In this issue of Blood, Evans et al1 report that fractal analysis of the mechanical properties of whole-blood clots defines a unique property of the incipient clot that can be used as a functional biomarker of hemostasis.
Approximately 2500 years ago, Heraclitus expressed the philosophy, “Ta panta rhei” or “everything flows,” as well as a longer statement of the same basic idea, “No man ever steps in the same river twice, for it's not the same river and he's not the same man.” Rheology, a term adopted from the former quotation in the original Greek, describing the science of the deformation and flow of materials, has become an important aspect of hematology. In hemostasis, the measurement of the clotting time, also known as the gel point, under various defined conditions has long been used to characterize coagulation and to diagnose clotting disorders. To measure the gel point accurately, many instruments, using various mechanical, optical, and electrical methods, have been developed. Nevertheless, because deficiencies in the accuracy and reproducibility of all existing methods persist, new ideas and techniques are needed. Furthermore, the results of such studies have often not been rigorously and quantitatively related to important pathologic conditions.
Mechanical methods are often used to determine the gel point in clinical coagulation laboratories. However, the viscoelastic properties of clots, the conjoint viscous and elastic behaviors not assessed adequately in these methodologies, are also among the most sensitive measures of differences in coagulation and clot structure and can provide much additional information, such as the kinetics of coagulation, assessment of platelet health, clot retraction, and fibrinolysis.2,3 As a result, rheologic instruments have been developed and used for many years to characterize both the process of clotting and clot properties.4,5 Monitoring anticoagulant or antithrombotic (antiplatelet) therapy has become an important function of these instruments, in addition to characterization of the hemostatic system before, during, and after surgery. These new technologies have come to the forefront at a time when there is also more emphasis on point-of-care and high-throughput instruments that simultaneously measure multiple hemostatic parameters.
Recently, it has been shown that mechanical properties of clots are altered in patients with coronary artery disease6 and venous thrombosis,7 and in their relatives.8 In general, it seems that more rigid clots made of thinner fibers with more branch points and smaller pores lyse more slowly and are associated with both arterial and venous thrombosis. These studies suggest that such viscoelastic measurements might be used for screening, diagnosis, and monitoring therapy.
The article by Evans et al1 shows that the application of fractal analysis to the measured viscoelastic properties of “incipient clots” made from whole blood yields a parameter reflecting mechanical properties, the fractal dimension, which can be used as a biomarker for hemostasis, enabling the effects of disease and therapeutic manipulation to be monitored in a novel and informative way not otherwise available using conventional clinical technology.
The research reported here involves 2 new approaches that may pose a challenge to the clinical hematologist: fractal analysis and Fourier transform mechanical spectroscopy. The potential clinical significance of this study, however, makes it worthwhile for readers to gain some understanding of the meaning of these terms, for these or related methods are likely to be carried forward into practice.
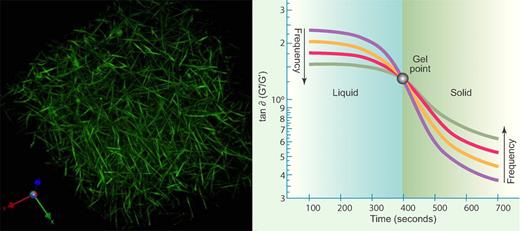
Fourier transform mechanical spectro-scopy is a technique involving oscillatory deformations over a range of frequencies by using a composite waveform to measure viscoelastic properties.5 This technique is especially well suited to detect the gel point because of one fundamental difference in the viscoelastic properties of a liquid and a solid. The ratio of the viscous and elastic components of the modulus (ratio of stress to strain) decreases with increasing frequency for a liquid but increases with frequency for a solid, so the gel point is precisely that point in between when that ratio is independent of frequency (see figure, right panel). This methodology identifies the “incipient clot” that is the structural template for the growing clot structure, and provides a more accurate measure of the gel point than any other method heretofore described.5
Gel point and fractal microstructure of blood clots. (Left) A 3-dimensional reconstruction of a hydrated fibrin network obtained using fluorescent confocal microscopy. Fractal analysis has long been used to characterize the growth of branched networks like this. (Image from A. Stout, K. Gersh, and J. Weisel, Department of Cell and Developmental Biology, School of Medicine, University of Pennsylvania, Philadelphia.) (Right) Fourier Transform mechanical spectroscopy of clotting blood. Oscillatory shear measurements of the ratio of viscous and elastic responses, or loss tangent (tan δ) of coagulating blood. The initial, preincipient clot response is characteristic of a viscoelastic fluid, with increasing frequency of oscillation causing tan δ to decrease. Tan δ becomes frequency independent as the incipient clot is established at the gel point, and thereafter, the frequency dependence is characteristic of a viscoelastic solid, with increasing frequency causing tan δ to increase. (Professional illustration by Paulette Dennis).
Gel point and fractal microstructure of blood clots. (Left) A 3-dimensional reconstruction of a hydrated fibrin network obtained using fluorescent confocal microscopy. Fractal analysis has long been used to characterize the growth of branched networks like this. (Image from A. Stout, K. Gersh, and J. Weisel, Department of Cell and Developmental Biology, School of Medicine, University of Pennsylvania, Philadelphia.) (Right) Fourier Transform mechanical spectroscopy of clotting blood. Oscillatory shear measurements of the ratio of viscous and elastic responses, or loss tangent (tan δ) of coagulating blood. The initial, preincipient clot response is characteristic of a viscoelastic fluid, with increasing frequency of oscillation causing tan δ to decrease. Tan δ becomes frequency independent as the incipient clot is established at the gel point, and thereafter, the frequency dependence is characteristic of a viscoelastic solid, with increasing frequency causing tan δ to increase. (Professional illustration by Paulette Dennis).
Fractals, which are used widely in biology to describe the nonlinear growth of branching network structures, arise from repeated transformations of a geometrical figure that leads to self-similar patterns, such that there are identical patterns at different observation scales (see figure, left panel). The fractal dimension, an indication of how completely a fractal appears to fill space as one goes down to finer and finer spatial scales, integrates structural/mechanical elements as a measure of the network complexity. At the gel point, polymeric clusters establish sufficient connectivity to become sample-spanning, conferring the properties of a solid on the system.9
It is significant that Evans et al1 have studied clotting simply in whole unadulterated blood. Analysis of viscoelastic data from whole blood from healthy subjects revealed that the fractal dimension has a clearly defined value, within a narrow range, 1.74 (± 0.07), which the authors refer to as a “Healthy Index.” The addition of unfractionated heparin to whole blood caused the expected changes in laboratory markers of coagulation and a progressive decease in fractal dimension as well as an increase in clotting time. In fact, the fractal dimension, together with activated partial thromboplastin time, was the most significant predictor of anti-factor Xa activity. These results also indicate that heparin affects clot structure in addition to clotting time.
In conclusion, this study introduces a novel method that allows the measurement of a single parameter, the fractal dimension, which can be used to characterize the complex relationship between the clotting system and the microstructure of incipient clots in healthy uncoagulated blood. Because many diseases affect hemostasis and hence clot properties, the next step will be extending these studies to thrombotic and hemorrhagic states to determine whether this functional biomarker remains consistently valid and can therefore provide insights into the diagnosis and management of these conditions.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal