Abstract
Elevated levels of fibrinogen are associated with increased risk of cardiovascular disease, whereas low fibrinogen can lead to a bleeding disorder. We investigated whether microRNAs (miRNAs), known to act as post-transcriptional regulators of gene expression, regulate fibrinogen production. Using transfection of a library of 470 annotated human miRNA precursor molecules in HuH7 hepatoma cells and quantitative measurements of fibrinogen production, we identified 23 miRNAs with down-regulating (up to 64% decrease) and 4 with up-regulating effects (up to 129% increase) on fibrinogen production. Among the down-regulating miRNAs, we investigated the mechanism of action of 3 hsa-miR-29 family members and hsa-miR-409-3p. Overexpression of hsa-miR-29 members led to decreased steady-state levels of all fibrinogen gene (FGA, FGB, and FGG) transcripts in HuH7 cells. Luciferase reporter gene assays demonstrated that this was independent of miRNA-fibrinogen 3′-untranslated region interactions. In contrast, overexpression of hsa-miR-409-3p specifically lowered fibrinogen Bβ mRNA levels, and this effect was dependent on a target site in the fibrinogen Bβ mRNA 3′-untranslated region. This study adds to the known mechanisms that control fibrinogen production, points toward a potential cause of variable circulating fibrinogen levels, and demonstrates that a screening approach can identify miRNAs that regulate clinically important proteins.
Introduction
Blood coagulation terminates in the controlled conversion of fibrinogen to fibrin, an insoluble polymer that gives structural stability, strength, and adhesive surfaces to growing clots. In healthy persons, plasma fibrinogen concentrations vary between 1.5 and 3.5 g/L. Patients with fibrinogen levels below this range (ie, hypofibrinogenemia and afibrinogenemia, OMIM no. 202400) when symptomatic, have a bleeding disorder. An increase in fibrinogen levels of 1 g/L above the normal range is associated with a near doubling of the risk of cardiovascular disease, independent of traditional risk factors.1 The mechanisms involved are unknown but most likely include increased blood viscosity, platelet aggregation, thrombophilia, and proliferation of vascular endothelial and smooth muscle cells.2,3
The fibrinogen molecule is composed of 2 copies of 3 polypeptides (Aα, Bβ, and γ), which fold into a functional hexamer.4 The 3 chains are encoded by 3 genes (FGA, FGB, and FGG) clustered in a compact locus spanning 50 kb on 4q32 in humans. Aα and γ fibrinogen have minor alternatively spliced isoforms: AαE and γ′. Fibrinogen is produced in hepatocytes and undergoes coordinated transcriptional regulation of its 3 genes, both in the basal state and when expression increases with inflammatory stress.5 Functional transcription factor binding sites have been described within the 3 promoter regions. The CCAAT-box/enhancer-binding protein and the hepatocyte nuclear factor-1 play important roles in the transcriptional regulation of basal expression of FGA and FGB,6,7 and all 3 promoters contain interleukin-6–responsive elements, mediating the rapid increase of fibrinogen gene transcription during the acute phase response.8-10 Interestingly, expression of all 3 fibrinogen genes follows a circadian transcription pattern,11 suggesting an additional level of fibrinogen transcriptional regulation that is not yet characterized. Besides studies focusing on transcription regulation, a study investigating the decay of fibrinogen mRNAs suggested that the degradation of fibrinogen transcripts might also be coordinately regulated.12 Thus, unknown regulatory mechanisms, acting at the posttranscriptional level, might exist for controlling fibrinogen biosynthesis.
A major addition to understanding post-transcriptional gene regulation has come from the discovery and characterization of microRNAs (miRNAs). These small noncoding RNAs (∼ 22 nucleotides) are trans-acting factors able to influence the productivity of metazoan protein-coding genes via translational repression, by directing mRNA degradation, or by a combination of both mechanisms.13 More than 750 miRNAs have been annotated in the human genome so far (miRBase, Release 14.0, September 2009) and have been shown to be involved in diverse processes, including development,14 cell differentiation,15 cell proliferation,16 and metabolism.17 More than one-third of human messenger RNAs (mRNAs) are predicted to be miRNA targets.18 Proteomic-based studies have demonstrated that individual miRNAs can regulate the expression of multiple proteins, the majority of which show less than 2-fold changes in protein output.19,20 Therefore, this broad spectrum of miRNA targets most probably includes transcripts encoding proteins that influence human disease at modestly altered expression levels, proteins such as fibrinogen.
To determine whether miRNAs regulate fibrinogen production and/or secretion, we screened for functional activity using a library of annotated human miRNA precursors, rather than a set of in silico predictions. Our screen follows a straightforward strategy; the effect of 470 precursor miRNA molecules on fibrinogen biosynthesis is tested in vitro, and further studies are then performed on candidates that lower fibrinogen levels in the screen. With this approach, we identified 23 miRNAs down-regulating fibrinogen production and 4 miRNAs up-regulating fibrinogen production. We then investigated the mechanism of action for 4 identified down-regulators, 3 hsa-miR-29 family members and hsa-miR-409-3p.
Methods
Cell culture
HuH7 and HEK-293T cells were cultured in Dulbecco modified Eagle medium (DMEM; Invitrogen) supplemented with 10% heat-inactivated fetal bovine serum, 100 U/mL of penicillin, and 100 μg/mL streptomycin (Invitrogen).
In vitro screen and ELISA assay
On the day before transfection, HuH7 cells were seeded at 104 cells per well, in 96-well plates. Transfections were made with 30 nM Pre-miR miRNA Precursor Molecules (Pre-miR Precursor Library-Human V3; Ambion) using Lipofectamine 2000 (Invitrogen) according to the manufacturer's protocol. At 24 hours after transfection, serum-containing medium was removed, cells washed with phosphate-buffered saline and serum-free medium added (DMEM; Invitrogen). After a further 24 hours, the cell-conditioned medium was harvested. Cells were lysed (lysis buffer: Triton X-100, 0.1% Tris, pH 8.0, 50mM, NaCl 100mM, Complete protease inhibitors, Roche Diagnostics); and total protein concentration in the cell lysate was measured by protein assay (micro BCA protein assay kit; Pierce Chemical), following the manufacturer's protocol. Fibrinogen concentration in the cell-conditioned medium was determined using an enzyme-linked immunosorbent assay (ELISA). Polyclonal rabbit anti–human fibrinogen antibodies (A0080; Dako) were used as capture antibodies to coat 96-well microplates (Nunc) at a 0.5 ng/μL concentration. Polyclonal goat anti–human fibrinogen antibodies, conjugated with horseradish peroxidase (ab7539; Abcam), were used for detection at a 1:10 000 dilution.
Screen data normalizations
Each miRNA precursor molecule was transfected in technical triplicates in 3 independent experiments. A ratio of fibrinogen concentration in the cell media divided by the total protein concentration of the associated cell lysate was determined for each well (fibrinogen/total protein). Each 96-well plate was used to measure the activity of 20 miRNAs as well as a negative control miRNA precursor molecule (Pre-miR miRNA Precursor molecules-Negative Control #2; Ambion). This negative control was considered as reference for a miRNA precursor molecule that does not show any regulation potential on fibrinogen production. It was thus used to normalize the ratio (fibrinogen/total protein) for miRNAs tested on each transfection plate. To compare values obtained for all 470 tested miRNAs, 2 further control miRNA precursors were used on each individual transfection plate: hsa-miR-29c, which showed a down-regulation potential; and hsa-miR-106a, which showed an up-regulation potential in a first experiment (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). The average normalized fold change (fibrinogen/total protein) values calculated over all transfection experiments (n = 75) for hsa-miR-29c (−0.576 in log2) and for hsa-miR-106a (0.344 in log2) were used to perform a linear transformation for each transfection plate.
miRNA liver expression
Total RNA was extracted from HuH7 cells using TRIzol reagent (Invitrogen) following the manufacturer's protocol. Human liver total RNA was purchased from Ambion. First, human liver miRNA expression was analyzed in triplicate using Illumina microRNA expression profiling panel (based on miRbase release 9.0) according to the manufacturer's recommendations (Illumina). This assay quantifies the relative expression of 858 miRNAs. A total of 200 ng of RNA from each cell culture was first labeled and then hybridized to each array using standard Illumina protocols. Sample arrays were scanned on an Illumina BeadArray reader. The raw data were processed and quantile normalized using Beadstudio software Version 3.2 (Illumina). A normalized log2 expression value associated with a P value is obtained for each miRNA. Quality and reproducibility of each expression profile were examined, and we considered as expressed only samples with P value less than or equal to .05 and with an intensity value greater than 500. Second, we analyzed the expression profiling of 384 miRNAs by TaqMan Human MicroRNA Array, Version 1.0 (Applied Biosystems), according to the manufacturer's instructions. Briefly, 100 ng from normal human liver total RNA was used as template for 8 multiplex reverse transcriptions containing up to 48 specific primers, using the Multiplex RT for TaqMan MicroRNA Assays Kit (Applied Biosystems). Each cDNA was amplified by quantitative polymerase chain reaction (PCR) using specific primers from the TaqMan microRNA Assays Human Panel on an Applied Biosystems 7900 Fast Real Time PCR system (Applied Biosystems). Raw threshold cycle (CT) values were generated using SDS, Version 2.0 software (Applied Biosystems). CT values were converted to quantity (q) with the formula q = 2−ΔCt. We classified miRNA expression as expressed (q > 2−(35−normalization factor)) or as nonexpressed (q < 2−(35−normalization factor), undetermined CT value or nonexponential amplification curves).
Analysis of apoptosis by propidium iodide staining and flow cytometry
A total of 1.5 × 106 HuH7 cells were seeded in 10-cm-diameter cell-culture dishes. The next day, cells were transfected with 30nM Pre-miR miRNA Precursor Molecules (Ambion) using Lipofectamine 2000 (Invitrogen) as transfectant, according to the manufacturer's protocol. Twenty-four hours after transfection, plates were washed with phosphate-buffered saline and DMEM (Invitrogen) was added. After another 24 hours, cells were trypsinized and harvested, fixed in chilled 70% ethanol, stained for 30 minutes at room temperature in the dark with propidium iodide (20 ng/μL; Sigma-Aldrich), and analyzed by fluorescence-activated cell sorter.21
Fibrinogen SYBR Green quantitative RT assay
A total of 500 ng of total RNA was reverse transcribed (SuperScript II; Invitrogen), primed with random hexamers (Invitrogen). The 5 fibrinogen mRNA levels were measured by quantitative reverse-transcriptase (RT)–PCR (SYBR Green master mix; Applied Biosystems). Each gene was amplified in 6 replicates per sample in 384-well plates, and input was normalized using 3 genes: β2-microglobulin (B2M), TATA box binding protein (TBP), and the eukaryotic translation elongation factor 1 alpha (EEF1A1). CT values were obtained using SDS, Version 2.1 software (Applied Biosystems) and replicates with a deviation of plus or minus 0.25 CT, with respect to the median, were considered outliers and excluded from further analyses. Relative mRNA levels were calculated using the ΔΔCT method.22
Luciferase gene reporter plasmids
PCR primers (supplemental Table 1) for the amplification of the complete 3′-untranslated regions (UTRs) of the 5 human fibrinogen-3′-UTRs were designed. The amplified fragments were inserted after the stop codon of the firefly luciferase gene in the XbaI site of the pTAL luc (firefly) reporter plasmid (Clontech). A fibrinogen β 3′-UTR reporter plasmid with a deleted seed sequence for the predicted hsa-miR-409-3p target site was obtained by overlap extension PCR of the 3′-UTR sequences upstream and downstream of the seed sequence. The assembled product was then cloned into the XbaI site of the pTAL luc (firefly) reporter plasmid (Clontech).
The FGG 5′-UTR was amplified with specific primers (supplemental Table 1), the amplicon was directly inserted before the start codon of the firefly luciferase gene into the NcoI site of the pTAL luc (firefly) reporter plasmid (Clontech). Each construct was verified by direct sequencing.
Luciferase reporter gene assay
HEK-293T cells were seeded at 104 cells per well in 96-well opaque plates (PerkinElmer Life and Analytical Sciences) the day before transfection. Each well was transfected with 200 ng of pTAL luc (firefly) reporter plasmid containing a fibrinogen 3′-UTR or 5′-UTR, 10 ng of transfection control plasmid expressing renilla luciferase gene (pRL-SV40, Promega), and 90, 30, or 10nM miRNA precursor (Pre-miR miRNA Precursor molecule; Ambion). Transient cotransfections were performed using Lipofectamine 2000 transfection reagent (Invitrogen) according to the manufacturer's instructions. At 48 hours after transfection, the activities of firefly and renilla luciferase were measured using the Dual-Luciferase Assay Kit (Promega). Luminescence intensities were quantified on a Victor 3 multilabel counter (PerkinElmer Life and Analytical Sciences). The luciferase activity ratio (firefly/renilla) for each transfection condition was normalized to the ratio obtained with the respective firefly luciferase plasmid without the addition of a miRNA precursor molecule.
Results
Screening the fibrinogen regulatory potential of 470 miRNAs in vitro
To assess whether miRNAs regulate fibrinogen production, we used a library of 470 known human miRNA precursors that we screened for activity in an in vitro assay. Briefly, each precursor miRNA was transfected into HuH7 human hepatoma cells, which produce endogenous fibrinogen, and the quantity of fibrinogen secreted into the cell culture medium assessed by an ELISA test. A ratio of secreted fibrinogen over total cell protein was calculated to account for any differences in well-to-well cell numbers. The reproducibility and the sensitivity of our method were tested with 10 miRNAs (supplemental Figure 1). Two of these 10 miRNAs, hsa-miR-29c and hsa-miR-106a, showing the lowest and the highest fibrinogen values in this experiment, were selected as internal controls for the larger screen. As a positive control for our experimental system, which is dependent on the successful transfection of HuH7 with precursor miRNAs, we transfected HuH7 cells with hsa-miR-1 and hsa-miR-34a, both known to down-regulate the levels of the hepatocyte growth factor receptor, Met.20,23 We found that transfection of HuH7 cells with both hsa-miR-1 and hsa-miR-34a reduced the levels of MET mRNA and Met protein compared with nontransfected HuH7 cells and cells transfected with a control miRNA (data not shown).
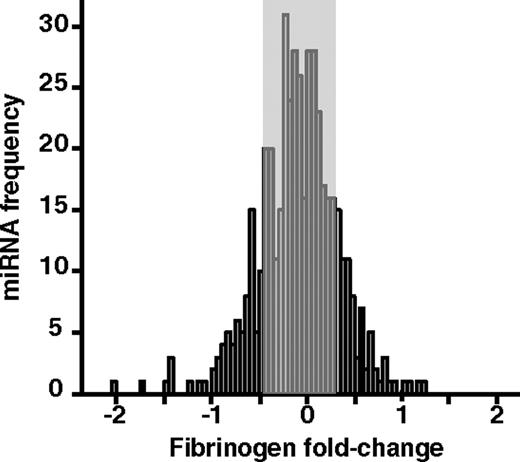
Data from our screen were plotted on a frequency distribution, with the fold change of fibrinogen/total protein plotted as the variable, and a negative control miRNA as the normalizing value (Figure 1). The general shape of the distribution is centered on zero with a slightly larger population of potential down-regulators than up-regulators, in accordance with other miRNA-based studies that used screening strategies.24,25 The screen suggests that most miRNAs have no regulatory potential (close to zero), as shown by the high frequency of miRNAs near the control value. Considering miRNAs able to decrease or increase fibrinogen production by at least 25%, compared with the control condition (negative control miRNA, “Screen data normalizations”), 39 miRNAs appear to have a significant down-regulatory effect (2-sided t test, P ≤ .05), with a maximal decrease of 64% (fold change in log2 of −1.46). In addition, 11 miRNAs show significant up-regulation (2-sided t test, P ≤ .05) of fibrinogen production, with a maximal increase of 129% (fold change in log2 of 1.2). The minimum 25% increase or decrease threshold we chose is based on the sensitivity of our in vitro assay, considering the consistency of the ELISA and protein assays.
Fibrinogen regulatory potential of 470 precursor miRNA molecules in vitro. The frequency distribution of 470 miRNA precursors is plotted for fibrinogen production fold change on the x-axis (log2). The gray area represents miRNAs that are considered to have no effect on fibrinogen production, using a threshold of at least 25% decrease or increase. Each precursor molecule was tested at 30nM in 3 independent transfection experiments, using HuH7 cells. Fold change in fibrinogen production was calculated relative to a negative control miRNA precursor used to normalize data.
Fibrinogen regulatory potential of 470 precursor miRNA molecules in vitro. The frequency distribution of 470 miRNA precursors is plotted for fibrinogen production fold change on the x-axis (log2). The gray area represents miRNAs that are considered to have no effect on fibrinogen production, using a threshold of at least 25% decrease or increase. Each precursor molecule was tested at 30nM in 3 independent transfection experiments, using HuH7 cells. Fold change in fibrinogen production was calculated relative to a negative control miRNA precursor used to normalize data.
To select candidate miRNAs regulating fibrinogen production for further investigation, we assessed their expression in human hepatocytes, the cell type where in vivo fibrinogen biosynthesis occurs. miRNA expression in human liver and HuH7 cell RNA extracts was measured by quantitative real-time PCR and multiplex assays (supplemental Table 2). Respectively, 59% (23 of 39 miRNAs) and 36% (4 of 11 miRNAs) of the potential down- and up-regulators revealed by the functional screen are expressed in human liver. Using these criteria, 23 potential down-regulators, with a maximal decrease of 64% (fold change in log2 of −1.46) and 4 miRNAs showing up-regulatory potential with a maximal increase of 129% increase (fold change in log2 of 1.2), on the fibrinogen production were identified (Table 1).
miRNAs affecting fibrinogen synthesis
| miRNA ID . | Fold change in fibrinogen production (log2)* . | Relative change in fibrinogen production, percentage† . | P‡ . | Expression in liver and HuH7 . |
|---|---|---|---|---|
| Down-regulators | ||||
| hsa-miR-29a | −1.46 | −63.6 | .0132 | E |
| hsa-miR-218 | −1.23 | −57.3 | .0144 | E |
| hsa-miR-409-3p | −0.88 | −45.5 | .0448 | E§ |
| hsa-miR-200c | −0.86 | −44.8 | .0146 | E |
| hsa-miR-29c | −0.83 | −43.7 | .0434 | E |
| hsa-miR-425-5p | −0.82 | −43.2 | .0114 | E |
| hsa-miR-195 | −0.80 | −42.7 | .0058 | E |
| hsa-miR-199b | −0.79 | −42.2 | .0428 | E |
| hsa-miR-31 | −0.77 | −41.5 | .0208 | E |
| hsa-miR-429 | −0.77 | −41.3 | .0388 | E |
| hsa-miR-197 | −0.67 | −37.0 | .0180 | E |
| hsa-miR-28 | −0.63 | −35.3 | .0176 | E |
| hsa-miR-22 | −0.63 | −35.2 | .0086 | E |
| hsa-miR-574 | −0.59 | −33.7 | .0420 | E |
| hsa-miR-23b | −0.58 | −33.2 | .0028 | E |
| hsa-let-7e | −0.57 | −32.7 | .0500 | E |
| hsa-let-7d | −0.56 | −32.0 | .0484 | E |
| hsa-miR-132 | −0.53 | −30.8 | .0048 | E |
| hsa-miR-215 | −0.46 | −27.3 | .0310 | E |
| hsa-miR-24 | −0.45 | −26.6 | .0264 | E |
| hsa-miR-182 | −0.44 | −26.4 | .0194 | E |
| hsa-miR-19b | −0.44 | −26.4 | .0346 | E |
| hsa-miR-194 | −0.42 | −25.5 | .0412 | E |
| Up-regulators | ||||
| hsa-miR-769-5p | 0.34 | 26.2 | .0066 | E |
| hsa-miR-592 | 0.44 | 35.9 | .0472 | E |
| hsa-miR-126 | 0.46 | 37.6 | .0022 | E |
| hsa-miR-365 | 1.20 | 129.3 | .0474 | E |
| miRNA ID . | Fold change in fibrinogen production (log2)* . | Relative change in fibrinogen production, percentage† . | P‡ . | Expression in liver and HuH7 . |
|---|---|---|---|---|
| Down-regulators | ||||
| hsa-miR-29a | −1.46 | −63.6 | .0132 | E |
| hsa-miR-218 | −1.23 | −57.3 | .0144 | E |
| hsa-miR-409-3p | −0.88 | −45.5 | .0448 | E§ |
| hsa-miR-200c | −0.86 | −44.8 | .0146 | E |
| hsa-miR-29c | −0.83 | −43.7 | .0434 | E |
| hsa-miR-425-5p | −0.82 | −43.2 | .0114 | E |
| hsa-miR-195 | −0.80 | −42.7 | .0058 | E |
| hsa-miR-199b | −0.79 | −42.2 | .0428 | E |
| hsa-miR-31 | −0.77 | −41.5 | .0208 | E |
| hsa-miR-429 | −0.77 | −41.3 | .0388 | E |
| hsa-miR-197 | −0.67 | −37.0 | .0180 | E |
| hsa-miR-28 | −0.63 | −35.3 | .0176 | E |
| hsa-miR-22 | −0.63 | −35.2 | .0086 | E |
| hsa-miR-574 | −0.59 | −33.7 | .0420 | E |
| hsa-miR-23b | −0.58 | −33.2 | .0028 | E |
| hsa-let-7e | −0.57 | −32.7 | .0500 | E |
| hsa-let-7d | −0.56 | −32.0 | .0484 | E |
| hsa-miR-132 | −0.53 | −30.8 | .0048 | E |
| hsa-miR-215 | −0.46 | −27.3 | .0310 | E |
| hsa-miR-24 | −0.45 | −26.6 | .0264 | E |
| hsa-miR-182 | −0.44 | −26.4 | .0194 | E |
| hsa-miR-19b | −0.44 | −26.4 | .0346 | E |
| hsa-miR-194 | −0.42 | −25.5 | .0412 | E |
| Up-regulators | ||||
| hsa-miR-769-5p | 0.34 | 26.2 | .0066 | E |
| hsa-miR-592 | 0.44 | 35.9 | .0472 | E |
| hsa-miR-126 | 0.46 | 37.6 | .0022 | E |
| hsa-miR-365 | 1.20 | 129.3 | .0474 | E |
E indicates expressed.
The fold change represents the calculated ratio (Fbg/Tot Prot) in log2 compared with the reference condition transfected with a negative control miRNA precursor molecule.
This relative change represents the percentage of decrease or increase in fibrinogen production compared with the reference condition transfected with a negative control miRNA precursor molecule.
Two-sided t test, tested miRNA (n = 3) versus negative control miRNA (n = 3).
This miRNA is expressed in liver but not in HuH7 cells.
Regulatory effects of hsa-miR-29 family members on fibrinogen production
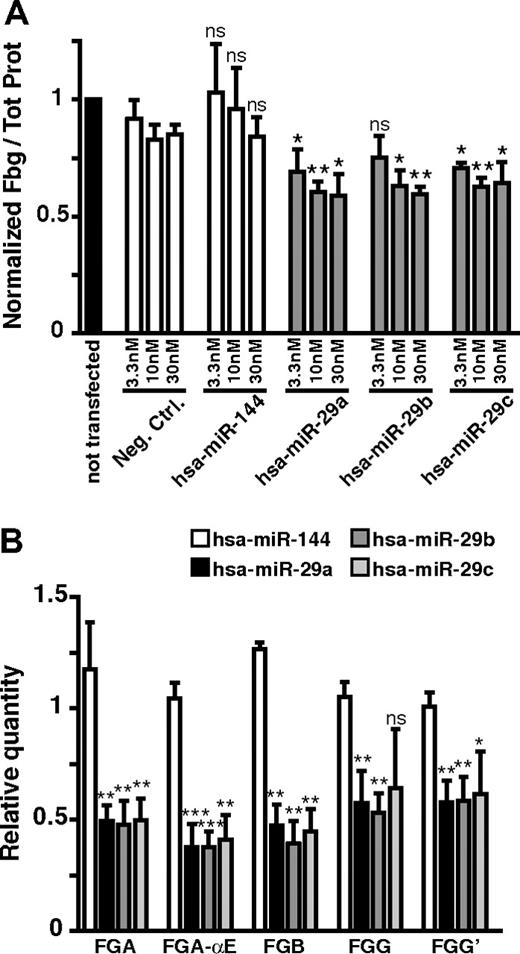
Of the 23 down-regulating miRNAs identified, hsa-miR-29a had the strongest down-regulating potential with a significant decrease of 64% (fold change in log2 of −1.46, P = .0132, 2-sided t test) on fibrinogen production (Table 1). This miRNA is a member of a family composed of 3 miRNAs, hsa-miR-29a, b, and c. Interestingly, hsa-miR-29c appears in fifth position in our screen, with a significant decrease effect of 44% (fold change in log2 of −0.83, P > .043, 2-sided t test) on fibrinogen production; whereas hsa-miR-29b, also expressed in the human liver (supplemental Table 2), shows a 63% decrease effect (fold change in log2 of −1.44, P > .08, 2-sided t test). We decided to investigate the mechanisms of action of these 3 miRNAs on the biosynthesis of fibrinogen. For this purpose, we first repeated the in vitro assay using 3 different precursor molecule concentrations (3.3, 10, and 30nM) using hsa-miR-144 as a control (a miRNA that had no effect on fibrinogen production in our screen). We observed significant 24%, 30%, and 30% decreases in fibrinogen production when HuH7 cells were transfected with 30nM hsa-miR-29c, hsa-miR-29a, and hsa-miR-29b, respectively, compared with cells transfected with a negative control precursor molecule (Figure 2A). A similar effect was also observed at lower concentrations (3.3 and 10nM). These results confirm that transfection of hsa-miR-29 family members can reduce fibrinogen production.
Down-regulation of fibrinogen production by hsa-miR-29 family members. (A) The normalized secreted fibrinogen (Fbg) levels for hsa-miR-29a, hsa-miR-29b, and hsa-miR-29c. Precursors were transfected at 3 concentrations (3.3, 10, and 30nM, gray bars), and secreted fibrinogen was analyzed. A negative control miRNA precursor (Neg. Ctrl.) and hsa-miR-144, which had no effect on fibrinogen production in the screen, were used as negative controls (▭). A nontransfected control was used as the normalizing condition (▬). P values were calculated for experimental data versus values for Neg. Ctrl. (B) Relative steady-state levels of each of the 5 fibrinogen transcripts (FGA, FGA-αE, FGB, FGG, and FGG′) were quantified by quantitative RT-PCR from RNA samples of HuH7 cells transfected with 30nM hsa-miR-29. hsa-miR-144 was used as a negative control. P values were calculated for experimental data versus values for hsa-miR-144: *P < .05, **P < .01, ***P < .001 (2-sided t test). Error bars represent SD; n = 3. ns indicates not significant.
Down-regulation of fibrinogen production by hsa-miR-29 family members. (A) The normalized secreted fibrinogen (Fbg) levels for hsa-miR-29a, hsa-miR-29b, and hsa-miR-29c. Precursors were transfected at 3 concentrations (3.3, 10, and 30nM, gray bars), and secreted fibrinogen was analyzed. A negative control miRNA precursor (Neg. Ctrl.) and hsa-miR-144, which had no effect on fibrinogen production in the screen, were used as negative controls (▭). A nontransfected control was used as the normalizing condition (▬). P values were calculated for experimental data versus values for Neg. Ctrl. (B) Relative steady-state levels of each of the 5 fibrinogen transcripts (FGA, FGA-αE, FGB, FGG, and FGG′) were quantified by quantitative RT-PCR from RNA samples of HuH7 cells transfected with 30nM hsa-miR-29. hsa-miR-144 was used as a negative control. P values were calculated for experimental data versus values for hsa-miR-144: *P < .05, **P < .01, ***P < .001 (2-sided t test). Error bars represent SD; n = 3. ns indicates not significant.
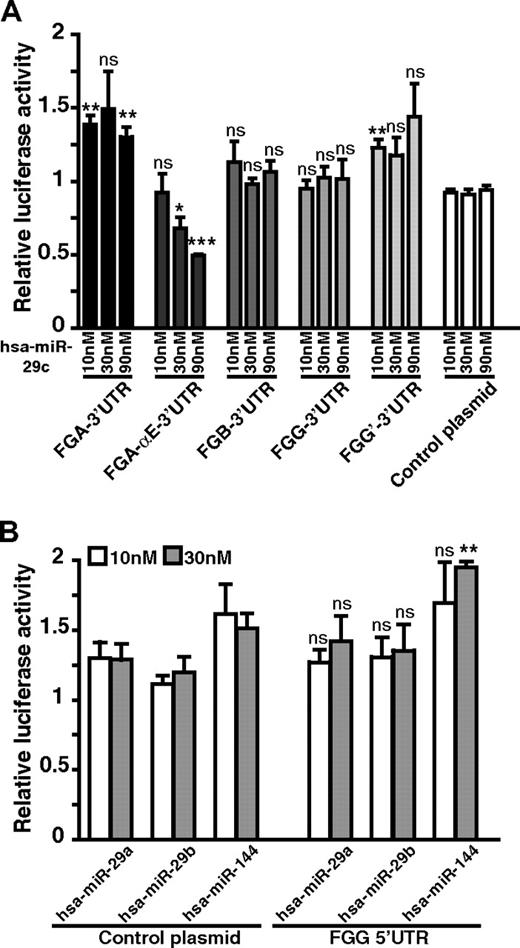
The down-regulation of fibrinogen production mediated by hsa-miR-29s (a, b, or c) could result either from direct effects of these miRNAs on 1 or several fibrinogen transcripts (FGA, FGA-αE, FGB, FGG, and FGG′) or indirectly by hsa-miR-29 (a, b, or c) targeting 1 or more unidentified regulator(s) of fibrinogen biosynthesis. To distinguish between these 2 mechanisms, we first analyzed the effect of the 3 hsa-miR-29 family members on the level of the 5 individual fibrinogen transcripts. We isolated total RNA from HuH7 cells transfected with 30nM precursor molecules and performed quantitative RT-PCR specific for each fibrinogen mRNA. Compared with the control condition transfected with hsa-miR-144, a decrease of all 5 fibrinogen transcripts was observed when HuH7 cells were transfected with hsa-miR-29a, hsa-miR-29b, or hsa-miR-29c (Figure 2B). The FGB mRNA level is on average reduced by 56% compared with the nontransfected control, whereas FGA and FGG transcript levels show reductions of 51% and 42%, respectively. The 2 isoforms FGA-αE and FGG′ mRNA levels are decreased by 61% and 41%, respectively. We then investigated whether the observed decrease of mRNA levels is the result of a direct effect via the 3′-UTR of each mRNA. Using the TargetScan26 algorithm prediction (release 5.1, April 2009), potential hsa-miR-29 target sites on fibrinogen transcript 3′-UTRs were assessed. Only the FGA-αE-3′-UTR has a predicted target site for the 3 hsa-miR-29 family members. To verify this prediction, fibrinogen 3′-UTRs (FGA, FGA-αE, FGB, FGG, and FGG′) were cloned after the stop codon of the firefly luciferase reporter gene and before the SV40 poly-A signal. Cotransfections in HEK-293T cells, including the reporter plasmid, miRNA precursor molecules, and a transfection control plasmid (encoding renilla luciferase) were made. In agreement with the TargetScan prediction, we detected a direct effect of hsa-miR-29c (hsa-miR-29a and hsa-miR-29b were not tested) on luciferase activity from the reporter plasmid carrying the FGA-αE-3′-UTR but not for the reporter plasmids carrying the other fibrinogen mRNA 3′-UTRs (Figure 3A). Because the extended version of the Aα chain represents only 1% to 2% of total Aα production,27 we conclude that a direct effect of hsa-miR-29 family members on FGA-αE transcripts cannot explain the decrease observed in our fibrinogen production assay.
Fibrinogen UTR gene reporter assays to detect direct effects of hsa-miR-29 family miRNAs. (A) The relative luciferase activity normalized with a nontransfected condition. Firefly luciferase reporter gene plasmids containing the fibrinogen 3′-UTRs (FGA, FGA-αE, FGB, FGG, and FGG′) were individually cotransfected with the hsa-miR-29c precursor molecule (10, 30, and 90nM) and a renilla luciferase transfection control plasmid in HEK-293T cells; n = 3. (B) A similar experiment using a reporter plasmid containing the FGG 5′-UTR sequence cloned upstream of the firefly luciferase reporter gene or a control plasmid without the FGG 5′-UTR cotransfected with 10nM or 30nM miRNA precursor molecules. P values were calculated for experimental data versus values for control plasmid conditions: *P < .05, **P < .01, ***P < .001 (2-sided t test). Error bars represent SD; n = 3. ns indicates not significant.
Fibrinogen UTR gene reporter assays to detect direct effects of hsa-miR-29 family miRNAs. (A) The relative luciferase activity normalized with a nontransfected condition. Firefly luciferase reporter gene plasmids containing the fibrinogen 3′-UTRs (FGA, FGA-αE, FGB, FGG, and FGG′) were individually cotransfected with the hsa-miR-29c precursor molecule (10, 30, and 90nM) and a renilla luciferase transfection control plasmid in HEK-293T cells; n = 3. (B) A similar experiment using a reporter plasmid containing the FGG 5′-UTR sequence cloned upstream of the firefly luciferase reporter gene or a control plasmid without the FGG 5′-UTR cotransfected with 10nM or 30nM miRNA precursor molecules. P values were calculated for experimental data versus values for control plasmid conditions: *P < .05, **P < .01, ***P < .001 (2-sided t test). Error bars represent SD; n = 3. ns indicates not significant.
We also used the RNA22 algorithm28 that predicts target sites within the 5′-UTR and coding sequences of mRNAs, to detect hsa-miR-29 target site(s) within fibrinogen transcripts. A potential target site for hsa-miR-29a and hsa-miR-29b was predicted within the 5′-UTR common to both FGG transcripts. To test whether this particular site was functional, we cloned the FGG 5′-UTR sequence in the luciferase reporter plasmid, upstream of the firefly luciferase gene. Cotransfections were then performed in HEK-293T cells, including the reporter plasmid, the hsa-miR-29a or hsa-miR-29b miRNA precursor, and a transfection control plasmid (encoding renilla luciferase). Neither hsa-miR-29a nor hsa-miR-29b had a direct effect on luciferase activity using this reporter gene (Figure 3B). These reporter gene experiments imply that the effects observed on other fibrinogen transcript levels, by quantitative RT-PCR, arise from distinct indirect mechanisms.
Recent in vitro studies have shown that the 3 hsa-miR-29 family members can induce apoptosis by targeting p85α and CDC42, both of which negatively regulate p53. This mechanism is dependent on a wild-type allele of p53.29 HuH7 cells carry a mutated allele of p5330 and therefore are not expected to activate such mechanisms in the presence of hsa-miR-29. To investigate whether hsa-miR-29c can induce apoptosis in HuH7 cells, we measured propidium iodide staining of cells transfected with hsa-miR-29c (30nM) precursor molecules. This assay detects subdiploid cells as a marker of apoptosis. We did not observe any induction of apoptosis in these cells, compared with control conditions (supplemental Figure 2), demonstrating that the reduced fibrinogen levels measured for cells transfected with hsa-miR-29c were not the result of apoptosis.
From these in vitro experiments and in silico predictions, we can conclude that hsa-miR-29 family members are able to decrease fibrinogen biosynthesis by indirect mechanisms.
hsa-miR-409-3p directly targets the FGB 3′-UTR in vitro
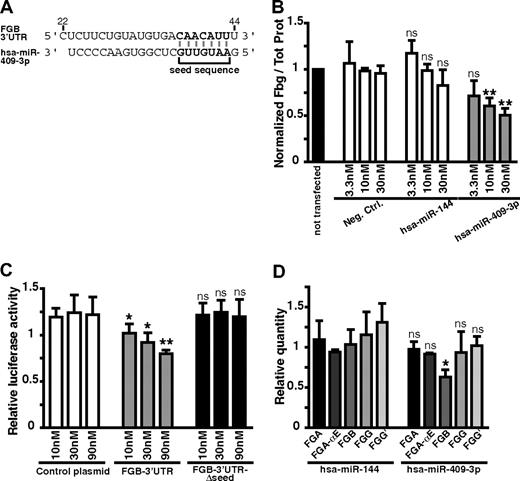
Using the TargetScan algorithm, 1 target site within the FGB 3′-UTR was predicted for hsa-miR-409-3p, one of the most potent fibrinogen down-regulating miRNAs identified in our screen, with a significant decrease effect of 46% (fold change in log2 of −0.88, P < .045, 2-sided t test) on fibrinogen production (Table 1). Interestingly, FGB has been proposed to play a key rate-limiting role in fibrinogen assembly.31 We therefore investigated further the mechanism of action of hsa-miR-409-3p on fibrinogen production. First, we quantified the effect of hsa-miR-409-3p on fibrinogen production in vitro by transfecting HuH7 cells with 3.3, 10, and 30nM precursor molecules (Figure 4A). A significant decrease in secreted fibrinogen of 33%, 39%, and 47% was measured for the respective concentrations (Figure 4B).
hsa-miR-409-3p regulates fibrinogen production via a target site in the FGB 3′-UTR. (A) Sequence details between the nucleotide 22 and 44 after the stop codon of the predicted target site, detected with TargetScan, for hsa-miR-409-3p in FGB 3′-UTR. (B) Normalized secreted fibrinogen levels for 2 control conditions: a negative control miRNA precursor (Neg. Ctrl.) and hsa-miR-144 (▭) and for hsa-miR-409-3p ( ). Precursors were transfected at 3 concentrations (3.3, 10, and 30nM), and secreted fibrinogen was analyzed. Nontransfected control (▬) was used to normalize results; n = 3. P values were calculated for experimental data versus values for Neg. Ctrl. (C) HEK-293T cells were cotransfected with firefly luciferase reporter plasmids carrying the FGB 3′-UTR (
). Precursors were transfected at 3 concentrations (3.3, 10, and 30nM), and secreted fibrinogen was analyzed. Nontransfected control (▬) was used to normalize results; n = 3. P values were calculated for experimental data versus values for Neg. Ctrl. (C) HEK-293T cells were cotransfected with firefly luciferase reporter plasmids carrying the FGB 3′-UTR ( ), the FGB-3′-UTR without the predicted target sequence of hsa-miR-409-3p (FGB-3′-UTR-Δseed, ▬), or a plasmid with no 3′-UTR (control plasmid, ▭); n = 5. P values were calculated for experimental data versus values for control plasmid conditions. (D) The relative quantity of each of the 5 fibrinogen transcripts quantified by quantitative RT-PCR from RNA samples of HuH7 cells transfected with 30nM of hsa-miR-409-3p, or hsa-miR-144 used here as a negative control; n = 3. P values were calculated for experimental data versus values for hsa-miR-144: *P < .05, **P < .01 (2-sided t test). Error bars represent SD. ns indicates not significant.
), the FGB-3′-UTR without the predicted target sequence of hsa-miR-409-3p (FGB-3′-UTR-Δseed, ▬), or a plasmid with no 3′-UTR (control plasmid, ▭); n = 5. P values were calculated for experimental data versus values for control plasmid conditions. (D) The relative quantity of each of the 5 fibrinogen transcripts quantified by quantitative RT-PCR from RNA samples of HuH7 cells transfected with 30nM of hsa-miR-409-3p, or hsa-miR-144 used here as a negative control; n = 3. P values were calculated for experimental data versus values for hsa-miR-144: *P < .05, **P < .01 (2-sided t test). Error bars represent SD. ns indicates not significant.
hsa-miR-409-3p regulates fibrinogen production via a target site in the FGB 3′-UTR. (A) Sequence details between the nucleotide 22 and 44 after the stop codon of the predicted target site, detected with TargetScan, for hsa-miR-409-3p in FGB 3′-UTR. (B) Normalized secreted fibrinogen levels for 2 control conditions: a negative control miRNA precursor (Neg. Ctrl.) and hsa-miR-144 (▭) and for hsa-miR-409-3p ( ). Precursors were transfected at 3 concentrations (3.3, 10, and 30nM), and secreted fibrinogen was analyzed. Nontransfected control (▬) was used to normalize results; n = 3. P values were calculated for experimental data versus values for Neg. Ctrl. (C) HEK-293T cells were cotransfected with firefly luciferase reporter plasmids carrying the FGB 3′-UTR (
). Precursors were transfected at 3 concentrations (3.3, 10, and 30nM), and secreted fibrinogen was analyzed. Nontransfected control (▬) was used to normalize results; n = 3. P values were calculated for experimental data versus values for Neg. Ctrl. (C) HEK-293T cells were cotransfected with firefly luciferase reporter plasmids carrying the FGB 3′-UTR ( ), the FGB-3′-UTR without the predicted target sequence of hsa-miR-409-3p (FGB-3′-UTR-Δseed, ▬), or a plasmid with no 3′-UTR (control plasmid, ▭); n = 5. P values were calculated for experimental data versus values for control plasmid conditions. (D) The relative quantity of each of the 5 fibrinogen transcripts quantified by quantitative RT-PCR from RNA samples of HuH7 cells transfected with 30nM of hsa-miR-409-3p, or hsa-miR-144 used here as a negative control; n = 3. P values were calculated for experimental data versus values for hsa-miR-144: *P < .05, **P < .01 (2-sided t test). Error bars represent SD. ns indicates not significant.
), the FGB-3′-UTR without the predicted target sequence of hsa-miR-409-3p (FGB-3′-UTR-Δseed, ▬), or a plasmid with no 3′-UTR (control plasmid, ▭); n = 5. P values were calculated for experimental data versus values for control plasmid conditions. (D) The relative quantity of each of the 5 fibrinogen transcripts quantified by quantitative RT-PCR from RNA samples of HuH7 cells transfected with 30nM of hsa-miR-409-3p, or hsa-miR-144 used here as a negative control; n = 3. P values were calculated for experimental data versus values for hsa-miR-144: *P < .05, **P < .01 (2-sided t test). Error bars represent SD. ns indicates not significant.
The predicted binding site for hsa-miR-409-3p on the FGB 3′-UTR sequence was tested in a luciferase gene reporter assay. We cotransfected HEK-293T cells with a reporter plasmid containing the FGB 3′-UTR downstream of a firefly luciferase reporter gene with the hsa-miR-409-3p precursor and a transfection control plasmid (encoding renilla luciferase). A 34% decrease in luciferase activity was measured when hsa-miR-409-3p was transfected with the FGB 3′-UTR reporter plasmid. However, this decrease was not detected when the seed sequence for hsa-miR-409-3p was deleted from the reporter gene 3′-UTR (Figure 4C). To assess whether the interaction between hsa-miR-409-3p and the FGB transcript leads to lowering of steady-state FGB mRNA levels, we performed a quantitative RT-PCR on total RNA extracted from HuH7 cells transfected with 30nM hsa-miR-409-3p (Figure 4D). A 37% decrease in FGB transcript was measured, whereas none of the other fibrinogen mRNAs was affected. Taken together, these experiments demonstrate that hsa-miR-409-3p can directly target the FGB 3′-UTR, leading to lower FGB mRNA levels and thus reducing total fibrinogen protein production.
Discussion
The most important finding of our study is that miRNAs can regulate fibrinogen production. This has not been described previously for control of fibrinogen synthesis. Using an in vitro screening strategy, we identified miRNAs acting both directly at the FGB 3′-UTR and indirectly on steady-state fibrinogen transcript levels. Our screen yields a catalog of many other miRNAs that can change fibrinogen production, both as up- and down-regulators, suggesting the existence of further direct and indirect modifiers.
The 3 fibrinogen genes are regulated by elements in their proximal promoters, but several studies have also proposed coregulation of the 3 chains.8,11,32 This seems a reasonable regulatory strategy for subunits of a common multimeric protein but implies more than just transcription factor-promoter interactions and also requires tissue specificity. Each fibrinogen chain is also up-regulated as part of the acute-phase inflammatory response.8,9 Fibrinogen regulation has been studied mainly at the transcriptional level. Despite experimental evidence suggesting regulation of fibrinogen transcript decay,12 there is, to our knowledge, no study that has investigated mechanisms involved in post-transcriptional fibrinogen regulation.
miRNAs can be considered to be among the most abundant gene-regulatory molecules in animal cells.13 It has been estimated that one-third of human transcripts are miRNA targets.18 A unique feature of their action is the potential for rapid and reversible regulatory outcomes and maintenance of steady-state protein levels.33 A combinatorial model involving gene expression regulation by a “transcription factor code” and post-transcriptional regulation with a “miRNA code” has been proposed to precisely delineate individual cell type expression of protein-coding genes.34 miRNAs are therefore attractive candidate regulators for proteins, such as fibrinogen, which require both tissue-specific expression and coregulation of several protein subunits.
Our screening approach enabled the identification of 23 potential down-regulators and 4 up-regulators of fibrinogen production. We used total protein-normalized secreted fibrinogen from HuH7 cells as the screen readout. This was used as a surrogate model of fibrinogen secretion from hepatocytes, a necessary step ensuring physiologic circulating fibrinogen levels. miRNAs were not selected for the screen a priori using in silico–based predictions. Instead, all miRNAs in a commercially available library were used. Previously, 2 high-throughput proteomic studies described the accuracy of in silico prediction of miRNA targets, by comparison of predictions with quantitative protein measurements.19,20 Several studies have also combined in silico predictions with differential miRNA expression patterns and identified physiologic relevant novel regulators.35-37 Here we were interested primarily in the capacity of individual miRNAs to alter fibrinogen production by direct or indirect means. We therefore ignored predictive algorithms for the first screen, used a limited number of in silico tests to identify potential direct fibrinogen regulators, and then investigated the validity of certain predictions. The choice for further investigation after the screen was also directed by the presence or absence of miRNA expression in human liver RNA. Similar screening strategies have been conducted with the use of large libraries of plasmid-based miRNA expression vectors38 or lentiviral vectors.24
Mechanisms underlying up-regulatory effects of miRNAs are still poorly understood.39 miRNA overexpression resulting in an increase in fibrinogen production probably involves an indirect mechanism (eg, by binding to a target site in a negative regulator of fibrinogen production). We did not examine further the 4 potential up-regulators identified in our screen but investigated further the mechanism of action of the 3 hsa-miR-29 family members and hsa-miR-409-3p. The 3 hsa-miR-29 family members are able to mediate a simultaneous decrease of all 5 fibrinogen transcript levels. As the fibrinogen transcripts are known to be coregulated, it is tempting to suggest that hsa-miR-29 can influence the expression and activity of a common fibrinogen regulatory molecule. Direct hsa-miR-29-mediated down-regulation of this “regulator” could indirectly lower all 5 fibrinogen transcript levels. The list of hsa-miR-29 targets predicted by miRBase40 and TargetScan26 did not yield any obvious candidates for this putative regulator (eg, hepatocyte nuclear factor-1 and CCAAT-box/enhancer-binding protein are not targets of the hsa-miR-29 family). We therefore investigated whether hsa-miR-29 targets predicted by TargetScan26 and PicTar41 were associated with specific biologic processes using Gene Ontology analysis42 or enriched in members of the KEGG pathways.43 Several pathways related to DNA-dependent regulation of transcription emerged from this analysis but no obvious biologically relevant pathway. Although we do not know the mechanism involved, it is clear that reduced steady-state fibrinogen mRNA levels in hsa-miR-29–transfected cells can explain the reduced fibrinogen protein production detected in our miRNA library screen.
Among the miRNAs that modify fibrinogen production in our screen, a predicted target sequence was identified for hsa-miR-409-3p using the TargetScan algorithm. The target includes a 7-mer seed sequence in the 3′-UTR of the FGB transcript, 37 to 43 nucleotides downstream of the FGB stop codon. Using a luciferase reporter gene assay, we demonstrated that the down-regulating effects of hsa-miR-409-3p can be mediated by the FGB 3′-UTR and that deletion of the seed sequence abolishes the effect. By sequence alignment, this 3′-UTR target site is found intact in primates (human, chimp, and rhesus monkey), rabbit, horse, and armadillo. This relatively modest conservation among mammals does not exclude it as a functional target site in vivo because several lines of experimental evidence suggest that functional miRNA binding sites are not necessarily deeply conserved.44
Our data support a mechanism of action for hsa-miR-409-3p that could include translation repression, mRNA target destabilization, and/or degradation.45 The measured decrease in steady-state FGB mRNA levels, when cells are transfected with hsa-miR-409-3p, suggests miRNA-mediated degradation of FGB transcripts. When the influence of a single miRNA has been measured at the proteomic level, the expression of mRNAs bearing the miRNA seed sequence correlates with lowered protein output.19,20 These results indicate that most of the targets could be repressed at both the mRNA and the translational levels. This is compatible with our results using hsa-miR-409-3p transfection: both steady-state FGB transcript levels and secreted fibrinogen are affected.
A hypothetical mechanism underlying the decrease in miRNA-targeted transcript levels has been proposed in which miRNAs mediate removal of the poly-A tail from mRNA, a direct consequence being the loss of poly-A-binding protein that ultimately leads to 5′-decapping, exposing the mRNA to exonuclease digestion.46 In the particular case of the fibrinogen β-chain, a significant decrease in transcript level and in translation is expected to affect significantly the production of the fibrinogen hexamer. Given that the level of the β-chain is thought to be rate-limiting for fibrinogen hexamer assembly/production,31 its decrease most probably explains the reduction in secreted fibrinogen that we measured from hsa-miR-409-3p–transfected HuH7 cells.
Using our screening approach, we can only uncover gain-of-function effects for the miRNAs in the library used. This probably leads to a non-negligible rate of false-positive discovery because of general perturbation of transcript production by nonphysiologic miRNA-mRNA interactions and saturation of the endogenous factors involved in miRNA-mediated regulation, such as the RNA-induced silencing complex. Competition for the RNA-induced silencing complex between transfected and endogenous miRNAs has been described.47
It would be interesting to perform mechanistic experiments on all potential regulatory miRNAs selected from our screen, to evaluate precisely the false-positive discovery rate of our strategy. On the other hand, it would be extremely difficult to evaluate the false-negative discovery rate of our approach because it would require mechanistic studies on too many miRNAs. Despite these limitations, our study can be considered as a proof of principle for large miRNA precursor molecule screening when searching for novel miRNA regulators of selected genes.
Fine-tuning regulation of fibrinogen is of clinical interest because mild changes in fibrinogen levels can influence risk of coronary heart disease and stroke. Indeed, epidemiologic studies have calculated that the hazard ratio for coronary heart disease and stroke is as high as 1.8 for every 1-g/L increase in plasma fibrinogen levels.1 Along this line of thought, it would be of major interest to apply a similar approach to the regulation of other proteins of hemostasis, perhaps unveiling common regulators for several factors. The identification of miRNAs able to modulate fibrinogen production in vitro reveals a new level of regulation of fibrinogen biosynthesis. Further investigations for 4 selected miRNAs demonstrated 2 different modes of action: hsa-miR-409-3p targets and modifies the steady-state level of the FGB transcript, whereas the hsa-miR-29 family members are able to mediate a coordinated decrease of all 5 fibrinogen mRNAs. We think that our screening approach could be useful for the identification of regulatory miRNAs acting on other clinically relevant proteins, particularly those that influence human disease at modestly altered expression levels.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Maryline Gagnebin, Corinne Di Sanza, and Luciana Romano for their technical assistance and the genomics platform of the University of Geneva for their assistance.
This work was supported by the Dr Henri Dubois-Ferrière-Dinu Lipatti Foundation, the Swiss National Science Foundation (grant 31-A0119845), and the European Union AnEUploidy project (S.E.A.).
Authorship
Contribution: A.F. and C.B. designed and performed research and analyzed data; E.M. provided expertise and helped with the statistical analysis of the data; S.E.A. and R.J.F. contributed to the design and analysis of the study; M.N.-A. designed and directed the study; and A.F., C.B., R.J.F., and M.N.-A. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Marguerite Neerman-Arbez, Department of Genetic Medicine and Development, 1 Rue Michel Servet, 1211 Geneva, Switzerland; e-mail: Marguerite.Neerman-Arbez@unige.ch.
References
Author notes
A.F. and C.B. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal