Abstract
The specification of arterial, venous, and lymphatic endothelial cell fate is critical during vascular development. Although the homeobox transcription factor, Prox1, is crucial for the specification and maintenance of lymphatic endothelial cell identity, little is known regarding the mechanisms that regulate Prox1 expression. Here we demonstrate that miR-181a binds the 3′ untranslated region of Prox1, resulting in translational inhibition and transcript degradation. Increased miR-181a activity in primary embryonic lymphatic endothelial cells resulted in substantially reduced levels of Prox1 mRNA and protein and reprogramming of lymphatic endothelial cells toward a blood vascular phenotype. Conversely, treatment of primary embryonic blood vascular endothelial cells with miR-181a antagomir resulted in increased Prox1 mRNA levels. miR-181a expression is significantly higher in embryonic blood vascular endothelial cells compared with lymphatic endothelial cells, suggesting that miR-181 activity could be an important mechanism by which Prox1 expression is silenced in the blood vasculature during development. Our work is the first example of a microRNA that targets Prox1 and has implications for the control of Prox1 expression during vascular development and neo-lymphangiogenesis.
Introduction
The lymphatic vasculature is an integral component of the cardiovascular system and fulfills critical roles during development and disease.1-3 Lymphatic vessels return interstitial fluid and protein to the bloodstream, absorb lipids from the digestive tract, and transport cells of the immune system during immune surveillance and inflammation. Aberrant lymphangiogenesis is a feature of the human disorders lymphedema, inflammatory diseases, and tumor metastasis.1-3 The identification of key prolymphangiogenic and antilymphangiogenic signals is currently the subject of intense investigation and will underpin the generation of novel therapeutic agents targeted to treat lymphatic vascular diseases.
The specification of arterial, venous, and lymphatic endothelial cell (LEC) fate is critical during vascular development. The homeobox transcription factor Prox1 is essential both for the specification of LEC fate and the maintenance of LEC identity; Prox1 is considered a master regulator of LEC phenotype.4-6 Despite the importance of Prox1 for lymphangiogenesis, little is known about the mechanisms by which Prox1 expression is controlled in endothelial cells, although recent work has demonstrated that the transcription factors Sox187 and Coup-TFII8,9 are critical for the induction of Prox1 expression in lymphatic endothelial progenitor cells located within the embryonic veins.
MicroRNAs (miRNAs) are endogenous, non–protein-coding small RNAs that play critical roles in the post-transcriptional regulation of target genes by directing target mRNAs for cleavage, translational repression, or destabilization.10 A select number of microRNAs have been shown to play key roles in embryonic vascular development11-13 and postnatal/pathologic angiogenesis14,15 and pose attractive targets for the generation of novel therapeutic agents to treat vascular diseases and cancer.16,17 The miR-181 family of microRNAs is expressed in muscle,18 eye,19 and the hemopoietic compartment20,21 ; established target genes in these tissues include HoxA1118 and Tcl1.22
In this study, we demonstrate that the microRNA miR-181a is expressed in endothelial cells and binds to the Prox1 3′-untranslated region (UTR), resulting in rapid and efficient transcript degradation and translational inhibition. This work reveals that miR-181a activity may comprise one mechanism by which Prox1 expression is finely tuned in endothelial cells and normally silenced in the blood vasculature. Our work is the first example of a microRNA that targets Prox1 and has important implications for the control of Prox1 expression during vascular development and in settings of neo-lymphangiogenesis.
Methods
Animal studies
Experiments using mice were performed using C57Bl/6 mice and were approved and conducted in accordance with the SA Pathology Animal Ethics Committee and Australian National Health and Medical Research Council guidelines.
DNA constructs
A 1.35-kb element of the Prox1 3′-UTR was amplified using forward 5′-CTCGAGTAGAGATTGCAACGCTCTTTTG-3′ and reverse 5′-CGCCGGCGGGGCCTGGATCACACTCTTA-3′ primers, cloned into the pGEMT-Easy vector (Promega), and the sequence verified. The predicted miR-181a “seed” site was mutated by further amplification of this construct using forward 5′- CTCGAGTAGAGATTGCAACGCTCTTTTATCAGTATGGATAGAAGAATTCC-3′ and reverse 5′-CGCCGGCGGGGCCTGGATCACACTCTTA-3′ primers, cloned into the pGEMT-Easy vector, and the mutation confirmed by sequence analysis. The wild-type and mutated 3′-UTRs were subcloned into the NotI-XhoI sites of the psiCHECK2 vector (Promega).
Cell isolation and culture
HeLa cells were maintained in Dulbecco Modified-Eagle Medium (DMEM) supplemented with 10% fetal bovine serum (FBS). Embryonic mouse LECs and blood endothelial cells (BECs) were isolated from embryonic day (E) 16.5 or E18.5 dermis as follows: embryonic skin was dissected in Hanks balanced salt solution supplemented with 10mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid and 5% FBS and digested for 30 minutes at 37°C in DMEM supplemented with 20% FBS, collagenase II (2.5 mg/mL, Worthington Biochemicals), collagenase IV (2.5 mg/mL, Worthington Biochemicals), and DNase (1 mg/mL, Worthington Biochemicals). Digested skin was filtered through a 40-μm cell strainer, and remaining cells were washed twice with DMEM/20% FBS and resuspended in Hanks balanced salt solution supplemented with 10mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid and 5% FBS. Hemopoietic cells, including LYVE-1(+) macrophages, were depleted from the cell mixture using the MiniMACS system (Miltenyi Biotec) and antibodies to mouse F4/80 (Invitrogen) and CD45 (BD Biosciences PharMingen) according to the manufacturer's instructions. LECs and BECs were subsequently purified using MiniMACS and anti–mouse LYVE-1 (AngioBio), followed by anti–mouse CD31 (BioLegend), respectively. Isolated primary dermal LECs and BECs were either used immediately for RNA and protein isolation or plated on fibronectin (Roche Diagnostics)–coated dishes in EBM-2 medium supplemented with EGM-2 MV SingleQuots (Lonza) and grown at 37°C/5% CO2. Adult LECs and BECs were isolated from the ears of female mice essentially as described, with the exception that skin was digested for 60 minutes.
Transfections
All cells were transfected using Lipofectamine 2000 (Invitrogen), according to the manufacturer's instructions. HeLa cells were plated in 24-well dishes at 4 × 105 cells/well and grown overnight at 37°C/5% CO2 before cotransfection with 0.1 μg plasmid and 25 pmol pre-miR miRNA precursor or negative control (Ambion). Cells were harvested 48 hours after transfection and subjected to luciferase assays. LECs were plated on fibronectin coated 24-well dishes or 8-well chamber slides at approximately 105 cells/well, cultured for 48 hours, and subsequently transfected with 25 pmol (42.6nM final concentration) pre-miR miRNA precursor. Cells were harvested 64 hours later and subjected to RNA and protein analyses. BECs were plated on fibronectin-coated 24-well dishes at approximately 2 × 105 cells/well, cultured for 72 hours, and transfected with 30 pmol (50nM final concentration) anti-miR miRNA inhibitor (Ambion). Cells were harvested after 64 hours and subjected to RNA analysis.
Immunofluorescence
For analysis of cell purity, primary LECs and BECs isolated from E16.5 embryos were grown on fibronectin-coated chamber slides for 5 days (LECs) or 2 days (BECs), fixed with 4% phosphate-buffered paraformaldehyde and incubated with antibodies against Prox1 (AngioBio), CD31 (BioLegend), CD45 (BD Biosciences PharMingen), LYVE-1 (AngioBio), and CD34 (eBioscience). Transfected LECs were fixed 64 hours after transfection with 4% paraformaldehyde and incubated with antibodies against Prox1 (AngioBio) and CD31 (BioLegend). Alexa-Fluor-488 and Alexa-Fluor-555–conjugated secondary antibodies (Invitrogen) were used for visualization. Cells were mounted in Prolong Gold with 4,6-diamidino-2-phenylindole (Invitrogen). Images were captured at room temperature with an Olympus DP70 camera attached to an Olympus BX51TF microscope, using an Olympus UPlanApo 20×/0.7 ∞/0.17 lens. Images were processed with Olysia Bioreport software Version 3.2 (Olympus).
Luciferase assay
Luminescent signals arising from psiCHECK2-transfected cells were analyzed using the Dual Luciferase Reporter Assay System (Promega) and quantified with a FLUOstar Optima Microplate Reader (BMG Labtech). Renilla luciferase signal was normalized to signal arising from the intraplasmid firefly luciferase transfection reporter cassette.
RNA analysis
Total RNA was isolated from transfected cells using TRIzol reagent (Invitrogen) according to the manufacturer's directions. RNA quality was assessed using a Bioanalyser (Agilent Technologies); all samples achieved an RNA Integrity Number (RIN) score more than 9.5. For investigation of mRNA expression, total RNA was reverse transcribed using Superscript III Reverse Transcriptase (Invitrogen) with a mixture of oligo dT and random hexamer primers. Primers used for real-time reverse-transcribed polymerase chain reaction (RT-PCR) analysis are shown in Table 1. PCR was performed with RT2 Real-Time SYBR Green/Rox PCR master mix (SA Biosciences) and analyzed on a Corbett Rotor-Gene 6000. Data were normalized to the housekeeping gene Actb as previously described.23 Quantification of mature miR-181 isoforms in primary embryonic and adult mouse LECs and BECs was performed using a miRCURY LNA Universal RT microRNA cDNA synthesis kit (Exiqon), SYBR Green master mix, Universal RT (Exiqon), LNA PCR primer sets (miR-181a: Exiqon catalog no. 204566, miR-181b: catalog no. 204530, miR-181c: catalog no. 204683), and normalized to U6 snRNA (Exiqon catalog no. 203907).
Primers used for real-time RT-PCR analysis
| Gene . | Forward primer . | Reverse primer . |
|---|---|---|
| Prox1 | 5′-CTGGGCCAATTATCACCAGT-3′ | 5′-GCCATCTTCAAAAGCTCGTC-3′ |
| Vegfr3 | 5′-CTGGCCAGAGGCACTAAGAC-3′ | 5′-CAGGGTGTCCTCTGGGAATA-3′ |
| Itga9 | 5′-GCCAGGCCGGAATAGCAGGC-3′ | 5′-GTGGCCAGCCGTCACTGCAT-3′ |
| Cd34 | 5′-TCCCCATCAGTTCCTACCAA-3′ | 5′-CAGTTGGGGAAGTCTGTGGT-3′ |
| Nrp1 | 5′-AAACCTTGGTGGAATTGCTG-3′ | 5′-TGGCTTCCTGGAGATGTTCT-3′ |
| Flt1 | 5′-AGCACCTTGACCTTGGACAC-3′ | 5′-CAGGGGATGATGAGCTGTCT-3′ |
| Pecam1 | 5′-AACAGAAACCCGTGGAGATG-3′ | 5′-GTCTCTGTGGCTCTCGTTCC-3′ |
| Actb | 5′-GATCATTGCTCCTCCTGAGC-3′ | 5′-GTCATAGTCCGCCTAGAAGCAT-3′ |
| Pdpn | 5′-ATGGCTTGCCAGTAGTCACC-3′ | 5′-TCCTCCACAGGAAGAGGATG-3′ |
| Vcam1 | 5′-TACACCATCCGCCAGGCACA-3′ | 5′-GAGTGCAAGGAGTTCGGGCG-3′ |
| Emr1 | 5′-CCATTGCCCAGATTTTCATC-3′ | 5′-GGTCAGTCTTCCTGGTGAGG-3′ |
| Gene . | Forward primer . | Reverse primer . |
|---|---|---|
| Prox1 | 5′-CTGGGCCAATTATCACCAGT-3′ | 5′-GCCATCTTCAAAAGCTCGTC-3′ |
| Vegfr3 | 5′-CTGGCCAGAGGCACTAAGAC-3′ | 5′-CAGGGTGTCCTCTGGGAATA-3′ |
| Itga9 | 5′-GCCAGGCCGGAATAGCAGGC-3′ | 5′-GTGGCCAGCCGTCACTGCAT-3′ |
| Cd34 | 5′-TCCCCATCAGTTCCTACCAA-3′ | 5′-CAGTTGGGGAAGTCTGTGGT-3′ |
| Nrp1 | 5′-AAACCTTGGTGGAATTGCTG-3′ | 5′-TGGCTTCCTGGAGATGTTCT-3′ |
| Flt1 | 5′-AGCACCTTGACCTTGGACAC-3′ | 5′-CAGGGGATGATGAGCTGTCT-3′ |
| Pecam1 | 5′-AACAGAAACCCGTGGAGATG-3′ | 5′-GTCTCTGTGGCTCTCGTTCC-3′ |
| Actb | 5′-GATCATTGCTCCTCCTGAGC-3′ | 5′-GTCATAGTCCGCCTAGAAGCAT-3′ |
| Pdpn | 5′-ATGGCTTGCCAGTAGTCACC-3′ | 5′-TCCTCCACAGGAAGAGGATG-3′ |
| Vcam1 | 5′-TACACCATCCGCCAGGCACA-3′ | 5′-GAGTGCAAGGAGTTCGGGCG-3′ |
| Emr1 | 5′-CCATTGCCCAGATTTTCATC-3′ | 5′-GGTCAGTCTTCCTGGTGAGG-3′ |
RT-PCR indicates reverse transcribed–polymerase chain reaction.
Western blotting
All protein was isolated using TRIzol reagent. After RNA extraction, protein was recovered from the remaining organic phase according to the manufacturer's instructions. For analysis of protein levels in transfected cells, equal amounts of protein isolated from 5 separate transfections were pooled and electrophoresed on 8% sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels before being transferred to polyvinylidene difluoride (Perkin Elmer). Western blots were performed according to standard protocols using anti-Prox1 (AngioBio) and anti–β-actin (Sigma-Aldrich) antibodies, followed by anti–rabbit AP (GE Healthcare) and anti–mouse Cy5 (GE Healthcare). Signal was detected using ECF Western Blot substrate (GE Healthcare), and blots were directly scanned on a Typhoon Imager (GE Healthcare). Densitometry was performed with ImageQuant TL software (GE Healthcare).
Results
miR-181a binds to the Prox1 3′-UTR, resulting in transcript repression
miRNAs are endogenous, non–protein-coding small RNAs that play critical roles in the post-transcriptional regulation of target genes by directing target mRNAs for cleavage, translational repression, or destabilization.10 We and others24 have observed that the expression of Prox1 mRNA does not always correlate with the presence of Prox1 protein in embryonic tissues, a key indication that Prox1 mRNA may be subject to post-transcriptional control. To assess whether Prox1 mRNA may be repressed by an miRNA, TargetScan (www.targetscan.org) was used to search for conserved miRNA binding sites in the Prox1 3′-UTR. A highly conserved 8mer site complementary to the seed region of the miR-181 family was identified just downstream of the Prox1 stop codon (Figure 1A).
miR-181a binds to the Prox1 3′-UTR, resulting in transcript repression. (A) Cross-species sequence alignment of the proposed miR-181 binding site located in the 3′-UTR (nucleotides 1-50 after the stop codon shown) of the Prox1 transcript. The miR-181 binding site is boxed and shaded. Con indicates conserved residues (100% conservation across species). (B) Luciferase reporter assay illustrating that miR-181a binds to the 3′-UTR of Prox1, resulting in transcript repression and reduced luciferase expression in HeLa cells. miR-181a does not repress luciferase activity when coexpressed with a Prox1 mutant construct in which the miR-181a binding site is mutated: Prox1 3′-UTR (mutant; mutated residues highlighted in bold and underlined). Renilla luciferase signal is normalized to the internal firefly luciferase transfection control; n = 6. **P < .001. Data are mean ± SEM. P values were calculated using Student paired t test.
miR-181a binds to the Prox1 3′-UTR, resulting in transcript repression. (A) Cross-species sequence alignment of the proposed miR-181 binding site located in the 3′-UTR (nucleotides 1-50 after the stop codon shown) of the Prox1 transcript. The miR-181 binding site is boxed and shaded. Con indicates conserved residues (100% conservation across species). (B) Luciferase reporter assay illustrating that miR-181a binds to the 3′-UTR of Prox1, resulting in transcript repression and reduced luciferase expression in HeLa cells. miR-181a does not repress luciferase activity when coexpressed with a Prox1 mutant construct in which the miR-181a binding site is mutated: Prox1 3′-UTR (mutant; mutated residues highlighted in bold and underlined). Renilla luciferase signal is normalized to the internal firefly luciferase transfection control; n = 6. **P < .001. Data are mean ± SEM. P values were calculated using Student paired t test.
To assess whether miR-181a was capable of binding to and repressing Prox1 mRNA, a 1.35-kb fragment of the Prox1 3′-UTR, containing the single predicted consensus miR-181a binding site, was cloned into the psiCHECK-2 luciferase reporter construct. Luciferase activity was then monitored in HeLa cells that do not express substantial levels of endogenous Prox1. Cotransfection of the Prox1 reporter construct together with miR-181a resulted in significantly reduced luciferase activity compared with cotransfection of the Prox1 reporter construct together with a control microRNA (Figure 1B). Furthermore, transfection of a Prox1 reporter construct into which a 4-bp mutation in the miR-181a binding site was engineered, together with miR-181a, abolished the repression of luciferase activity mediated by miR-181a on the wild-type construct (Figure 1B). Taken together, these data provide compelling evidence that miR-181a directly binds to and negatively regulates Prox1 expression.
miR-181a expression is elevated in embryonic BECs compared with LECs
To investigate in which tissues miR-181a regulation of Prox1 might be most relevant during development, the expression of miR-181a and Prox1 was examined by in situ hybridization and immunohistochemistry, respectively, in E14.5 mouse tissues. We observed that miR-181a and Prox1 protein were expressed in reciprocal expression patterns. For example, in the eye, miR-181 expression was low in the lens where Prox1 protein levels are high, whereas miR-181 expression was much higher in the retina (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article), where Prox1 protein levels are relatively low and restricted to retinal progenitor cells at this stage of development.25 As the initiation of Prox1 expression in a polarized population of venous endothelial cells is critical for the specification of LEC fate4,5 and the maintenance of Prox1 expression is crucial to preserve LEC identity throughout adulthood,6 we investigated whether miR-181a expression differed between primary embryonic blood and lymphatic vascular endothelial cells. Relative quantification PCR analyses using primary embryonic blood (BECs) and lymphatic (LECs) vascular endothelial cells purified from E16.5 and E18.5 mouse skin, revealed that miR-181a expression was approximately 2.5-fold higher in BECs compared with LECs at these embryonic stages (Figure 2A). Expression of 2 additional miR-181 isoforms, miR-181b and miR-181c, was also examined in BECs and LECs. Although expression of both miR-181b and miR-181c was slightly elevated in BECs compared with LECs, the levels of these isoforms present were substantially lower than that of miR-181a (Figure 2A), suggesting that miR-181a is the most biologically relevant miR-181 isoform in endothelial cells. Our subsequent analyses were therefore restricted to miR-181a. As miR-181a is expressed in cells of the hemopoietic lineage,20,21 we investigated whether potential carryover of hemopoietic cells into purified endothelial cell populations could contribute to the miR-181a expression measured in LECs and BECs; our relative quantification data revealed that the expression of miR-181a in purified LECs and BECs was substantially higher than that measured in hemopoietic cells isolated from the skin (supplemental Figure 2A), therefore discounting this possibility. Our discovery that miR-181a expression was significantly elevated in embryonic BECs compared with LECs led us to hypothesize that elevated miR-181 expression could be one mechanism used by BECs to mediate silencing of Prox1 in the embryonic blood vasculature.
Expression of miR-181 isoforms in primary LECs and BECs. (A) Quantification of miR-181 isoforms assessed by real-time RT-PCR in primary LECs and BECs isolated from (i) dermis at E16.5, (ii) dermis at E18.5, and (iii) adult ears. **P < .005. *P < .03. (Ai-ii) Data are mean ± SEM of 3 independent cell isolations from pools of 7 to 13 embryos each. (Aiii) Data are mean ± SEM of technical replicates for one isolation from 4 adult females and are representative of 3 independent experiments. P values were calculated using Student paired t test. (B) Quantification, as assessed by real-time RT-PCR, of (i) relative Prox1 mRNA levels and (ii) Pecam1 mRNA levels in LECs and BECs at indicated developmental time points. Data represent the fold difference in expression in LECs over BECs ± SEM; n = 3. (C) Analysis of Prox1 protein levels relative to β-actin by Western blot in LECs isolated at E16.5 and adult stages. Relative intensity of Prox1 staining normalized to β-actin is shown.
Expression of miR-181 isoforms in primary LECs and BECs. (A) Quantification of miR-181 isoforms assessed by real-time RT-PCR in primary LECs and BECs isolated from (i) dermis at E16.5, (ii) dermis at E18.5, and (iii) adult ears. **P < .005. *P < .03. (Ai-ii) Data are mean ± SEM of 3 independent cell isolations from pools of 7 to 13 embryos each. (Aiii) Data are mean ± SEM of technical replicates for one isolation from 4 adult females and are representative of 3 independent experiments. P values were calculated using Student paired t test. (B) Quantification, as assessed by real-time RT-PCR, of (i) relative Prox1 mRNA levels and (ii) Pecam1 mRNA levels in LECs and BECs at indicated developmental time points. Data represent the fold difference in expression in LECs over BECs ± SEM; n = 3. (C) Analysis of Prox1 protein levels relative to β-actin by Western blot in LECs isolated at E16.5 and adult stages. Relative intensity of Prox1 staining normalized to β-actin is shown.
Dynamic regulation of miR-181a and Prox1 protein levels in LECs during development
To investigate whether miR-181 expression was likely to contribute to the regulation of Prox1 expression in adult endothelial cells, the expression levels of miR-181 family members were quantitated in adult lymphatic and blood vascular endothelial cells isolated from ear skin. In contrast to the scenario in embryonic endothelial cells, miR-181a expression was elevated approximately 3.5-fold in adult LECs compared with BECs (Figure 2A). Although the level of miR-181a, as determined by relative quantification PCR, did not differ substantially between E18.5 and adult blood vascular endothelial cells, the level of miR-181a in adult LECs was elevated approximately 10-fold compared with E18.5 LECs (Figure 2A). As Prox1 expression is constantly required during embryogenesis and adulthood to maintain LEC identity,6 we next investigated the relative expression levels of Prox1 mRNA and protein in embryonic and adult LECs and BECs. Intriguingly, we observed that the expression of Prox1 mRNA was dynamically regulated in endothelial cells during development. Although Prox1 expression was elevated in LECs compared with BECs at all stages analyzed, the relative level of Prox1 expressed in LECs versus BECs was dramatically increased at E16.5 and E18.5 compared with E14.5 and adulthood (Figure 2B). This relative increase in Prox1 mRNA expression in LECs compared with BECs at E16.5 and E18.5 was the result of a combination of progressively increased Prox1 expression in LECs during development, combined with progressively diminished expression in BECs (supplemental Figure 2B). In contrast, expression of the pan-endothelial cell gene Pecam1 remained relatively constant in LECs compared with BECs throughout embryogenesis and in adulthood (Figure 2B). These data reflect the likelihood that the control of Prox1 expression in endothelial cells is subject to a combination of factors that are progressively fine-tuned during development, rather than a single “on-off” switch. In accordance with Prox1 mRNA expression levels, quantification of Prox1 protein revealed approximately 30-fold higher expression in E16.5 LECs than in adult LECs (Figure 2C). The decreased Prox1 mRNA and protein levels observed in adult LECs correlated with substantially increased miR-181a expression, suggesting that dynamic control of miR-181a expression may be one mechanism that contributes to the regulation of Prox1 within the LEC milieu, as well as to the silencing of Prox1 expression in the blood vascular endothelial cell environment.
Introduction of miR-181a to primary embryonic LECs decreases Prox1 mRNA and protein expression, resulting in reversion of LEC identity
To definitively determine whether miR-181a was able to regulate Prox1 expression in endothelial cells, miR-181a was introduced to cultured primary LECs isolated from E16.5 embryos. Primary embryonic LECs and BECs were isolated from the skin of embryos using a magnetic bead purification approach (J.K. and N.L.H., manuscript in preparation). Briefly, macrophages and hemopoietic cells were depleted from single-cell suspensions of embryonic mouse skin before the purification of LECs on the basis of LYVE-1 expression. Blood vascular endothelial cells were then isolated from the LYVE-1-negative cell fraction on the basis of Pecam1/CD31 expression. The purity of isolated embryonic LECs and BECs was assessed using both immunostaining and relative quantification PCR for markers characteristic of LECs, BECs, and macrophage identity (supplemental Figure 3) and was routinely found to be greater than 95%.
Ectopic miR-181a expression in primary LECs results in reduced Prox1 mRNA and protein levels. (A) Prox1 mRNA expression is substantially reduced in primary embryonic LECs after transfection of miR-181a, compared with transfection with a negative control (miR-neg) that has no established targets; n = 6. *P < .001. (B) Immunostaining of primary embryonic LECs transfected with control miR-neg (top panel) versus miR-181a (bottom panel), illustrating dramatic reduction in nuclear Prox1 as a result of miR-181 activity. CD31 (green) and Prox1 (red). Scale bar represents 100 μm. (Ci) Western blot illustrating that Prox1 protein is substantially reduced relative to β-actin in primary embryonic LECs after the introduction of miR-181a. (Cii) Quantification of reduction in Prox1 protein levels relative to β-actin levels in primary embryonic LECs after ectopic miR-181a expression, compared with miR-neg expression. Data are representative of 3 independent experiments. (Di) Dose-response analysis of Prox1 mRNA expression in primary embryonic LECs after transfection of increasing concentrations of miR-181a (black bars), compared with transfection with negative control miR-neg (gray bars). Significant reduction in Prox1 mRNA and protein levels is achieved at 5nM, the lowest level tested; n = 4. *P < .05. Data are mean ± SEM. P values were calculated using Student paired t test. (Dii) Prox1 mRNA and protein levels as assessed by Western blot are shown relative to the negative control.
Ectopic miR-181a expression in primary LECs results in reduced Prox1 mRNA and protein levels. (A) Prox1 mRNA expression is substantially reduced in primary embryonic LECs after transfection of miR-181a, compared with transfection with a negative control (miR-neg) that has no established targets; n = 6. *P < .001. (B) Immunostaining of primary embryonic LECs transfected with control miR-neg (top panel) versus miR-181a (bottom panel), illustrating dramatic reduction in nuclear Prox1 as a result of miR-181 activity. CD31 (green) and Prox1 (red). Scale bar represents 100 μm. (Ci) Western blot illustrating that Prox1 protein is substantially reduced relative to β-actin in primary embryonic LECs after the introduction of miR-181a. (Cii) Quantification of reduction in Prox1 protein levels relative to β-actin levels in primary embryonic LECs after ectopic miR-181a expression, compared with miR-neg expression. Data are representative of 3 independent experiments. (Di) Dose-response analysis of Prox1 mRNA expression in primary embryonic LECs after transfection of increasing concentrations of miR-181a (black bars), compared with transfection with negative control miR-neg (gray bars). Significant reduction in Prox1 mRNA and protein levels is achieved at 5nM, the lowest level tested; n = 4. *P < .05. Data are mean ± SEM. P values were calculated using Student paired t test. (Dii) Prox1 mRNA and protein levels as assessed by Western blot are shown relative to the negative control.
Introduction of miR-181a to primary embryonic LECs in culture resulted in rapid and highly significant down-regulation of Prox1 mRNA and protein within 48 hours, indicating that miR-181a targets the Prox1 transcript for both degradation and translational inhibition26 (Figure 3A-C). Although Prox1 mRNA was decreased by approximately 50% in miR-181a–transfected LECs (Figure 3A), quantification of Prox1 protein levels by Western analysis revealed that Prox1 protein was decreased by 75% after the introduction of miR-181a (Figure 3C). Immunostaining of miR-181a–transfected LECs revealed that Prox1 expression was barely detectable; the protein that was visible was apparent in a perinuclear rather than nuclear staining pattern, perhaps reflecting a site of stalled translation (Figure 3B). To determine the efficiency of miR-181a–mediated Prox1 regulation, Prox1 mRNA and protein were quantitated after serial titration of miR-181a in LECs. Near-maximal inhibition of Prox1 message and protein levels was observed at the lowest dose of miR-181a tested (5nM; Figure 3D), indicating that the targeting of Prox1 by miR-181a is highly efficient and likely to be of significant biologic relevance.
Because Prox1 expression has been shown to be critical for specifying the fate and maintaining the identity of LECs4,5 and endothelial cells are exquisitely sensitive to Prox1 dosage,5,6,27 we next investigated the effects of elevated miR-181a activity on LEC identity. Genes characteristic of LEC identity and previously shown to be increased in expression after ectopic Prox1 expression in BECs, including Vegfr3 and Itga9,28-30 were significantly reduced in expression as a result of miR-181a introduction to cultured LECs (Figure 4A-B). Conversely, the expression of blood vascular endothelial genes, including Cd34 and Nrp1, previously shown to be decreased in BECs after ectopic Prox1 expression,28,29 were substantially increased in LECs expressing miR-181a (Figure 4C-D). Taken together, these data indicate that increased miR-181a activity in embryonic LECs is sufficient to at least partially reverse LEC phenotype to that of blood vascular endothelial cells. The expression of pan-endothelial cell genes including Pecam1 and Vcam1, was not significantly affected by ectopic miR-181a expression (Figure 4E-F), suggesting that the reversion of LEC identity toward that of BECs was a direct result of specific, miR-181a mediated Prox1 suppression and that off-target effects were probably minimal.
Increased miR-181a activity in primary LECs results in reversion of LEC phenotype to BEC. Expression of Vegfr3 (A), Itga9 (B), Cd34 (C), Nrp1 (D), Vcam1 (E), and Pecam1 (F) mRNA relative to Actb, as assessed by real-time RT-PCR in primary embryonic LECs transfected with a negative control miR-neg (gray bars) or miR-181a (black bars); n = 3. Vegfr3: *P < .015. Itga9: *P < .017. Cd34: *P < .025. Nrp1: *P < .001. Data are mean ± SEM and are representative of 3 independent experiments. P values were calculated using Student paired t test.
Increased miR-181a activity in primary LECs results in reversion of LEC phenotype to BEC. Expression of Vegfr3 (A), Itga9 (B), Cd34 (C), Nrp1 (D), Vcam1 (E), and Pecam1 (F) mRNA relative to Actb, as assessed by real-time RT-PCR in primary embryonic LECs transfected with a negative control miR-neg (gray bars) or miR-181a (black bars); n = 3. Vegfr3: *P < .015. Itga9: *P < .017. Cd34: *P < .025. Nrp1: *P < .001. Data are mean ± SEM and are representative of 3 independent experiments. P values were calculated using Student paired t test.
Inhibition of endogenous miR-181a in BECs results in elevated Prox1 expression
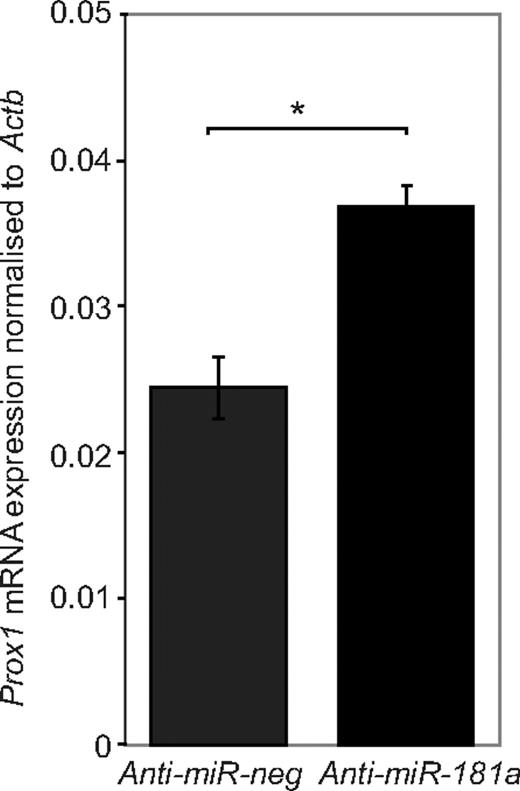
To determine whether a reduction in miR-181a activity in embryonic blood vascular endothelial cells modulated Prox1 expression, BECs isolated from E16.5 skin were transfected with miR-181a antagomir. Inhibition of endogenous miR-181a in embryonic blood vascular endothelial cells resulted in significantly increased Prox1 mRNA expression in embryonic BECs (Figure 5), suggesting that the regulation of Prox1 by miR-181a is likely to be of biologic significance. On the basis of previous work illustrating that endothelial cells are sensitive to changes in Prox1 gene dosage,6,27-29 we would predict that a subtle increase in Prox1 expression, even if not sufficient to completely drive BEC identity toward an LEC phenotype, would facilitate cells being increasingly responsive to additional combinatorial factors that further ramp up Prox1 expression and specify LEC identity. Our data suggest that miR-181a regulation of Prox1 mRNA and protein expression is likely to be one mechanism used to ensure silencing of Prox1 and therefore prevention of the acquisition of LEC identity in blood vascular endothelial cells.
Knockdown of endogenous miR-181a expression in primary BECs results in increased Prox1 levels. Prox1 mRNA expression is increased in primary embryonic BECs after transfection with anti–miR-181a, compared with a negative control anti-miRNA that has no established targets; n = 6. *P < .001. Data are mean ± SEM and are representative of 2 independent experiments. P value was calculated using Student paired t test.
Knockdown of endogenous miR-181a expression in primary BECs results in increased Prox1 levels. Prox1 mRNA expression is increased in primary embryonic BECs after transfection with anti–miR-181a, compared with a negative control anti-miRNA that has no established targets; n = 6. *P < .001. Data are mean ± SEM and are representative of 2 independent experiments. P value was calculated using Student paired t test.
Discussion
Our work has uncovered a novel mechanism of Prox1 post-transcriptional regulation mediated by miR-181a. miR-181a targeting of Prox1 mRNA for degradation in addition to translational inhibition provides an extremely efficient mechanism by which Prox1 protein levels may be ultimately fine-tuned.26 The recent finding that Prox1 regulates expression of its own gene8 also suggests that miR-181a–mediated reduction of Prox1 mRNA and protein further feeds back on Prox1 expression to progressively dial down Prox1 transcription. We demonstrated that elevated miR-181a activity in embryonic LECs resulted in reduced levels of Prox1 transcript and protein and reversion of LEC identity toward a blood vascular phenotype. Conversely, inhibition of endogenous miR-181a in embryonic blood vascular endothelial cells resulted in increased Prox1 expression, suggesting that miR-181a activity could be an important mechanism by which Prox1 expression is normally silenced in the blood vasculature during development. Our finding that miR-181a expression is elevated in embryonic blood vascular endothelial cells compared with LECs is supportive of this hypothesis. The relief of miR-181a–mediated Prox1 repression may be an important event during the initiation of LEC fate specification from blood vascular progenitor cells.
The results of our study also illustrated that Prox1 expression is dynamically regulated in LECs during embryonic development and in adulthood. The substantial decrease in Prox1 mRNA and protein levels observed in adult LECs compared with their embryonic counterparts was accompanied by a significant increase in miR-181a expression, suggesting that miR-181a–mediated repression may also contribute to the temporal control of Prox1 levels within LECs. Why would Prox1 expression be reduced in adult compared with embryonic lymphatic endothelium? One possibility is that, once genesis of the lymphatic vasculature is complete, reduced levels of Prox1 are sufficient to maintain LEC identity. “Dialing down” Prox1 levels would enable cells to be more responsive to subtle alterations in the transcriptional milieu and thereby to readily adopt a new identity in response to physiologic or pathologic cues. Our data highlighting that Prox1 levels are temporally regulated fit with the recent discovery that LEC identity is plastic rather than fixed6 and furthermore suggest that LEC identity could be more plastic during adulthood than during peak periods of lymphangiogenesis in the embryo. It remains an intriguing possibility that miR-181a expression may be modulated and therefore contribute to the abnormal, mixed lymphatic/blood vascular endothelial cell identity that is characteristic of pathologic conditions, including angiosarcomas,31 the tumor microvasculature,6,32 and Kaposi sarcoma herpesvirus infection of endothelial cells.33-35
Although the likelihood exists that miR-181a targets endothelial cell genes in addition to Prox1 to cooperatively regulate endothelial cell identity, the control of a “master regulator” of LEC identity provides a rapid and efficient mechanism by which endothelial cell identity may be specified or switched in response to physiologic stimuli. Searching for miR-181a target genes using Targetscan (www.targetscan.org) identified many potential targets of miR-181a, although none appears to be an established LEC-specific gene. Searching for microRNAs that potentially bind the 3′-UTR of Prox1 revealed a consensus binding site only for miR-181. Whether or not the expression of miR-181a is subject to differential regulation in endothelial cells during LEC fate specification, thereby contributing to the onset of Prox1 synthesis in venous LEC progenitor cells, remains to be established; modulation of miR-181a levels in the blood vasculature may provide a mechanism for a rapid switch in endothelial cell phenotype. How the expression of miR-181a is differentially regulated in LECs and BECs remains to be established. Finally, the discovery of miR-181a–mediated regulation of Prox1 highlights potential roles for the miR-181 family outside the vasculature in tissues, including the eye and muscle, in which both Prox136 and miR-18118,19 are expressed.
In conclusion, we have discovered a novel mechanism of Prox1 regulation in endothelial cells: that of miR-181a mediated post-transcriptional silencing. Our data suggest that miR-181 activity could be an important mechanism by which Prox1 expression is inhibited in the blood vasculature during embryonic development, as well as down-regulated in the lymphatic vasculature during adulthood. Modulation of Prox1 levels using microRNA-based technology may have future applications for the treatment of human disease.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank K. Wicks and the staff of the IMVS Veterinary Services Division for animal husbandry.
This work was supported by the National Health and Medical Research Council (Project Grant 453493; N.L.H.), Royal Adelaide Hospital Florey Research Fellowship (N.L.H.), and Cancer Council of South Australia (Project Grant 426761; M.M).
Authorship
Contribution: J.K., M.Z.M., and N.L.H. designed experiments; J.K. performed experiments; and J.K., M.Z.M., and N.L.H. analyzed data and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Natasha Harvey, Division of Haematology, Centre for Cancer Biology, SA Pathology, PO Box 14, Rundle Mall South Australia, 5000 Adelaide, Australia; e-mail: natasha.harvey@health.sa.gov.au.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal