Osteoporosis is a common problem in disorders characterized by iron overload, such as the thalassemias and hereditary hemochromatosis. The exact role of iron in the development of osteoporosis in these disorders is not established. In this issue of Blood, Tsay et al1 suggest that increased bone resorption caused by increased iron-associated oxidative stress may be a significant pathogenetic mechanism.
In their study, Tsay et al examined the effect of iron excess on bone using an iron-overloaded mouse model treated by weekly iron dextran injections for a period of 2 months. Iron overload had a pronounced dose-dependent effect. Iron-overloaded mice had increased reactive oxygen species (ROS) and elevated serumtumor necrosis factor alpha (TNF-α) and interleukin 6 (IL-6) concentrations. Exposure of the osteoclast-like RAW cell line to ferric iron resulted in increased TNF-α production. Thechanges observed in vivo were associated with increased bone resorption (see figure), increased oxidative stress, and evidence of systemic inflammation. Significantly, they were prevented by treatment with the antioxidant N-acetyl-L-cysteine, supporting the hypothesis that oxidative stress is involved in their pathogenesis. Osteoblast function was unaltered. In view of these observations, the authors conclude that iron overload in mice results in increased bone resorption, oxidative stress, and inflammation, leading to changes in bone microarchitecture and other properties.
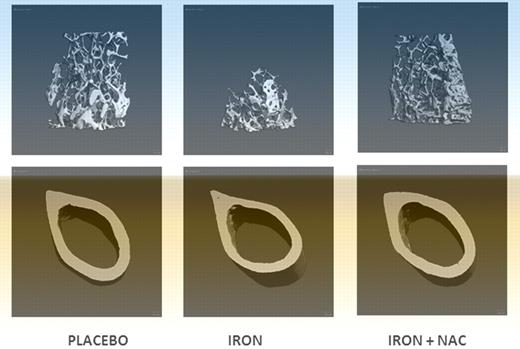
Treatment with an antioxidant prevents the bone loss induced by iron overload. Micro-CT images of placebo, iron dextran, and iron dextran + NAC animals. Trabecular bone at proximal femur and cortical bone at mid-diaphysis femur (image is Figure 4B from Tsay et al1 ).
Treatment with an antioxidant prevents the bone loss induced by iron overload. Micro-CT images of placebo, iron dextran, and iron dextran + NAC animals. Trabecular bone at proximal femur and cortical bone at mid-diaphysis femur (image is Figure 4B from Tsay et al1 ).
The association of osteoporosis with iron overload in general, and in thalassemia in particular, is poorly understood.2,3 Ineffective erythropoiesis and the associated vastly expanded erythropoietic mass in thalassemia may by themselves cause abnormalities in bone architecture. Ascorbate deficiency is caused by increased oxidative breakdown of ascorbic acid catalyzed by iron, leading to scurvy and the associated bone defects.4 However, the unaltered collagen synthesis found in the present study suggests that ascorbate levels have not decreased to the point of inhibiting hydroxylation of key proline residues in collagen, a rate-limiting step in collagen synthesis.
The relation between iron overload and increased oxidative stress is well documented.5 It is also known that cytokines such as TNF-α and IL-6 mediate bone loss primarily by increasing bone resorption.6 Tsay et al propose a model wherein iron excess leads to increased oxidative stress: oxidative stress in turn induces inflammatory changes in a dose-dependent fashion, then mediates bone loss through changes in bone remodeling. However, the evidence linking increased oxidative stress to increased cytokine production, and the role of iron in cytokine-mediated bone loss, is at present unclear.7 In fact, in hereditary hemochromatosis, cytokine production is impaired.8 Conversely, increased cytokine production in sickle cell disease compared with thalassemia is not related to the degree of iron overload, but to the underlying unique pathology of sickle cell disease.9
The study by Tsay et al addresses a significant problem encountered in chronic iron overload that so far has not received sufficient attention and its pathogenesis is poorly understood. The study is particularly strong in its methodology, offering carefully collected data supporting the claim that the bone defect associated with iron overload is caused by increased osteoclastic activity. The clear prevention of iron-associated bone abnormalities by the antioxidant N-acetyl-L-cysteine is strong evidence supporting the role of ROS in the observed bone abnormalities. By contrast, the experiments involving inflammatory cytokines are less convincing. This is not because of the correlations observed, but the lack of evidence supporting a mechanistic cause-and-effect relation.
Despite these caveats and the need for further studies to validate these concepts in the clinical settings, the above observations may have significant implications for the management of clinical complications encountered in patients with chronic iron overload.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal