Abstract
The prospective 1-year Evaluation of Patients' Iron Chelation with Exjade (EPIC) study enrolled a large cohort of 116 patients with aplastic anemia; the present analyses evaluated the efficacy and safety of deferasirox in this patient population. After 1 year, median serum ferritin decreased significantly from 3254 ng/mL at baseline to 1854 ng/mL (P < .001). Decreases occurred in chelation-naive (3229-1520 ng/mL; P < .001, last-observation-carried-forward analysis), and previously chelated (3263-2585 ng/mL; P = .21, last-observation-carried-forward analysis) patients and were reflective of dose adjustments and ongoing iron intake. Baseline labile plasma iron levels were within normal range despite high serum ferritin levels. The most common drug-related adverse events were nausea (22%) and diarrhea (16%). Serum creatinine increases more than 33% above baseline and the upper limit of normal occurred in 29 patients (25%), but there were no progressive increases; concomitant use of cyclosporine had a significant impact on serum creatinine levels. The decrease in mean alanine aminotransferase levels at 1 year correlated significantly with reduction in serum ferritin (r = 0.40, P < .001). Mean absolute neutrophil and platelet counts remained stable during treatment, and there were no drug-related cytopenias. This prospective dataset confirms the efficacy and well characterizes the tolerability profile of deferasirox in a large population of patients with aplastic anemia. This study was registered at www.clinicaltrials.gov as #NCT00171821.
Introduction
Aplastic anemia (AA) is a rare, potentially life-threatening bone marrow failure disorder characterized by pancytopenia and an associated increase in the risk of hemorrhage, infection, organ dysfunction, and death. Most cases of AA are acquired and immune-mediated, with a wide geographic distribution.1,2 The etiology of acquired AA is variable, involving environmental factors (eg, chemical exposure, medical drug use, infection) and host genetic risk factors,1 although many cases remain idiopathic. Treatment with immunosuppressive therapy or hematopoietic stem cell transplantation can be effective, but many patients with AA require blood transfusions as supportive therapy. Regular transfusions lead to the development of iron overload, which can cause significant damage to the heart, liver, and endocrine glands.3,4 The impact of iron overload-related complications on patient morbidity and mortality, and the demands they make on medical resources in patients with AA, have been highlighted in a number of studies.4-7 United Kingdom guidelines for the management of acquired AA suggest that iron chelation therapy for the treatment of iron overload should be considered when serum ferritin levels exceed 1000 ng/mL, although patients need to be assessed on an individual basis.8
Deferoxamine (DFO) is an effective iron chelator, but its demanding treatment regimen can result in suboptimal compliance, with ultimate failure to show clinically meaningful decreases in serum ferritin levels, as well as impaired quality of life.9 A few studies have been conducted with DFO in patients with AA; however, these studies only enrolled a small number of patients (n = 1-33); therefore, data with DFO in this population remain limited.5,10,11 Deferasirox is a once-daily, oral, iron chelator approved for the treatment of chronic iron overload resulting from blood transfusions. The effectiveness of deferasirox in reducing or maintaining body iron has been demonstrated in studies involving large numbers of patients with a variety of transfusion-dependent anemias.12,13 Earlier clinical studies of deferasirox have included smaller populations of patients with AA (n = 512 and n = 5014 ). The largest number of patients with AA ever included in an iron chelator trial (n = 116) were enrolled in the large prospective, multicenter, 1-year Evaluation of Patients' Iron Chelation with Exjade (EPIC) study. Data from the overall EPIC study population have been published previously.15 The objective of the present analysis is to specifically evaluate the efficacy and safety of deferasirox in the patient population with AA.
Methods
A full description of the EPIC study design and inclusion/exclusion criteria has been previously published15 ; however, the key information relevant to this analysis is summarized here.
Study design and dosing schedule
EPIC was a prospective, multicenter, open-label, 1-year study. The deferasirox starting dose was individualized according to blood transfusion frequency. The recommended initial dose was 20 mg/kg per day for patients receiving approximately 2 to 4 units/month (7-14 mL/kg per month for a body weight of approximately 60 kg, equivalent to 0.3 to 0.5 mg/kg per day of iron) of packed red blood cells. Initial doses of 10 or 30 mg/kg per day could be considered for patients receiving less or more frequent blood transfusions, respectively. Specific dose adjustments in steps of 5 to 10 mg/kg per day up to 40 mg/kg per day were allowed based on quarterly serum ferritin trends and continuous assessment of safety markers (including serum creatinine, liver function tests, skin rash, and auditory and ocular disturbances). Dose increases were recommended in patients with baseline serum ferritin values of more than 500 ng/mL who had an upward trend or patients with baseline serum ferritin values of more than 1000 ng/mL who had no downward trend after 3 months. Dose escalation to more than 40 mg/kg per day was permitted in exceptional circumstances and had to be approved individually by the Study Monitoring Committee. If serum ferritin levels fell to less than or equal to 500 ng/mL on 2 consecutive study visits, deferasirox treatment was suspended until levels exceeded 500 ng/mL.
Key inclusion and exclusion criteria
In total, 1744 iron-overloaded patients with various transfusion-dependent anemias, including patients with AA, were enrolled. Male or female patients (age, ≥ 2 years) with transfusional iron overload were included in the study if they fulfilled the following criteria: serum ferritin levels more than or equal to 1000 ng/mL, or less than 1000 ng/mL but with a history of multiple transfusions (> 20 transfusions or 100 mL/kg of red blood cells) and R2 magnetic resonance imaging-confirmed liver iron concentration (LIC) of more than or equal to 2 mg Fe/g dry weight. Patients were excluded from the study if they had a life expectancy of less than 1 year, alanine aminotransferase (ALT) levels of more than 300 U/L or serum creatinine levels above the upper limit of normal (ULN), or a history of nephrotic syndrome. Patients (or parents/guardians) provided written, informed consent before entering the study, which was conducted in accordance with Good Clinical Practice guidelines and the Declaration of Helsinki. The study was approved by an Institutional Review Board/Independent Ethics Committee/Research Ethics Board at each participating site. A Study Monitoring Committee supervised the trial conduct and made decisions regarding dose adjustments for individual patients who were outside of those outlined in the protocol.
Assessments
Serum ferritin was assessed every 4 weeks. Efficacy endpoints included the change in serum ferritin level from baseline to 52 weeks, the relationship between serum ferritin levels and other surrogate markers of iron overload, as well as changes in labile plasma iron (LPI) pre- to post-deferasirox administration. The correlation between changes from baseline in serum ferritin and ALT was also assessed as a post hoc analysis. Blood samples to assess changes in LPI levels were taken before administration (ie, at predicted daily peak) and 2 hours after administration of deferasirox, at baseline and at weeks 12, 28, and 52. Iron intake was derived based on the volume of transfused blood given and its concentration (ie, the hematocrit), and the amount of iron present in a red cell, using a prespecified and validated formula that has been described in more detail elsewhere.16 Safety was evaluated by regular monitoring and recording of all adverse events (AEs) and serious AEs, routine laboratory testing (including hematology, blood chemistry, and urine renal function assessments), and medical physical examination during study visits.
Statistical methods
Efficacy was assessed for the full analysis set, including all patients who had been successfully screened and started the study. If there was no serum ferritin value available at 52 weeks, the last available observation was used as the end of study assessment to calculate change from baseline (last-observation-carried-forward analysis), thereby providing a robust yet conservative endpoint to cope with the intention-to-treat principle. The correlation between change from baseline in serum ferritin versus ALT was assessed using Pearson correlation coefficients and regression analysis. All patients who received at least 1 dose of study medication were included in the safety population. The reported P values were based on 2-sided significance tests (Student t test).
Results
Patient characteristics and disposition
In total, 116 patients with AA were enrolled in the EPIC study (Table 1). These patients had been receiving transfusions for a mean duration of more than 6 years, yet more than two-thirds had never received iron chelation therapy. In the overall AA population, median serum ferritin level at baseline was 3254 ng/mL; baseline levels in chelation-naive and previously chelated patients were 3229 and 3263 ng/mL, respectively.
Demographic and baseline patient characteristics
| Characteristic . | Patients with AA (n = 116) . |
|---|---|
| Mean age ± SD, y | 33.3 ± 17.1 |
| Age group, y | |
| 2 to less than 16, n (%) | 16 (13.8) |
| 16 or older, n (%) | 100 (86.2) |
| Male/female, n | 67:49 |
| Race (White/Black/Asian), n | 32:4:80 |
| Chelation-naive, n (%) | 79 (68.1) |
| Previous iron chelation therapy, n (%) | |
| DFO monotherapy | 31 (26.7) |
| DFO + deferiprone combination | 6 (5.2) |
| Mean duration of previous iron chelation therapy ± SD, y | 4.3 ± 5.8 |
| Mean duration of transfusion history ± SD, y | 6.1 ± 5.7 |
| Mean no. of transfusion sessions in the year before study entry ± SD | 12.5 ± 13.0 (n = 114) |
| Mean total volume red blood cells transfused in the year before study entry ± SD, mL/kg | 116 ± 179 (n = 112) |
| Median baseline serum ferritin (range), ng/mL | 3254 (908-25 346) (n = 115) |
| Characteristic . | Patients with AA (n = 116) . |
|---|---|
| Mean age ± SD, y | 33.3 ± 17.1 |
| Age group, y | |
| 2 to less than 16, n (%) | 16 (13.8) |
| 16 or older, n (%) | 100 (86.2) |
| Male/female, n | 67:49 |
| Race (White/Black/Asian), n | 32:4:80 |
| Chelation-naive, n (%) | 79 (68.1) |
| Previous iron chelation therapy, n (%) | |
| DFO monotherapy | 31 (26.7) |
| DFO + deferiprone combination | 6 (5.2) |
| Mean duration of previous iron chelation therapy ± SD, y | 4.3 ± 5.8 |
| Mean duration of transfusion history ± SD, y | 6.1 ± 5.7 |
| Mean no. of transfusion sessions in the year before study entry ± SD | 12.5 ± 13.0 (n = 114) |
| Mean total volume red blood cells transfused in the year before study entry ± SD, mL/kg | 116 ± 179 (n = 112) |
| Median baseline serum ferritin (range), ng/mL | 3254 (908-25 346) (n = 115) |
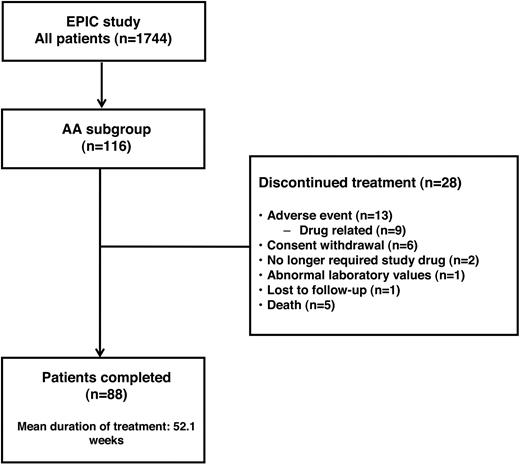
In total, 88 patients (75.9%) with AA completed the 1-year study (Figure 1). Of the 28 patients (24.1%) who discontinued, 18 (22.8%) of 79 were chelation-naive and 10 (27.0%) of 37 were previously chelated; reasons for discontinuation were similar in both groups. Thirteen patients (11.2%) discontinued because of the occurrence of AEs: 9 discontinuations were the result of AEs assessed by the investigator to be related to study drug (including skin rash [n = 4], hearing loss, nausea, upper abdominal pain, anorexia, abnormal serum creatinine levels), whereas 4 were the result of non–drug-related AEs (including pyrexia, leg fracture, subdural hematoma, and alcohol abuse). Investigators assessed that 2 patients no longer required study drug because of rapidly decreasing serum ferritin levels. The discontinuation resulting from abnormal laboratory values was the result of elevated serum creatinine levels and febrile neutropenia. Six patients withdrew consent; however, the investigators were not obliged to comment on the reasons for withdrawal, so no additional statement can be made about these discontinuations. Five patients died of non-treatment-related causes, including sepsis (n = 3), pneumonia and hepatic rupture (n = 1 for both).
Deferasirox dosing and iron intake
At the beginning of the study, 2 patients (1.7%) initially received a deferasirox dose of 10 mg/kg per day, 113 (97.4%) received 20 mg/kg per day, and 1 (0.9%) received 30 mg/kg per day. During the study, deferasirox dose was adjusted on 1 or more occasion in 85 patients (73.3%). Doses were increased 19 times in 17 patients, (14.7%) at a median of 21 weeks (range, 8-49 weeks) after treatment initiation. Doses were reduced in 31 patients (26.7%) because of laboratory abnormalities or AEs, most commonly abnormal serum creatinine levels (n = 10), skin rash (n = 5), and diarrhea (n = 4). Laboratory abnormalities or AEs led to temporary drug interruptions in 52 patients (44.8%). Among the 88 patients who completed 12 months of treatment, 26 (29.5%) were receiving deferasirox less than 20 mg/kg per day at the end of the study, 50 (56.8%) were receiving more than or equal to 20 to less than 30 mg/kg per day, and 12 (13.6%) were receiving more than or equal to 30 mg/kg per day. Over the course of the 1-year treatment period, the average actual dose of deferasirox was 17.6 plus or minus 4.8 mg/kg per day; 75 (64.7%) and 41 (35.3%) patients received average actual doses of less than 20 and more than or equal to 20 to less than 30 mg/kg per day, respectively.
During the treatment period, patients received a mean of 66.1 plus or minus 47.2 mL red blood cells/kg, equivalent to 0.25 plus or minus 0.19 mg/kg per day of iron. Mean transfusional iron intake was significantly different between patients who received an average actual deferasirox dose of less than 20 and more than or equal to 20 to less than 30 mg/kg per day (0.21 vs 0.31 mg/kg per day, respectively, P < .006, Wilcoxon 2-sided test).
Effect of deferasirox on serum ferritin levels
After 1 year of treatment, median serum ferritin levels decreased in the overall AA patient group from 3254 ng/mL at baseline to 1854 ng/mL, representing a significant decrease of −964 ng/mL (n = 115, P < .001; Table 2) based on last-observation-carried-forward analysis.
Change from baseline in median serum ferritin by average actual dose
| . | Average actual deferasirox dose . | ||
|---|---|---|---|
| < 20 mg/kg/d (n = 75) . | ≥ 20-< 30 mg/kg/d (n = 41) . | All (n = 116) . | |
| Baseline serum ferritin, ng/mL | 3263 (908-18 635) | 3238 (1129-25 346) | 3254 (908-25 346) |
| Serum ferritin at 1 y, ng/mL | 1819 (212-14 509) | 2191 (87-17 233) | 1854 (87-17 233) |
| Absolute change in serum ferritin, ng/mL* | −970 (−11 753 to −7883) | −884 (−15 704 to −13 894) | −964 (−15 704 to −13 894) |
| P | < .001 | < .278 | < .001 |
| Mean iron intake ± SD, mg/kg/d | 0.21 ± 0.18 | 0.31 ± 0.20 | 0.25 ± 0.19 |
| . | Average actual deferasirox dose . | ||
|---|---|---|---|
| < 20 mg/kg/d (n = 75) . | ≥ 20-< 30 mg/kg/d (n = 41) . | All (n = 116) . | |
| Baseline serum ferritin, ng/mL | 3263 (908-18 635) | 3238 (1129-25 346) | 3254 (908-25 346) |
| Serum ferritin at 1 y, ng/mL | 1819 (212-14 509) | 2191 (87-17 233) | 1854 (87-17 233) |
| Absolute change in serum ferritin, ng/mL* | −970 (−11 753 to −7883) | −884 (−15 704 to −13 894) | −964 (−15 704 to −13 894) |
| P | < .001 | < .278 | < .001 |
| Mean iron intake ± SD, mg/kg/d | 0.21 ± 0.18 | 0.31 ± 0.20 | 0.25 ± 0.19 |
Serum ferritin data are median (range).
Based on last-observation-carried-forward analysis.
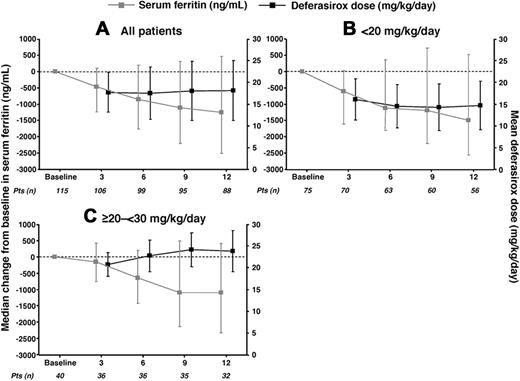
Median serum ferritin levels at baseline were similar in patients who received average actual deferasirox doses of less than 20 and more than or equal to 20 to less than 30 mg/kg per day, although patients in the less than 20 mg/kg per day group had a lower mean iron intake (Table 2). Serum ferritin levels decreased comparably in the 2 dose groups over 1 year of treatment; however, the decrease was significant in the less than 20 mg/kg per day group (P < .001) but not in the more than or equal to 20 to less than 30 mg/kg per day group (Table 2; Figure 2). Patients in the more than or equal to 20 to less than 30 mg/kg per day dose group had higher iron intake during the study, hence the need for higher doses to achieve a comparable reduction in serum ferritin. Median serum ferritin decreased from baseline to 1 year in both chelation-naive (3229-1520 ng/mL, n = 79; P < .001) and previously chelated patients (3263-2585 ng/mL, n = 37; P = .21).
Mean deferasirox dose (± SD) and median change in serum ferritin (± 25th/75th percentiles), by average actual dose categories (full analysis set). (A) All patients. (B) Dose less than 20 mg/kg per day. (C) Dose more than or equal to 20 to less than 30 mg/kg per day.
Mean deferasirox dose (± SD) and median change in serum ferritin (± 25th/75th percentiles), by average actual dose categories (full analysis set). (A) All patients. (B) Dose less than 20 mg/kg per day. (C) Dose more than or equal to 20 to less than 30 mg/kg per day.
Change in LPI with deferasirox treatment
LPI data were available for 104 patients with AA. LPI levels before deferasirox administration were within normal limits (ie, < 0.4μM)17 throughout the study and were decreased after deferasirox administration at baseline (0.23-0.01μM), week 12 (0.08-0.01μM), week 28 (0.06-0.01μM), and week 52 (0.04-0.03μM).
Relationship between serum ferritin and ALT
Mean serum ALT at baseline, 3, 6, 9, and 12 months was 62.5 plus or minus 50.7, 54.5 plus or minus 66.7, 41.1 plus or minus 40.3, 42.5 plus or minus 41.5, and 35.4 plus or minus 29.2 U/L, respectively. After 12 months of deferasirox treatment, there was a significant mean decrease from baseline in ALT of 26.8 plus or minus 42.9 U/L (P < .001); the decrease in the less than 20 and more than or equal to 20 to less than 30 mg/kg per day dose categories was 30.4 plus or minus 48.4 (P < .001) and 20.4 plus or minus 30.7 U/L (P < .371), respectively. There was a significant correlation between the absolute change in serum ferritin and ALT (r = 0.40, P < .001), which indicated that a decrease in serum ferritin of 500 ng/mL was associated with a decrease in ALT of 17.4 U/L.
Safety of deferasirox
Irrespective of the relationship with study drug, AEs were reported in 110 patients with AA (94.8%); the most common AEs were gastrointestinal (69.0%) and infections (59.5%). Sixty-four patients (55.2%) experienced AEs that were considered by the investigator to be drug related, the most common of which were gastrointestinal symptoms, such as nausea, diarrhea, and vomiting, as well as skin rash (Table 3). Drug-related AEs were observed in 44 (58.7%) and 20 (48.8%) patients who received actual deferasirox doses of less than 20 and more than or equal to 20 to less than 30 mg/kg per day, respectively. Most drug-related AEs were mild to moderate in nature (96.6%) and did not lead to study discontinuation. There were no serious AEs considered by the investigators to be drug related. Eleven patients underwent a dose reduction because of the occurrence of an AE, which was considered to be drug related in 5 patients (nausea, vomiting, rash, increased serum creatinine, increased ALT). Two patients (1.7%) had an increase in ALT more than 10 × ULN on 2 consecutive visits: 1 patient was receiving a dose of 20 mg/kg per day and the other 30 mg/kg per day at the time of the increase. Both patients had elevated ALT levels at baseline, and neither underwent a dose reduction because of elevated ALT levels. In general, there was no difference in the AE profile between chelation-naive and previously chelated patients.
Most common (> 5% overall) drug-related AEs by dose group
| . | < 20 mg/kg/d (n = 75), no. (%) . | ≥ 20-< 30 mg/kg/d (n = 41), no. (%) . | All (n = 116), no. (%) . |
|---|---|---|---|
| Nausea | 19 (25.3) | 7 (17.1) | 26 (22.4) |
| Diarrhea | 10 (13.3) | 8 (19.5) | 18 (15.5) |
| Rash | 9 (12.0) | 4 (9.8) | 13 (11.2) |
| Vomiting | 9 (12.0) | 1 (2.4) | 10 (8.6) |
| Dyspepsia | 8 (10.7) | 1 (2.4) | 9 (7.8) |
| Abdominal pain | 5 (6.7) | 2 (4.9) | 7 (6.0) |
| Upper abdominal pain | 4 (5.3) | 3 (7.3) | 7 (6.0) |
| Anorexia | 6 (8.0) | 1 (2.4) | 7 (6.0) |
| . | < 20 mg/kg/d (n = 75), no. (%) . | ≥ 20-< 30 mg/kg/d (n = 41), no. (%) . | All (n = 116), no. (%) . |
|---|---|---|---|
| Nausea | 19 (25.3) | 7 (17.1) | 26 (22.4) |
| Diarrhea | 10 (13.3) | 8 (19.5) | 18 (15.5) |
| Rash | 9 (12.0) | 4 (9.8) | 13 (11.2) |
| Vomiting | 9 (12.0) | 1 (2.4) | 10 (8.6) |
| Dyspepsia | 8 (10.7) | 1 (2.4) | 9 (7.8) |
| Abdominal pain | 5 (6.7) | 2 (4.9) | 7 (6.0) |
| Upper abdominal pain | 4 (5.3) | 3 (7.3) | 7 (6.0) |
| Anorexia | 6 (8.0) | 1 (2.4) | 7 (6.0) |
Change in serum creatinine
Twenty-nine patients (25.0%) had 2 consecutive serum creatinine increases more than 33% above baseline, which also exceeded the ULN; 28 of these had normal serum creatinine levels at baseline. Of the 29 patients, 20 (26.7%) of 75 and 9 (22.0%) of 41 received actual doses of less than 20 and more than or equal to 20 to less than 30 mg/kg per day and 22 (27.8%) of 79 and 7 (18.9%) of 37 were chelation-naive and previously chelated, respectively. There were no progressive increases in the mean serum creatinine over time. Two consecutive serum creatinine increases more than 33% above baseline and ULN were observed in 10 of 21 patients (47.6%) receiving concomitant cyclosporine treatment, compared with 19 of 95 patients (20.0%) not receiving cyclosporine (P < .001; Table 4). In a logistic regression model to assess factors potentially influencing serum creatinine levels, which also considered gender, age, and mean actual deferasirox dose, concomitant cyclosporine use was the only variable that reached statistical significance (P = .03). The odds ratio for the relative risk of having an event of 2 consecutive values increased by more than 33% and more than ULN was 3.14 for patients on concomitant cyclosporine. Ten patients had their dose of deferasirox reduced because of an increase in serum creatinine, and 4 of these patients were receiving cyclosporine; the last creatinine value assessed was within the normal range in 8 of these patients, including 3 of the 4 patients receiving cyclosporine.
Patients with increase in serum creatinine more than 33% above baseline on 2 consecutive visits and/or serum creatinine above ULN, with and without concomitant cyclosporine use
| Cyclosporine use . | n (%) . | Two values > ULN,* no. (%) . | Two consecutive values > 33%,* no. (%) . | Two consecutive values > 33% and > ULN,* no. (%) . |
|---|---|---|---|---|
| No cyclosporine | 95 (82) | 8 (8.4) | 34 (35.8) | 19 (20.0) |
| Concomitant cyclosporine | 21 (18) | 3 (14.3) | 3 (14.3) | 10 (47.6)† |
| Total | 116 (100) | 11 (9.5) | 37 (31.9) | 29 (25.0) |
| Cyclosporine use . | n (%) . | Two values > ULN,* no. (%) . | Two consecutive values > 33%,* no. (%) . | Two consecutive values > 33% and > ULN,* no. (%) . |
|---|---|---|---|---|
| No cyclosporine | 95 (82) | 8 (8.4) | 34 (35.8) | 19 (20.0) |
| Concomitant cyclosporine | 21 (18) | 3 (14.3) | 3 (14.3) | 10 (47.6)† |
| Total | 116 (100) | 11 (9.5) | 37 (31.9) | 29 (25.0) |
Categories are mutually exclusive.
P < .001 versus patients with no concomitant use of cyclosporine.
Other safety parameters
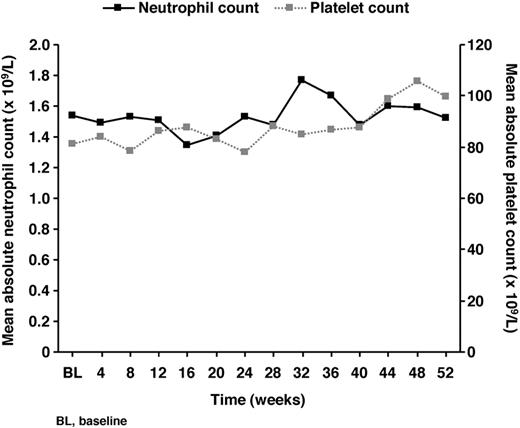
Five patients (4.3%) had ear disorders, only 1 of which (moderate deafness) was considered by the investigator to be related to study drug. There were 15 patients (12.9%) with eye disorders, none of which was considered related to study drug. Mean absolute neutrophil and platelet counts remained stable over the course of the study (Figure 3), and there were no cases of drug-related cytopenias in any patients with AA. Mean hemoglobin levels were maintained between 9 and 10 g/dL throughout deferasirox treatment.
Mean absolute neutrophil and platelet counts during deferasirox treatment.
Discussion
Previous clinical evaluation of deferasirox in patients with AA has been limited to a few studies in small numbers of patients; however, these studies have demonstrated the potential for deferasirox to decrease iron burden in this population.12,14 These findings have been confirmed in this large study of 116 iron-overloaded patients with AA, in which 1 year of treatment with deferasirox resulted in a statistically significant and clinically relevant reduction in serum ferritin levels that was reflective of dose adjustments and mean iron intake during the study. Baseline serum ferritin levels and the reduction in serum ferritin over the 1-year treatment period were similar in patients who received average actual deferasirox doses of less than 20 mg/kg per day or more than or equal to 20 to less than 30 mg/kg per day. However, the decrease was not significant in patients in the more than or equal to 20 to less than 30 mg/kg per day dose group, who needed higher doses because they were receiving higher iron intake than those in the less than 20 mg/kg per day dose group. These data support the clinical approach in patients with AA of selecting deferasirox starting dose based on transfusion requirements; appropriate dose titration at 3-month intervals based on serum ferritin levels and safety markers should subsequently be performed. This observation is in agreement with conclusions made based on the assessment of data from other patient types enrolled in the EPIC study.
Nevertheless, there remains some debate regarding the clinical complications of transfusional iron overload and subsequently the need for chelation therapy in patients with AA and other bone marrow failure disorders. Although this has only been clearly demonstrated in patients with β-thalassemia, most patients with AA will receive supportive care with blood transfusions8 ; toxic iron as a result of chronic transfusion therapy is expected to affect organs similarly in AA patients. In addition, survival rates are relatively long in transfused AA patients,18 leaving them at increased risk of developing iron overload and its related complications. This was highlighted in a survey by the Korean Iron Overload Study Group in patients with AA and myelodysplastic syndromes (MDS) with a history of more than 20 units of blood transfusions. A substantial proportion of patients were iron overloaded (331 of 1128 patients surveyed) despite the availability of DFO, and many of these patients (97 of 331 patients) also had organ damage.5 Serum ferritin levels correlated with the number and duration of transfusions, as well as the duration of transfusion dependence. In the EPIC study, AA patients had median baseline serum ferritin levels of more than 2500 ng/mL, which is known to be associated with significant negative outcomes.19,20 For the patients who had been previously chelated, these data suggest that prior chelation practices were not providing adequate management of their iron burden. Together with the lack of prior chelation therapy in more than two-thirds of patients, these data suggest that the need to treat iron overload, and its potential complications, may be underappreciated in the AA population. Further and longer-term data are needed to more fully evaluate the efficacy and safety of chelation therapy and to assess the impact on morbidity and mortality.
In the present study, after 1 year of deferasirox treatment, median serum ferritin levels had fallen to less than 2500 ng/mL in chelation-naive patients and were close to 2500 ng/mL in previously chelated patients. Data from this trial are based on the preplanned analysis of absolute change in serum ferritin from baseline. Although the assessment of serum ferritin has limitations (eg, levels can be increased under certain conditions such as infection and inflammation), it is simple and inexpensive and, when assessed serially, generally provides a reliable tool to assess total body iron and response to chelation therapy. In addition, the relationship between long-term control of serum ferritin and survival has been clearly demonstrated in cohort studies of chelated patients with thalassemia major.20-23 Advanced technologies, such as noninvasive magnetic resonance imaging, are becoming more available for measuring iron burden; however, the use of this technique in clinical practice is currently somewhat limited as the necessary equipment is not widely available and specific methods and calibrations are required. There is evidence of a relationship between changes in serum ferritin and LIC with deferasirox therapy in a range of transfusional iron overload conditions.12 Therefore, the serial measurement of serum ferritin remains the most accessible method and the one most widely used in clinical practice for determining iron overload and monitoring chelation therapy in many parts of the world.
Liver dysfunction is one of the major complications of iron overload in patients with AA,4 and elevated serum ALT is an important indicator of hepatocellular injury that can potentially progress to cirrhosis. In this cohort of AA patients, the observed reduction in serum ferritin over 1 year of deferasirox treatment was significantly correlated with a decrease in serum ALT, a finding that has been observed with deferasirox in other patient populations.24-26 This suggests that the management of iron overload with chelation therapy in patients with AA may have the potential to improve clinical outcomes. Further prospective studies are necessary to investigate the clinical benefits of deferasirox on liver and other organ function.
As LPI is redox-active, it increases the risk of reactive oxygen species production and can increase oxidative stress,27,28 which may cause oxidation of lipids, proteins, and DNA, cell death, and organ damage.29 Chelation therapy with deferasirox has been shown to produce sustained reduction in LPI in patients with β-thalassemia30 and MDS31-33 ; however, this is the first time that LPI levels have been assessed in patients with AA. Of note, in contrast to what has been observed in patients with β-thalassemia and MDS, baseline LPI levels were within the normal range (< 0.4μM17 ) in this AA population, despite high serum ferritin levels. The mechanism for this is conjectural at present, but ineffective erythropoiesis is known to contribute to the formation of non-transferrin-bound iron and therefore, presumably, to LPI formation. The relative lack of ineffective erythropoiesis in AA, compared with β-thalassemia major or MDS, may lead to a slower rate of iron release from bone marrow macrophages and hence a greater chance of released iron binding to transferrin.
Twenty-eight patients with AA (24.1%) discontinued during the 1-year treatment period, primarily the result of the occurrence of AEs; this rate is comparable with that of the overall EPIC population (20.4%).15 Approximately 55% of patients with AA experienced drug-related AEs; the most common were gastrointestinal disorders and rash, as could be anticipated from previous experience with deferasirox.12,13,34 Although more than 95% of AEs were mild or moderate in nature, they may still have had a potential impact on patients' quality of life; however, this was not assessed in the AA population using validated quality-of-life questionnaires. The incidence of drug-related AEs was similar to that observed in the overall EPIC population.15 As only a few, smaller studies have been conducted with other iron chelators in patients with AA, it is difficult to compare the safety profile of deferasirox in this population with any other iron chelator. Eleven patients had a deferasirox dose reduction because of an AE, but there were no serious AEs or deaths considered related to study drug. Absolute neutrophil and platelet counts remained stable during deferasirox treatment, and there were no cases of drug-related cytopenias, which is important because AA patients may develop or have worsened cytopenias with progression of their underlying disease. Nevertheless, it is advisable to monitor blood counts on a monthly basis when deferasirox is being administered to patients with AA. Although 25% of AA patients had an increase in serum creatinine more than 33% above baseline and more than ULN on 2 consecutive visits, there were no progressive increases. The only factor identified that had a significant impact on serum creatinine during the study was concomitant use of the immunosuppressive agent cyclosporine, a drug with a well-recognized potential to impair renal function.35 Careful monitoring of renal function is necessary in patients with AA who are receiving concomitant cyclosporine and deferasirox.
In conclusion, these data demonstrate that 1 year of treatment with deferasirox was generally effective in reducing iron burden and well tolerated in a large cohort of patients with AA and transfusional iron overload. In line with other chronically transfused patients,12,16 the reduction in serum ferritin was a function of transfusional iron intake and deferasirox dose, with higher deferasirox doses required to decrease iron burden in patients with higher iron intake.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Andrew Jones for medical editorial assistance with this manuscript.
This study was supported by Novartis Pharma AG. Financial support for medical editorial assistance was provided by Novartis Pharmaceuticals.
Authorship
Contribution: J.W.L. drafted the manuscript; S.-S.Y., Z.X.S., A.G., H.-C.H., and J.B.P. served as investigators on this trial (enrolling patients), contributed to data interpretation, reviewed the manuscript, and provided comments on this manuscript; D.H. and G.D. coordinated the execution of the trial and contributed to the analysis, interpretation, and reporting of the trial data; and B.R. served as the trial statistician.
Conflict-of-interest disclosure: J.B.P. reports receiving consulting fees, research grant funding, and lecture fees from Novartis Pharmaceuticals and consulting fees from Vifor International and Mundipharma. A.G. reports receiving consulting fees and honoraria from Novartis Pharmaceuticals, Genzyme, Amgen, and Celgene. D.H., G.D., and B.R. are full-time employees of Novartis Pharmaceuticals. The remaining authors declare no competing financial interests.
Centers and investigators who participated in the Evaluation of Patients' Iron Chelation with Exjade (EPIC) study are listed in the supplemental Appendix (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Correspondence: Jong Wook Lee, Division of Hematology, Department of Internal Medicine, Seoul St Mary's Hospital, The Catholic University of Korea, No. 505 Banpo-dong, Seocho-gu, Seoul 137-701, Korea; e-mail: jwlee@catholic.ac.kr.