the platelet thrombus. As shown in the fibrin binding study, SLK binds specifically to partially degraded fibrin, which is dotted around the platelet thrombus, with A11 lacking this binding. We have previously shown that tPA in a cerebral stroke model similar to what we have used in our current study, protects from infarction when given at 2 hours but not at 4 hours, with 4 of 12 mice dying as a result of intracranial hemorrhage as a complication of tPA use.14 Thus, SLK appears to have advantages over tPA with respect to its time window efficacy and associated hemorrhage complications, at least in mouse stroke models. Our observations with A11 and SLK are confirmed by protection against neuropathologically documented damage, as well as brain function.
The advantages of A11 and SLK over the previously described antiplatelet Abs include: (1) dissolution of the already formed platelet thrombus; (2) no measurable effect on platelet function or bleeding; (3) a human Ab that is unlikely to act as a foreign antigen giving rise to hypersensitivity; (4) absence of an Fc domain, so that it will not activate complement and an inflammatory phagocytic/cytokine response; (5) the small size of the scFv allows greater penetration of tissue, compared with the full-length Ab; and (6) preferential binding to activated versus resting platelets.
Because of these advantages, the scFv format is now being used widely for other targets and is entering clinical trials.28,29 The ease of cloning in bacteria offers the opportunity of genetic engineering, such as further optimization and construction of fusion molecules,30 as we have done with SLK. In contrast to the production of mAbs in hybridoma or other mammalian cells, scFvs can be produced in bacteria at low cost and are easily amenable to commercial scale up. In addition, a technical advantage is the ability to add identification flags (eg, His tag) to allow for high-purity production using affinity columns. Hence, we have described a novel antithrombotic agent for platelet thrombus clearance, via platelet membrane oxidative fragmentation, which can be exploited clinically to treat arterial platelet thrombi for cerebral stroke and/or myocardial infarction.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Yan Deng of the Imagine Core Facility of the New York University Skirball Institute of Biomolecular Medicine who assisted with obtaining confocal data.
This work was supported by the National Institutes of Health (grants AG20245, AG15408, DA020816, and DA004315).
This manuscript is dedicated to the memory of Dr Simon Karpatkin who tragically passed away on August 21, 2009.
National Institutes of Health
Authorship
Contribution: W.Z., Y.-S.L., M.A.N., S.D., J.Y., Y.J., and Z.L. performed research and analyzed data; S.K. and T.W. designed the research and analyzed data; and W.Z., S.K., and T.W. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Thomas Wisniewski, New York University School of Medicine, Millhauser Laboratory, Rm HN419, 550 First Ave, New York, NY 10016; e-mail: thomas.wisniewski@nyumc.org.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal