Abstract
The high frequency of allogeneic reactive CD8+ T cells in human and their resistance to immunosuppression might be one of the reasons why successful tolerance-inducing strategies in rodents have failed in primates. Studies on the requirement for T-helper cells in priming CD8+ T-cell responses have led to disparate findings. Recent studies have reported CD8+-mediated allograft rejection independently of T-helper cells; however, the mechanisms that govern the activation of these T cells are far from being elucidated. In this study, we demonstrated that lipopolysaccharide-treated dendritic cells (DCs) were able to induce proliferation and cytotoxic activity of allogeneic CD8+ T cells independently of CD4+ T cells, while adding mycophenolic acid (MPA) to LPS abolished this capacity and resulted in anergic CD8+ T cells that secreted high levels of interleukin-4 (IL-4), IL-5, IL-10, and transforming growth factor-β. Interestingly, we demonstrated that MPA inhibited the LPS-induced synthesis of tumor necrosis factor-α, IL-12, and interferon-γ (IFN-γ) in DCs. Importantly, we found that adding exogenous IFN-γ to MPA restored both the synthesis of cytokines and the ability to activate CD8+ T cells. However, adding IL-12 or tumor necrosis factor-α had no effect. These results suggest that IFN-γ has an important role in licensing DCs to prime CD4-independent CD8 allogeneic T cells via an autocrine loop.
Introduction
Many early studies focused on inhibiting CD4+ T-cell allorecognition pathways to induce transplantation tolerance in rodents.1 However, the activation of memory allogeneic reactive CD8+ T cells has proven a major obstacle to tolerance induction.2-4 Their high frequency in human adults and resistance to many immunosuppressive strategies might be one of the reasons why successful tolerance-inducing strategies in rodents have failed in primates.5 By inducing the apoptosis of graft cells, cytotoxic CD8+ T cells have a critical role in allograft rejection.6,7 The presence of alloreactive CD8+ T cells has also been correlated with the epithelial cell to mesenchymal cell transition in chronic rejection of human kidney8 and immunoresistant CD8+ T-cell responses in humans were associated with increased incidence of acute allograft rejection.9 Taken together, these findings suggest that CD8+ T cells might remain active in allograft recipients, despite chronic immunosuppression. Many experimental findings have thus reinforced the view that CD8+ T-cell responses require specific manipulation in addition to CD4+ T-cell responses for effective prevention of organ transplant rejection in both primates and humans.5,10 However, the mechanisms involved in the activation and memory development of alloreactive CD8+ T cells remain to be elucidated. Greater understanding of this process might therefore help to find new strategies to control CD8 activity and might have important implications for transplantation.
It is often assumed that CD4+ helper T cells are essential for the activation of cytolytic lymphocytes,11-13 especially for memory development, but the requirement for cognate help is not absolute and may be bypassed by alternative pathways, as suggested by studies showing that primary CD8+ T-cell responses against several pathogens are unimpaired in the absence of CD4+ cells.14-16 In allotransplantation, several studies have recently reported CD8+-mediated allograft rejection independently of CD4+ cells6,17-19 and their resistance to immunoregulation by agents that control CD4-dependent rejections have been noted.20,21 Despite the recognition of alloreactive CD4-independent, CD8-dependent im-mune responses as a barrier to allograft survival, little is known regarding the conditions that promote activation of this pathway, especially in humans.
Dendritic cells (DCs) are the main professional antigen-presenting cells (APCs) involved in the initiation of CD8+ T-cell responses to many antigens.22 Several studies have reported that human DCs can activate CD8+ T-cell cytotoxic activity independently of CD4+ helper cells.23,24 However, the signals required by DCs to acquire the ability to activate CD8+ cells have still not been completely defined. In this study, we investigated further the ability of human DCs to induce the differentiation of allogeneic CD8+ lymphocytes into effector cytotoxic cells in the absence of CD4+ T cells. We also explored the possibilities of modulating this ability by pretreating DCs with mycophenolic acid (MPA), the active metabolite of mycophenolate mofetil routinely used in transplantation and in autoimmune diseases.25,26 MPA inhibits inosine 5′-monophosphate-dehydrogenase (IMPDH) involved in de novo synthesis of guanosine nucleotides in T lymphocytes.
The results of the study presented here demonstrated that lipopolysaccharide (LPS)–treated DCs were able to induce cytotoxic allogeneic CD8+ T cells independently of CD4+ T cells. We also found that pretreatment of DCs with MPA abolished this capacity through the inhibition of interferon-γ (IFN-γ) synthesis in DCs. These results suggest that IFN-γ has an important role in licensing human DCs to prime CD4-independent CD8+ allogeneic T cells via an autocrine loop and may provide new approaches to modulating the CD8 cytotoxic alloresponse to promote allograft tolerance.
Methods
Reagents and cytokines
Cells were cultured in RPMI 1640 medium (Gibco) supplemented with 50 IU/mL penicillin (ICN), 50 IU/mL streptomycin (ICN), 2mM l-glutamine (BioWhittaker) and 10% heat-inactivated fetal calf serum (FCS; Gibco). Recombinant human interleukin-4 (IL-4) was obtained from R&D Systems (Abingdon) and rh IFN-γ obtained from Peprotech. Granulocyte macrophage colony-stimulating factor (GM-CSF) was obtained from Abcys (Reuil Malmaison), and LPS and MPA, from Sigma-Aldrich.
Generation of DCs
The blood of healthy volunteers was obtained by cytapheresis after signed informed consent. Human peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll-Hypaque density gradient centrifugation (Lymphoprep). Monocytes were prepared either by selective adhesion to plastic or purified by positive selection using anti-CD14–conjugated magnetic microbeads (Milteny Biotec). Immature DCs were generated by culturing monocytes for 5 days in RPMI 1640 containing 10% FCS, 50 IU/mL penicillin, 50 IU/mL streptomycin, 25 ng/mL IL-4, and 1000 IU/mL GM-CSF at 37°C in a humidified 5% CO2 atmosphere as previously described.27 Day 5 DCs were stimulated for 2 days with either LPS alone (50 ng/mL) or LPS with 100μM MPA (MPA-DCs). On day 7, DCs were washed extensively with RPMI/10% FCS before further culture.
Isolation of CD8+ T-cell subsets
CD8+ T cells were obtained from human PBMCs by positive selection using magnetic beads coated with an anti-CD8 antibody (Dynal) according to the manufacturer's instructions. Briefly, peripheral blood lymphocytes were resuspended in 1 mL phosphate-buffered saline (PBS) with 0.1% FCS and 2mM EDTA (ethylenediaminetetraacetic acid) for 25 minutes at 4°C, and Dynabeads were added. The cells were detached by incubating with Detachabeads for 45 minutes at room temperature. CD8+ CD45RA+ T cells were then purified from pre-purified CD8+ T cells by positive selection using magnetic beads coupled to an anti-CD45RA antibody (Dynal Biotech). The negative fraction (CD45RA−) was used as memory cells (staining with anti-CD45RO showed that more than 95% were positive for this marker confirming the memory phenotype, data not shown).
Proliferation assay
In the allogeneic mixed lymphocyte reaction (MLR) assay, mature DCs (mDCs) or MPA-DCs were distributed in 96-well plates at 3 × 104 cells in 100 μL per well, and allogeneic CD8+ T cells, CD8+ RO+ or CD8+ RA+ (105 cells/100 μL), were added to each well (when specified, IL-2 was added to the culture at 100 IU/mL; R&D Systems). T-cell proliferation was measured using 1 μCi [3H]-thymidine (Amersham Pharmacia, Biotech) during the last 18 hours of the 6-day culture. The levels of [3H]-thymidine incorporation into the cellular DNA were determined by liquid scintillation counting (Tri-Carb 2550 TR/LL, Packard). In some experiments, we carried out a second allogeneic MLR: allogeneic CD8+ T cells were first cocultured with mDCs or MPA-DCs for 6 days. At the end of coculture, cells were harvested, washed, and cocultured in 96-well plates with mDCs from the same donor or from a third party donor at a DC:T ratio of 1:3 (when specified, IL-2 was added to the wells at 100 IU/mL). CD8+ T-cell proliferation was then assessed as described in the paragraph.
Cytokine production assay
Determination of cytokine concentrations in culture supernatants was performed by enzyme-linked immunosorbent assay (ELISA) according to manufacturer's protocols. Reagents for IL-2, IL-4, IL-5, IL-10, IL-12, tumor necrosis factor-α (TNF-α), transforming growth factor-β (TGF-β), and IFN-γ measurement were obtained from eBiosciences. To activate latent TGF-β, the samples were acidified (1N HCl) and then neutralized (1N NaOH). Samples were assayed in triplicate and were quantified by comparison with standard curves obtained with purified recombinant cytokines. Optical densities were measured in an ELISA platereader at 450-nm wavelength. The results are presented as the means of triplicate wells.
Antibodies and flow cytometry
A total of 105 CD8+ cells (cocultured with mDCs or MPA-DCs for 6 days) were incubated with fluorescence-labeled conjugated mouse anti–human antibodies (mAbs) for 30 minutes at 4°C. The following mAbs were used for cytometric analysis: CD8-APC Cy7 (clone SK1, immunoglobulin [Ig]G1), CD25-APC (clone M-A251, IgG1), CD107a-PE (clone H4A3, IgG1a), CD134-PE (clone ACT35, IgG1), CD154-PE (clone TRAP1, IgG1), and CD39-APC (clone TU66, IgG2b) from BD Pharmingen; and CD28-PE (clone CD28.2, IgG1), CD69-PE (clone TP1.55.3, IgG2b), and CD103-FITC (clone 2G5, IgG2a) from Beckman Coulter. For intracellular cytokine staining, cells were incubated with Golgi Stop solution (Becton Dickinson) for the last 5 hours of culture. Cells were then harvested, surface-stained, and permeabilized with “fix and perm” solution from Caltag Laboratories. IFN-γ–APC (clone B27, IgG1), Perforin-phycoerythrin (PE; clone δG9, IgG2b), granzyme A–PE (clone CB9, IgG1), and granzyme B–fluorescein isothiocyanate (FITC; clone GB11, IgG1) anti–human antibodies were purchased from BD Pharmingen and CD152-PE (clone BNI3, IgG2a) with isotype control from Beckman Coulter. The following mouse anti–human mAbs were used in some experiments to stain DCs after 48 hours of maturation: CD209-PE (DC SIGN; clone AZND1, IgG1), CD80-PE (clone MAB104, IgG1), CD40-PE (clone mAb89, IgG1), CD54-PE (clone 84H10, IgG1), and CD58-PE (clone AICD58, IgG2a) from Beckman Coulter; and CD25-APC (clone M-A251, IgG1), CD83-FITC (clone HB15e, IgG1), CD86-PeCy5 (clone 2331, IgG1κ), and CD70-FITC (clone Ki-24, IgG3κ) from Becton Dickinson.
Data were analyzed for geometric mean fluorescence intensity (MFI) and the percentage of marker-positive cells (at least 30 000 cells per sample were analyzed).
Cytotoxicity assay by flow cytometry CFSE/7-AAD
PBMC target cells (106) were labeled with 2 μL of carboxyfluorescein succininyidyl ester (CFSE) (Sigma-Aldrich; 5μM stock concentration) for 10 minutes at 37°C in 1 mL of PBS. After quenching the labeling reaction by addition of FCS, cells were washed extensively and immediately used in the cytotoxicity assay. Effector cells (E) were seeded with a constant number of CFSE-labeled target cells (T) at different E:T ratios (4:1, 2:1, 1:1, 1:2, 1:4, and 1:10). In parallel, target cells were incubated alone to measure basal lysis. Cells were incubated in 96-well microplates in a total volume of 200 μL complete medium for 7 hours in a 5% CO2 atmosphere at 37°C. Cell mixtures were then washed in PBS and incubated in PBS containing 1 μg of 7-amino actinomycin D (7-AAD; Sigma-Aldrich). CFSE fluorescence and 7-AAD emission were detected in the FL-1 and FL-3 channels, respectively. For each E:T ratio, 30 000 target cells were acquired. Analysis was performed with BD FACS Diva software Version 6.1.2. The percentage of specific lysis (PSL) was determined by the formula: PSL = 100 × (% sample lysis − % basal lysis)] / (100 − % basal lysis).
Statistical analysis
Data for each experiment were expressed as mean values (± SD) and the results were analyzed for statistical significance using the paired nonparametric Wilcoxon test. The level of significance was set at P < .05.
Results
MPA did not affect maturation marker expression induced by LPS on DCs but inhibited cytokine synthesis
Full activation of CD8+ T cells required a cognate interaction between the T-cell receptor and the major histocompatibility complex–peptide complex and costimulatory signals. We first analyzed the effect of MPA on the human DC maturation phenotype after stimulation with LPS. We found that MPA-DCs and LPS-DCs expressed similar levels of CD83, CD25, CD80, CD86, CD40, CD54, and CD58 (Figure 1A). Recent studies in rodents have suggested that the interaction between CD70 on DC and CD70L (CD27) on T cells might be an important costimulatory signal for CD8 activation.28 We therefore analyzed the role of this interaction in our experimental model. We found that neither mDCs nor MPA-DCs expressed this molecule as reported by Arimoto-Miyamoto et al29 (Figure 1A right histogram).
MPA did not affect maturation marker expression induced by LPS on DCs but inhibited cytokine synthesis. (A) MPA did not affect maturation marker expression induced by LPS on DCs. Surface marker expression was analyzed on human DC after maturation with LPS (50 ng/mL) in the presence or absence of MPA (MPA-DCs or mDCs, respectively). Black histograms and gray histograms represent the expression of the cell-surface markers indicated and isotype control mAbs, respectively. (B) MPA inhibited cytokine synthesis by DCs. The levels of IL-12, IFN-γ, and TNF-α were detected by ELISA in supernatants of mDC and MPA-DC cultures. The results are expressed in arbitrary units where the value 100 was given to mDCs. Statistical analyses were performed using the Wilcoxon test for paired nonparametric data. Significance is indicated by P value. (C) Intracytoplasmic IFN-γ in MPA-DCs and in mDCs. Cells were double stained for DC-SIGN and intracytoplasmic IFN-γ. The percentage of double positive cells is indicated on the figure. The results are representative of 1 of 5 experiments.
MPA did not affect maturation marker expression induced by LPS on DCs but inhibited cytokine synthesis. (A) MPA did not affect maturation marker expression induced by LPS on DCs. Surface marker expression was analyzed on human DC after maturation with LPS (50 ng/mL) in the presence or absence of MPA (MPA-DCs or mDCs, respectively). Black histograms and gray histograms represent the expression of the cell-surface markers indicated and isotype control mAbs, respectively. (B) MPA inhibited cytokine synthesis by DCs. The levels of IL-12, IFN-γ, and TNF-α were detected by ELISA in supernatants of mDC and MPA-DC cultures. The results are expressed in arbitrary units where the value 100 was given to mDCs. Statistical analyses were performed using the Wilcoxon test for paired nonparametric data. Significance is indicated by P value. (C) Intracytoplasmic IFN-γ in MPA-DCs and in mDCs. Cells were double stained for DC-SIGN and intracytoplasmic IFN-γ. The percentage of double positive cells is indicated on the figure. The results are representative of 1 of 5 experiments.
In addition to phenotype maturation, we also investigated the cytokine secretion profile of human DCs stimulated with LPS in the presence or absence of MPA. We first showed that immature DCs did not produce any detectable level of IL-12p70, IFN-γ, or TNF-α whereas stimulation with LPS resulted in significantly increased production of these cytokines (data not shown and Figure 1B). Interestingly, we found that adding MPA to LPS significantly inhibited these cytokine secretions (Figure 1B). In addition, double staining for DC-specific intercellular adhesion molecule-3-grabbing nonintegrin (DC-SIGN) and intracytoplasmic IFN-γ showed that MPA strongly decreased the number of double positive cells (33.4% for MPA-DC vs 71.2% for mDCs), confirming that MPA affected the production of IFN-γ in DCs (Figure 1C).
LPS-treated DCs induced CD4-independent CD8+ T-cell proliferation whereas pretreatment of DCs with MPA abolished this capacity
Purified CD8+ T lymphocytes were mixed with allogeneic DCs (pretreated with either LPS or both MPA and LPS). T-cell proliferation was assessed on day 6. As shown in Figure 2A, we first demonstrated that mDCs supported the proliferation of purified allogeneic CD8+ cells independently of CD4 cells, whereas MPA-DCs did not (14 375 counts per minute [cpm] ± 790 vs 3086 cpm ± 245). It has previously been suggested that contaminating components of adherent PBMCs (especially natural killer cells) might have a role in allogeneic CD8+ T-cell activation in the absence of CD4+ helper T cells.30 To obtain highly purified DCs, we positively selected CD14 monocytes (> 97% purity as assessed by DC-SIGN expression). As shown in Figure 2A, highly purified mDCs were as effective as DCs derived from adherent monocytes in inducing CD8+ T-cell proliferation (dark hatched bar) whereas highly purified MPA-DCs did not (Figure 2A light hatched bar). This suggested that the inhibition of CD8 proliferation by MPA-DCs was truly due to its impact on DCs.
LPS-treated DCs induced CD4-independent CD8+ T-cell proliferation whereas pretreatment of DCs with MPA abolished this capacity. MPA-DCs and mDCs designate DCs matured with LPS (50 ng/mL) for 2 days in the presence or absence of MPA (100μM). (A) MPA-DCs induced CD8+ T-cell unresponsiveness. mDCs and MPA-DCs obtained after monocyte adhesion (filled bars) or after CD14-positive selection (hatched bars) were cocultured for 6 days with allogeneic purified CD8+ T lymphocytes at a DC:T ratio of 1:3. T-cell proliferation was measured by incorporation of 1 μCi [3H]-thymidine added for the last 18 hours of culture. (B) Naive and memory CD8+ T cells were unresponsive to MPA-DCs. CD8+ CD45RA+ (filled bars) and CD8+ CD45RO+ (hatched bars) T cells were isolated from purified CD8+ T lymphocytes and then cocultured with allogeneic MPA-DCs or mDCs for 6 days at a DC:T ratio of 1:3. T-cell proliferation was measured by [3H]-thymidine incorporation. (C) IL-2 prevented CD8+ T-cell hyporesponsiveness. Purified CD8+ T lymphocytes were cocultured with allogeneic MPA-DCs or mDCs in the presence or absence of exogenous IL-2 (100 IU/mL). T-cell proliferation was measured as described above. (D) MPA-DCs induced donor-specific anergy. First coculture of allogeneic CD8+ T cells with MPA-DCs was performed, followed by a second stimulation in the presence (black bar) or absence (gray bar) of exogenous IL-2 (100 IU/mL) with mDCs from the donor used in the first coculture. Donor-specific unresponsiveness was assessed by further stimulation using mDCs from the first DC-donor (mDCa; gray bar) or a third party (mDCb; hatched bars). T-cell proliferation was measured on day 5 by incorporation of [3H]-thymidine. Results are expressed as mean cpm ± SD obtained from triplicate wells and are representative of 1 of 7 experiments. *P < .05.
LPS-treated DCs induced CD4-independent CD8+ T-cell proliferation whereas pretreatment of DCs with MPA abolished this capacity. MPA-DCs and mDCs designate DCs matured with LPS (50 ng/mL) for 2 days in the presence or absence of MPA (100μM). (A) MPA-DCs induced CD8+ T-cell unresponsiveness. mDCs and MPA-DCs obtained after monocyte adhesion (filled bars) or after CD14-positive selection (hatched bars) were cocultured for 6 days with allogeneic purified CD8+ T lymphocytes at a DC:T ratio of 1:3. T-cell proliferation was measured by incorporation of 1 μCi [3H]-thymidine added for the last 18 hours of culture. (B) Naive and memory CD8+ T cells were unresponsive to MPA-DCs. CD8+ CD45RA+ (filled bars) and CD8+ CD45RO+ (hatched bars) T cells were isolated from purified CD8+ T lymphocytes and then cocultured with allogeneic MPA-DCs or mDCs for 6 days at a DC:T ratio of 1:3. T-cell proliferation was measured by [3H]-thymidine incorporation. (C) IL-2 prevented CD8+ T-cell hyporesponsiveness. Purified CD8+ T lymphocytes were cocultured with allogeneic MPA-DCs or mDCs in the presence or absence of exogenous IL-2 (100 IU/mL). T-cell proliferation was measured as described above. (D) MPA-DCs induced donor-specific anergy. First coculture of allogeneic CD8+ T cells with MPA-DCs was performed, followed by a second stimulation in the presence (black bar) or absence (gray bar) of exogenous IL-2 (100 IU/mL) with mDCs from the donor used in the first coculture. Donor-specific unresponsiveness was assessed by further stimulation using mDCs from the first DC-donor (mDCa; gray bar) or a third party (mDCb; hatched bars). T-cell proliferation was measured on day 5 by incorporation of [3H]-thymidine. Results are expressed as mean cpm ± SD obtained from triplicate wells and are representative of 1 of 7 experiments. *P < .05.
Because the requirements for activation of naive and memory CD8+ cells are quite different, we decided to assess whether MPA-DCs could affect both memory and naive CD8+ T cells. CD45RA+-depleted T cells (memory) and positively selected CD45RA+ T cells (naive) were used as responders in MLR with allogeneic MPA-DCs. As shown in Figure 2B, mDCs induced proliferation of both naive CD8+ (CD45RA+) and memory CD8+ (CD45RO+) T cells at similar levels while MPA-DCs were unable to induce responsiveness in either of the 2 subsets of CD8.
As IL-2 is important in the activation and in the proliferation of T cells, we hypothesized that cells activated by MPA-DCs did not produce enough IL-2 to sustain their own proliferation. As shown in Figure 2C, adding exogenous IL-2 during MLR with MPA-DCs resulted in a strong response by allogeneic CD8+ T cells. This suggested that poor IL-2 availability during coculture with MPA-DCs might be involved in the hyporesponsiveness.
As reported in seminal studies by Schwartz and Jenkins,31 anergy is a state in which T cells are incapable of producing their own IL-2 on restimulation with antigen, and this is reversed by stimulating the cells in the presence of exogenous IL-2. We therefore added exogenous IL-2 during the second MLR and found that T cells became fully reactive to the first donor's mature DCs (Figure 2D black bar). Taken together, this suggested that at least some of the first donor-reactive CD8+ T cells became anergic.
Finally, we tested whether CD8+ T-cell anergy induced by MPA-DCs was restricted to T cells specifically reactive to alloantigens expressed by DC. As shown in Figure 2D, CD8+ T cells first activated by first party allogeneic MPA-DCs proliferated abundantly in response to mDCs from a third party (hatched bars), whereas they remained nonresponsive to restimulation with mDCs from the first donor (filled bars), demonstrating the antigen-specificity of the hyporesponsiveness. Taken together, these findings strongly suggest that MPA-DCs induce alloantigen-specific anergy of CD8+ T cells.
Phenotype characteristics of CD8+ T cells after 6 days of coculture with mDCs or MPA-DCs
We analyzed the phenotype characteristics of CD8+ T cells after 6 days' coculture with mDCs or MPA-DCs. Markers such as CD25, CD28, CD69, CD152 (CTLA-4), CD154 (CD40L), and CD134 (Ox40) associated with T-cell activation were tested. In addition, we also analyzed CD103 (β7 integrin), which has previously been reported as expressed by a subpopulation of CD8+ T cells with regulatory ability.32 Moreover, we assessed the expression of CD39 demonstrated to define distinct cytotoxic subsets within alloactivated human CD8+ T cells.33 Not surprisingly, we found that MPA-DCs and mDCs induced different levels of expression of activation markers, such as CD25 and CD69, on allogeneic CD8+ T cells (19.4% ± 9% vs 36.7% ± 7%, P = .008, and 16.9% ± 9% vs 27.1% ± 11%, P = .018, respectively, n = 6; Figure 3). These results confirmed that MPA strongly reduced the ability of mDCs to activate purified CD8 cells. We also examined the influence of MPA-DCs on the expression of several costimulatory molecules of CD8+ T cells. As shown in Figure 3, MPA-DCs induced lower percentages of positive cells for CD134 (11.7% ± 5% vs 23.8% ± 7%, P = .016) and for intracellular CD152 (9.3% ± 5% vs 39.2% ± 9%, P = .016). Interestingly, the percentage of CD28high cells was reduced in response to MPA-DCs (13.3% ± 9% vs 40.9% ± 9%, P = .008). The expressions of CD154 and CD103 were slightly decreased in CD8+ MPA-DCs compared with CD8+ mDCs while the expression of CD62L and TNF-related activation-induced cytokine, or receptor activator for nuclear factor κB ligand were similar in the 2 populations (data not shown). A similar proportion of the cells expressed Foxp3 in the 2 populations (6%, data not shown). Finally, CD8+ MPA-DCs exhibited much lower expression of CD39 compared with CD8+ mDCs on day 6 of culture (Figure 3).
Phenotype characteristics of CD8+ after 6 days of coculture with either mDCs or MPA-DCs. Purified CD8+ T lymphocytes were cocultured for 6 days with allogeneic MPA-DCs or mDCs. At the end of coculture, cells were harvested and expression of CD25, CD69, CD28, CD152, CD154, CD103, CD134, and CD39 was assessed by FACS analysis for each condition: CD8+ mDCs (left column of each histogram) and CD8+ MPA-DCs (right column). Each point represents 1 experiment and the black bar represents the mean of 6 independent experiments. Wilcoxon test was used to calculate statistical differences between groups. The P value is indicated on each histogram of the figure. *P < .05.
Phenotype characteristics of CD8+ after 6 days of coculture with either mDCs or MPA-DCs. Purified CD8+ T lymphocytes were cocultured for 6 days with allogeneic MPA-DCs or mDCs. At the end of coculture, cells were harvested and expression of CD25, CD69, CD28, CD152, CD154, CD103, CD134, and CD39 was assessed by FACS analysis for each condition: CD8+ mDCs (left column of each histogram) and CD8+ MPA-DCs (right column). Each point represents 1 experiment and the black bar represents the mean of 6 independent experiments. Wilcoxon test was used to calculate statistical differences between groups. The P value is indicated on each histogram of the figure. *P < .05.
CD8+ T cells stimulated by MPA-DCs secreted high levels of IL-4, IL-5, IL-10, and TGF-β and low levels of IL-2, TNF-α, and IFN-γ
Having demonstrated that MPA-DCs were poor stimulators of allogeneic CD8+ T cells, we next investigated how MPA-DCs could alter the cytokine synthesis pattern of CD8+ T cells. CD8+ T cells were therefore activated by mDCs or MPA-DCs for 6 days and production of different cytokines was measured by ELISA.
The results presented in Figure 4 show that the levels of IL-2, TNF-α (Figure 4A), and IFN-γ (Figure 4B) were lower in the supernatants of CD8+ activated with MPA-DCs, whereas TGF-β, IL-4, IL-5, and IL-10 levels were significantly higher (P < .05; Figure 4). Because IFN-γ was produced by both DC and CD8+ T cells, we assessed intracytoplasmic production in CD8+ T cells. We found that CD8+ T cells activated with MPA-DCs produced less IFN-γ than those activated with mDCs (Figure 4B). We also analyzed the secretion of IL-17 that was barely detectable, and the levels were not significantly different between the 2 conditions (data not shown). Finally, we could not detect any significant level of IL-6 in any condition (data not shown). Furthermore, these results indicated that MPA-DCs induced CD8+ T cells characterized by low level of IFN-γ secretion and high production of IL-4, suggesting a Th2-type cytokine profile as reported by Vukmanovic-Stejic et al.34
CD8+ T cells cocultured with MPA-DCs synthesized high levels of TGF-β, IL-4, and IL-5, and low levels of TNF-α, IL-2, and IFN-γ. CD8+ T cells were cocultured with either MPA-DCs or mDCs for 6 days. (A) Levels of TGF-β, IL-2, IL-4, IL-5, IL-10, and TNF-α in supernatants were analyzed by ELISA. The samples were acidified and then neutralized to activate latent TGF-β1. The results are expressed in picograms per milliliter as mean ± SD of 9 experiments. (B) IFN-γ synthesis was also assessed by ELISA and intracytoplasmic staining. Results are expressed in percentage of positive cells. Wilcoxon test was used to calculate statistical differences between groups. The P value is indicated on each histogram of the figure. *P < .05
CD8+ T cells cocultured with MPA-DCs synthesized high levels of TGF-β, IL-4, and IL-5, and low levels of TNF-α, IL-2, and IFN-γ. CD8+ T cells were cocultured with either MPA-DCs or mDCs for 6 days. (A) Levels of TGF-β, IL-2, IL-4, IL-5, IL-10, and TNF-α in supernatants were analyzed by ELISA. The samples were acidified and then neutralized to activate latent TGF-β1. The results are expressed in picograms per milliliter as mean ± SD of 9 experiments. (B) IFN-γ synthesis was also assessed by ELISA and intracytoplasmic staining. Results are expressed in percentage of positive cells. Wilcoxon test was used to calculate statistical differences between groups. The P value is indicated on each histogram of the figure. *P < .05
mDCs induced cytotoxic CD8+ T cells independently of CD4+ T cells, whereas MPA-DCs induced lower cytotoxic activity in allogeneic CD8+ T cells
Finally, we investigated whether CD8+ mDC and CD8+ MPA-DC populations had different cytotoxic activity against targets using a test based on double staining with CFSE/7-AAD, as previously described by Lecoeur et al.35 As shown in Figure 5A, the PSL induced by CD8+ mDCs was 50.2% compared withonly 28.8% with CD8+ MPA-DCs at a E:T ratio of 1:1. Figure 5B demonstrates that PBMC lysis correlated well with the E:T ratio and that PSL induced by CD8+[MPA-DC]s was consistently lower than with CD8+mDCs whatever the E:T ratio used (Figure 5B black symbols). We also performed experiments with PBMC target cells from a third party and found that the 2 subsets were not able to lyse PBMCs from an unrelated donor, thus confirming that the cytotoxicity of CD8+ was antigen-specific (Figure 5B empty symbols).
mDCs induced cytotoxic CD8+ T cells independently of CD4+ T cells whereas MPA-DCs induced lower cytotoxic activity in allogeneic CD8+ T cells. Effector cells (E) were incubated at different E:T ratios in the presence of CFSE-labeled PBMC target cells (T) for 7 hours, as described in the cytotoxicity assay section. (A) Target cell lysis was quantified by analyzing 7-AAD staining in CFSE-positive cells. CFSE and 7-AAD histograms from a representative experiment are shown and the percentages of 7-AAD-positive cells are indicated in each histogram. Basal lysis was measured on target cells incubated in the absence of effectors (target alone). (B) To assess the specificity of CD8 cytotoxic function, CD8+ mDCs (triangles) or CD8+ MPA-DCs (squares) were mixed with PBMCs from the first DC donor (black symbols) or from an unrelated donor (empty symbols) for 7 hours. PBMC lysis was assessed by CFSE/7-AAD staining and the percentage of specific target lysis was calculated as described in “Methods.” Results of 1 of 8 independent experiments are presented for 6 different E:T ratios. (C) Percentage of specific lysis induced by naive (CD45RA+) or memory (CD45RO+) CD8+ T cells cocultured with either mDCs or MPA-DCs was determined using PBMCs from the first DCs donor at a E:T ratio of 1:1. Results of 1 of 3 independent experiments are presented. (D) For intracellular staining, cells were incubated with Golgi Stop solution for the last 5 hours of culture. At the end of coculture, cells were harvested and expression of the CD107a PE, granzyme A PE, granzyme B FITC, and Perforin PE markers was assessed by FACS analysis. Each point represents 1 experiment and the black bar represents the mean of 6 independent experiments. The P value is indicated on each histogram of the figure. *P < .05
mDCs induced cytotoxic CD8+ T cells independently of CD4+ T cells whereas MPA-DCs induced lower cytotoxic activity in allogeneic CD8+ T cells. Effector cells (E) were incubated at different E:T ratios in the presence of CFSE-labeled PBMC target cells (T) for 7 hours, as described in the cytotoxicity assay section. (A) Target cell lysis was quantified by analyzing 7-AAD staining in CFSE-positive cells. CFSE and 7-AAD histograms from a representative experiment are shown and the percentages of 7-AAD-positive cells are indicated in each histogram. Basal lysis was measured on target cells incubated in the absence of effectors (target alone). (B) To assess the specificity of CD8 cytotoxic function, CD8+ mDCs (triangles) or CD8+ MPA-DCs (squares) were mixed with PBMCs from the first DC donor (black symbols) or from an unrelated donor (empty symbols) for 7 hours. PBMC lysis was assessed by CFSE/7-AAD staining and the percentage of specific target lysis was calculated as described in “Methods.” Results of 1 of 8 independent experiments are presented for 6 different E:T ratios. (C) Percentage of specific lysis induced by naive (CD45RA+) or memory (CD45RO+) CD8+ T cells cocultured with either mDCs or MPA-DCs was determined using PBMCs from the first DCs donor at a E:T ratio of 1:1. Results of 1 of 3 independent experiments are presented. (D) For intracellular staining, cells were incubated with Golgi Stop solution for the last 5 hours of culture. At the end of coculture, cells were harvested and expression of the CD107a PE, granzyme A PE, granzyme B FITC, and Perforin PE markers was assessed by FACS analysis. Each point represents 1 experiment and the black bar represents the mean of 6 independent experiments. The P value is indicated on each histogram of the figure. *P < .05
Because CD4-independent memory CD8+ T cells are powerful mediators of allograft rejection,2,6 we checked whether MPA-DCs could reduce the cytotoxic activity in this subset. As shown in Figure 5C, both naive and memory CD8+ T cells exhibited a reduced cytotoxic function in response to MPA-DCs.
To provide a more complete assessment of the functionality of CD8+ T cells, we also analyzed markers associated with cytotoxic granules such as granzymes, perforin36 and CD107 expressed on the surface of effector cytotoxic cells after degranulation.37,38 Figure 5D shows that the percentage of positive cells expressing perforin was significantly decreased in CD8+ T cells stimulated by MPA-DCs (9.83% ± 2.1% vs 17.7% ± 2.6%; P = .016). Similarly, levels of expression of granzymes A and B were decreased in CD8+ MPA-DCs compared with CD8+ mDCs (7.23% ± 1% vs 16.3% ± 2.8%, and 10.15% ± 1.8% vs 20.23% ± 2.4%, respectively; P = .016). Finally, the percentage of CD107-positive cells was also lower in CD8+ MPA-DCs (15.05% ± 9% compared with 29.3% ± 10% in CD8+ T cells activated by mDCs, P = .031; Figure 5D). Taken together, these findings demonstrated that MPA diminished the ability of DCs to induce cytotoxic functions in CD8+ cells.
CD8+ T cells activated by MPA-DCs had no regulatory activity
Given that the CD8+ MPA-DCs were able to produce regulatory cytokines such as IL-10 and TGF-β, and in view of their low proliferative capacity, we examined their ability to regulate allogeneic CD4+ T-cell proliferation. CD8+ cells preactivated by MPA-DCs were mixed with responding autologous CD4+ T cells and mature DCs obtained from the same donor as MPA-DCs (the donor used to stimulate CD8+ T cells). As shown in Figure 6A, addition of CD8+ MPA-DCs did not affect the proliferation of responding CD4+ T cells activated with mDCs over 5 days whatever the ratio used. We also checked that CD8+MPA-DCs remained unresponsive after restimulation with mature DC (data not shown). Moreover, same results were obtained with different responder populations such as PBMCs, total T cells, or also CD8+ T cells (data not shown).
CD8+ T cells cocultured with MPA-DCs had no regulatory capacity in vitro. Purified CD8+ T cells were cocultured for 6 days with allogeneic MPA-DCs. (A) CD8+ MPA-DCs did not suppress the proliferation of responder CD4+ T cells. At day 6, CD8+ MPA-DCs were tested for their suppressive activity in MLR. Responder CD4+ T cells (designated naive CD4+) were cocultured with allogeneic mature DCs for 5 days at a DC:T ratio of 1:3 and were used as positive control. CD8+ MPA-DCs were added to the MLR at different ratios. T-cell proliferation was assessed by 3H-thymidine incorporation during the last 18 hours of the 5 day MLR (mean cpm ± SD of triplicate wells). Data are representative of 1 of 6 experiments. (B) CD8+ MPA-DCs did not inhibit the secretion of IFN-γ in MLR. At the end of the second MLR, the level of IFN-γ (picograms per milliliter) was detected by ELISA in supernatants. Each point represents 1 experiment and the black bars represent the mean of 5 independent experiments. Wilcoxon test was used to calculate statistical differences between groups. *P < .05 and NS = Not Significant. (C) CD8+ MPA-DCs did not inhibit intracellular IFN-γ expression of CD4+ T cells. Responder CD4+ T cells were cocultured with allogeneic mDC in the presence (right dot plot) or absence (left dot plot) of CD8+ MPA-DCs at a ratio of 1:1 for 5 days. At the end of coculture, cells were harvested and CD4 FITC/IFN-γ APC double staining was assessed by FACS analysis for the 2 conditions. The percentage of IFN-γ-positive cells among CD4-positive cells is shown in panel C. Results are representative of 1 of 4 independent experiments.
CD8+ T cells cocultured with MPA-DCs had no regulatory capacity in vitro. Purified CD8+ T cells were cocultured for 6 days with allogeneic MPA-DCs. (A) CD8+ MPA-DCs did not suppress the proliferation of responder CD4+ T cells. At day 6, CD8+ MPA-DCs were tested for their suppressive activity in MLR. Responder CD4+ T cells (designated naive CD4+) were cocultured with allogeneic mature DCs for 5 days at a DC:T ratio of 1:3 and were used as positive control. CD8+ MPA-DCs were added to the MLR at different ratios. T-cell proliferation was assessed by 3H-thymidine incorporation during the last 18 hours of the 5 day MLR (mean cpm ± SD of triplicate wells). Data are representative of 1 of 6 experiments. (B) CD8+ MPA-DCs did not inhibit the secretion of IFN-γ in MLR. At the end of the second MLR, the level of IFN-γ (picograms per milliliter) was detected by ELISA in supernatants. Each point represents 1 experiment and the black bars represent the mean of 5 independent experiments. Wilcoxon test was used to calculate statistical differences between groups. *P < .05 and NS = Not Significant. (C) CD8+ MPA-DCs did not inhibit intracellular IFN-γ expression of CD4+ T cells. Responder CD4+ T cells were cocultured with allogeneic mDC in the presence (right dot plot) or absence (left dot plot) of CD8+ MPA-DCs at a ratio of 1:1 for 5 days. At the end of coculture, cells were harvested and CD4 FITC/IFN-γ APC double staining was assessed by FACS analysis for the 2 conditions. The percentage of IFN-γ-positive cells among CD4-positive cells is shown in panel C. Results are representative of 1 of 4 independent experiments.
We also assessed the secretion of IFN-γ in these MLRs. As shown in Figure 6B, high IFN-γ concentrations were found in the supernatants of CD4+ T cells activated with mDCs, and addition of CD8+ MPA-DCs had no effect on IFN-γ secretion (18 467 ± 4803 pg/mL vs 15 468 ± 2852 pg/mL; P > .05, n = 5). We checked that CD8+ T cells first activated with MPA-DCs secreted very low levels of this cytokine after restimulation with mature DCs (1501 ± 686‖pg/mL), suggesting that the concentration observed in MLRs with CD4+ cells mainly originated from the CD4+ T lymphocytes. However, to assess definitively that CD8+ MPA-DCs did not affect the level of IFN-γ production by CD4+ cells, we carried out double staining for CD4 and intracytoplasmic IFN-γ in MLRs in the presence or absence of CD8+ MPA-DCs and found similar fractions of CD4+ T cells expressing this cytokine in both conditions (Figure 6C). Taken together, these results showed that MPA-DCs could not generate regulatory CD8+ T cells.
IFN-γ synthesis was crucial for the ability of DCs to activate CD8+ T cells
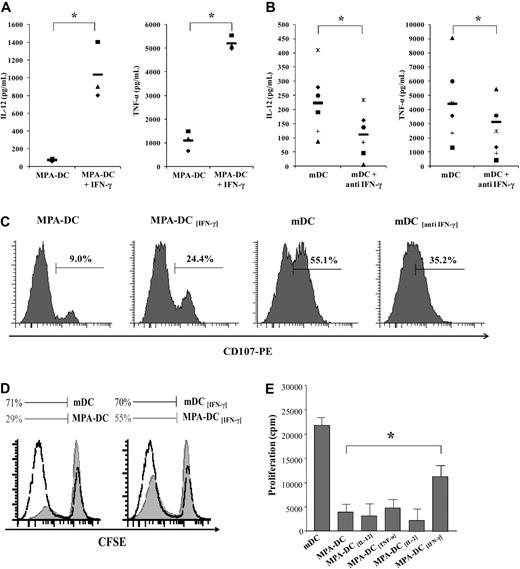
To explore whether deficit in IFN-γ synthesis by MPA-DCs was involved in their inability to activate CD8+ T cells, DC maturation was induced in the presence of exogenous IFN-γ along with LPS and MPA. As shown in Figure 7, recombinant human IFN-γ totally restored the production of TNF-α and IL-12 in MPA-DCs (Figure 7A) while addition of a blocking anti–IFN-γ antibody with LPS (designated mDC[anti IFN-γ]) strongly decreased IL-12 and TNF-α secretions (Figure 7B). We then analyzed the early expression of CD107 (a cytotoxic associated marker) in allogeneic CD8+ T cells. At 24 hours of coculture, we found that pre-treatment of MPA-DCs with IFN-γ increased CD107 ex-pression compared with stimulation with MPA-DCs (24.4% vs 9%; Figure 7C) whereas anti–IFN-γ–pretreated DCs decreased CD107 expression compared with stimulation with untreated mDCs (35.2% vs 55.1%).
IFN-γ synthesis was crucial for the ability of DCs to activate CD8+ T cells. (A-B) IFN-γ regulated IL-12 and TNF-α synthesis in DCs. When mentioned, recombinant human IFN-γ (10 ng/mL) or blocking anti–IFN-γ antibody (10 μg/mL) were added to immature DCs 1 hour before LPS in the presence or absence of MPA for 2 days. Levels of IL-12p70 and TNF-α in supernatants were analyzed by ELISA. n = 3 (A) and n = 6 (B). (C) IFN-γ increased the ability of MPA-DCs to induce cytotoxic CD8+ T cells whereas anti–IFN-γ decreased this ability in mDCs. When mentioned, recombinant human IFN-γ (10 ng/mL) or blocking anti–IFN-γ antibody (10 μg/mL) were added to immature DCs 1 hour before LPS in the presence or absence of MPA for 2 days. After maturation, DCs were mixed with allogeneic purified CD8+ T cells for 24 hours and expression of CD107 was analyzed by FACS. One representative experiment of 3 is presented. (D-E) IFN-γ partially restored the ability of MPA-DCs to induce CD8+ T-cell proliferation whereas IL-12, TNF-α, and IL-2 had no effect. Immature DCs were incubated with rhIL-12 or rhTNF-α or rhIL-2 or rhIFN-γ and matured with LPS in the presence of MPA for 2 days. After maturation, DCs were mixed with allogeneic purified CD8+ T cells for 5 days. T-cell proliferation was assessed by CFSE dilution (D) or 3H-thymidine incorporation (E) during the last 18 hours of MLR (mean cpm ± SD of triplicate wells). Results are representative of 3 donors. *P < .05.
IFN-γ synthesis was crucial for the ability of DCs to activate CD8+ T cells. (A-B) IFN-γ regulated IL-12 and TNF-α synthesis in DCs. When mentioned, recombinant human IFN-γ (10 ng/mL) or blocking anti–IFN-γ antibody (10 μg/mL) were added to immature DCs 1 hour before LPS in the presence or absence of MPA for 2 days. Levels of IL-12p70 and TNF-α in supernatants were analyzed by ELISA. n = 3 (A) and n = 6 (B). (C) IFN-γ increased the ability of MPA-DCs to induce cytotoxic CD8+ T cells whereas anti–IFN-γ decreased this ability in mDCs. When mentioned, recombinant human IFN-γ (10 ng/mL) or blocking anti–IFN-γ antibody (10 μg/mL) were added to immature DCs 1 hour before LPS in the presence or absence of MPA for 2 days. After maturation, DCs were mixed with allogeneic purified CD8+ T cells for 24 hours and expression of CD107 was analyzed by FACS. One representative experiment of 3 is presented. (D-E) IFN-γ partially restored the ability of MPA-DCs to induce CD8+ T-cell proliferation whereas IL-12, TNF-α, and IL-2 had no effect. Immature DCs were incubated with rhIL-12 or rhTNF-α or rhIL-2 or rhIFN-γ and matured with LPS in the presence of MPA for 2 days. After maturation, DCs were mixed with allogeneic purified CD8+ T cells for 5 days. T-cell proliferation was assessed by CFSE dilution (D) or 3H-thymidine incorporation (E) during the last 18 hours of MLR (mean cpm ± SD of triplicate wells). Results are representative of 3 donors. *P < .05.
We also found that pretreatment of MPA-DCs with IFN-γ increased their ability to induce CD8+cells proliferation as shown by either CFSE dilution (55% vs 29% of CD8+ T cells had proliferated; Figure 7D) or [3H] incorporation (8320 ± 510 cpm vs 2826 ± 650 cpm; P = .05, Figure 7E). Interestingly and in contrast with what happened with IFN-γ, the pretreatment of MPA-DCs with either rh IL-12 or TNF-α or IL-2 did not influence their ability to activate CD8+ T cells (Figure 7E).
Taken together, these findings indicate that in the absence of CD4+ T cells, IFN-γ synthesis by DCs is of crucial importance to induce CD8+cells activation via an autocrine loop and that the modulation of IFN-γ synthesis induced by MPA in DCs has a pivotal role.
Discussion
The activation of memory allogeneic-reactive CD8+ T cells appears to be a major obstacle to tolerance induction.2-4 The mechanisms involved in the activation of alloreactive CD8+ T cells are far from being elucidated, and improving understanding of these mechanisms might therefore be valuable for transplantation.
The requirement for helper T cells in priming cytotoxic T lymphocyte (CTL) responses has come under intense scrutiny and has led to disparate findings. In organ transplantation, many early studies emphasized the role of CD4-dependent CD8+ T cells,39 but the role of CD4-independent CD8+ T cells in allograft rejection has been reported in mouse models.19 However, in humans there remain doubts as to whether CD8+ T cells can generate cytotoxic CD8+ responses to an allograft in the absence of T-helper cells. Interestingly, our results showed that human monocyte-derived DCs activated with LPS were able to induce cytotoxic effector functions in allogeneic CD8+ T cells in the absence of help from CD4+ T cells. These results were consistent with previous reports23,24,28 but were in contrast with those of Kelleher et al.40 In fact, Kelleher's study showed that LPS activation of DCs was insufficient to generate CTLs from naive polyclonal CD8+ T cells, but they studied a peptide-specific response characterized by a low frequency of precursors. In our model, the high frequency of alloreactive CD8+ precursors via the direct pathway41 might be sufficient to provide their own help for activation into effector cells by DCs, as suggested by a study of Wang et al.42 Much of the variability in requirement for CD4 T cells in generating CD8+ T-cell responses might also lie in the type of APCs and in the way in which they are activated by exposure to different types of antigens or conditions of inflammation.43 Several studies have highlighted the critical role of the costimulatory molecule CD70 in the promotion of CD8+ cytotoxic T-cell activation.28 We therefore analyzed its expression but, in contrast with the situation in mice, we were unable to demonstrate any expression of CD70 on human mature DCs, suggesting that CD70 is probably not involved in the generation of cytotoxic CD8+ T cells by LPS-matured DCs, which is in accordance with a recently published paper.29
We also demonstrated in this study that MPA-DCs induced CD8+ T-cell anergy. While anergy in CD4+ T cells has been widely reported, it is less well characterized in CD8+ T cells. Anergy is defined as an active process leading to the inability of antigen-specific T cells to produce IL-2 and to expand clonally on rechallenge with fully competent APCs.44 Anergy occurs when T-cell receptors are ligated by antigen in the absence of effective costimulation signals (second signal). However, we found that, in contrast to what we have previously reported with TNF-α, MPA did not affect the up-regulation of costimulatory molecules such as CD40, CD80, and CD86 in the presence of LPS,27 suggesting that CD8+ T-cell anergy could be induced despite high expression of these costimulatory molecules. The inhibition of the synthesis of several DC-derived cytokines by MPA might thus have participated in the ability of DCs to induce anergy. These cytokines might have a role either by acting directly on T cells (by giving a survival or proliferation signal) or by licensing the DCs in their ability to activate CD8+ T cells. We explored the second hypothesis further, in particular the involvement of IL-12 and IFN-γ. After demonstrating that MPA inhibited the synthesis of IFN-γ in human DCs, we found that adding exogenous IFN-γ to MPA and LPS exclusively during the DC maturation phase restored synthesis of IL-12 and TNF-α in DCs and their ability to activate CD8+ T cells, whereas exogenous IL-12 had no effect. These results suggested that IFN-γ had a crucial role in licensing DCs to induce primary allogeneic CTL expansion independently of CD4+ T cells. Several studies in B6 mice recipients in which CD4+ help was inhibited by costimulation blockade showed that CD8+ T-cell-mediated rejection was dependent on IFN-γ signals delivered to recipient cells.45 However, the mechanisms by which IFN-γ promotes the development of anti–donor CD8+ T cells in the setting of costimulation blockade have not been elucidated to date. Our results raise the possibility that the DC might indeed be the target of IFN-γ.
We next questioned the origin of IFN-γ and we demonstrated by intracytoplasmic staining that DC produced IFN-γ after LPS stimulation in accordance with other studies.46,47 Synthesis of IFN-γ by DCs has not been extensively studied and its role has not been firmly established. These results suggest that DC-derived IFN-γ may be an autocrine starter signal that may activate DCs to a sufficient level to stimulate alloreactive CD8+ cells if present in a sufficient number. These activated T cells secrete cytokines that may in turn amplify the activation of neighboring APCs, initiating a positive feedback loop on DCs independently of CD40 signaling, as suggested by a study by Ruedl et al.48
A recent study in humans suggested a role for the direct pathway of antigen presentation in long term allograft injury,49 and it has been shown in mice that CD8+ T cells can be activated via the direct pathway in the absence of donor-derived DCs.50 Taken together, these results suggest that the activation of CD8+ T cells via the direct pathway might be active and lead to tissue damage for a much longer period than previously thought in immunosuppressed transplant patients. It therefore appears important to inhibit this response over long periods. In this context, the ability of MPA to directly modulate the capacity of DCs to activate allogeneic CD4-independent memory CD8+ T cells might be involved in its benefit in clinical transplantation. Finally, these results provide encouragement for further investigations into the potency of MPA-treated DCs to modulate the alloimmune response in experimental transplantation models.
In conclusion, our findings indicate that LPS is sufficient to raise human DCs to a certain activation state capable of expanding alloreactive CD8+ T cells and inducing fully functional cytotoxic cells in the absence of CD4+ T cells. Our results also suggest that IFN-γ has an important role in licensing DCs to prime allogeneic CD4-independent CD8+ T cells via an autocrine loop. We also demonstrated that inhibition of IFN-γ secretion in DCs by MPA resulted in profound T-cell anergy in both memory and naive allogeneic CD8+ T cells.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank the Etablissement Français du Sang du Center Atlantique of Tours for providing blood samples from healthy donors and Doreen Raine for revising the English text.
The work described in this paper was supported by François Rabelais University of Tours.
Authorship
Contribution: R.L. carried out all the experiments, performed the statistical analysis, and drafted the manuscript; F.V.-R. and H.N. participated in the coordination of the study; F.H. and R.F. carried out some experiments; Y.L. took part in the design of the study; C.B. conceived and coordinated the study and participated in the writing of the manuscript; and all authors read and approved the final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Roxane Lemoine, Université François Rabelais, EA 4245 Cellules Dendritiques, Immunomodulation et Greffes, UFR de Médecine, 10 Boulevard Tonnellé, 37032 Tours Cedex, France; e-mail: roxlem@orange.fr.


![Figure 2. LPS-treated DCs induced CD4-independent CD8+ T-cell proliferation whereas pretreatment of DCs with MPA abolished this capacity. MPA-DCs and mDCs designate DCs matured with LPS (50 ng/mL) for 2 days in the presence or absence of MPA (100μM). (A) MPA-DCs induced CD8+ T-cell unresponsiveness. mDCs and MPA-DCs obtained after monocyte adhesion (filled bars) or after CD14-positive selection (hatched bars) were cocultured for 6 days with allogeneic purified CD8+ T lymphocytes at a DC:T ratio of 1:3. T-cell proliferation was measured by incorporation of 1 μCi [3H]-thymidine added for the last 18 hours of culture. (B) Naive and memory CD8+ T cells were unresponsive to MPA-DCs. CD8+ CD45RA+ (filled bars) and CD8+ CD45RO+ (hatched bars) T cells were isolated from purified CD8+ T lymphocytes and then cocultured with allogeneic MPA-DCs or mDCs for 6 days at a DC:T ratio of 1:3. T-cell proliferation was measured by [3H]-thymidine incorporation. (C) IL-2 prevented CD8+ T-cell hyporesponsiveness. Purified CD8+ T lymphocytes were cocultured with allogeneic MPA-DCs or mDCs in the presence or absence of exogenous IL-2 (100 IU/mL). T-cell proliferation was measured as described above. (D) MPA-DCs induced donor-specific anergy. First coculture of allogeneic CD8+ T cells with MPA-DCs was performed, followed by a second stimulation in the presence (black bar) or absence (gray bar) of exogenous IL-2 (100 IU/mL) with mDCs from the donor used in the first coculture. Donor-specific unresponsiveness was assessed by further stimulation using mDCs from the first DC-donor (mDCa; gray bar) or a third party (mDCb; hatched bars). T-cell proliferation was measured on day 5 by incorporation of [3H]-thymidine. Results are expressed as mean cpm ± SD obtained from triplicate wells and are representative of 1 of 7 experiments. *P < .05.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/116/16/10.1182_blood-2010-02-268623/4/m_zh89991059050002.jpeg?Expires=1767700160&Signature=kOQXyZUJL~NqFcbeAQvgpgrdMgmP-Mx72AFsTl7uBLzwFv39oB0BGMlFdpS3Xtdxzvb58ushG49RtpDFJn48oGx1U1u7iYv5RBnNaby3R0fXjsfTHK0mCBo5tEld5Edg8y7N1xPubvvt1lfmfmPJ2C1TLBYOOxedU0iojk~~UKBZJOsWitX-3UnfGq~amh37kidQLBkE~B5LfWV3Dr~85RaKiCUvgKeHIYqSbBn8aB-8~OMffQg8OYaRTl2YwbWA0COf53wO4PZmSUYAH1g3lLDV0QktKx0sxwhuroua1ZbR19CMbQq2VPiLO6IB1twRRHjJtJSbwos5NlkqB6eusA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal