Abstract
Diverse human cancers with poor prognosis, including many lymphoid and myeloid malignancies, exhibit high levels of Mcl-1. To explore the impact of Mcl-1 overexpression on the hematopoietic compartment, we have generated vavP-Mcl-1 transgenic mice. Their lymphoid and myeloid cells displayed increased resistance to a variety of cytotoxic agents. Myelopoiesis was relatively normal, but lymphopoiesis was clearly perturbed, with excess mature B and T cells accumulating. Rather than the follicular lymphomas typical of vavP-BCL-2 mice, aging vavP-Mcl-1 mice were primarily susceptible to lymphomas having the phenotype of a stem/progenitor cell (11 of 30 tumors) or pre-B cell (12 of 30 tumors). Mcl-1 overexpression dramatically accelerated Myc-driven lymphomagenesis. Most vavP-Mcl-1/ Eμ-Myc mice died around birth, and transplantation of blood from bitransgenic E18 embryos into unirradiated mice resulted in stem/progenitor cell tumors. Furthermore, lethally irradiated mice transplanted with E13 fetal liver cells from Mcl-1/Myc bitransgenic mice uniformly died of stem/progenitor cell tumors. When treated in vivo with cyclophosphamide, tumors coexpressing Mcl-1 and Myc transgenes were significantly more resistant than conventional Eμ-Myc lymphomas. Collectively, these results demonstrate that Mcl-1 overexpression renders hematopoietic cells refractory to many cytotoxic insults, perturbs lymphopoiesis and promotes malignant transformation of hematopoietic stem and progenitor cells.
Introduction
MCL-1, an antiapoptotic member of the Bcl-2 protein family, was discovered as an immediate early response gene expressed in human myeloid leukemia cells induced to differentiate by phorbol ester.1 Early studies using a human MCL-1 minigene demonstrated that overexpression of Mcl-1 predisposed mice to a range of late-onset B-cell lymphomas,2,3 and elevated Mcl-1 has subsequently been associated with poor prognosis and drug resistance in a wide variety of human tumors, particularly multiple myeloma,4 acute myeloid leukemia,5 acute lymphoblastic leukemia,6 chronic lymphocytic leukemia,7,8 and melanoma.9 Moreover, in a recent screen of more than 3000 human tumors of diverse tissue types, the MCL-1 locus was found to be amplified in almost 11% of cases.10
Mcl-1 is the most divergent of the antiapoptotic Bcl-2-like proteins. Homology with Bcl-2 is restricted to the C-terminal moiety of Mcl-1, and its unique N-terminal region (∼ 150 amino acids) bears PEST domains known to target proteins for rapid turnover. Indeed, Mcl-1 has a much shorter half-life (t1/2≤ 3 hours11 ) than either Bcl-2 or Bcl-xL (∼ 20 hours12,13 ). Although the structure of the Bcl-2-like moiety of Mcl-1 is very similar to that of other antiapoptotic family members, the surface-exposed BH3-domain binding groove in its helical bundle is more open.14,15 Like Bcl-2, Bcl-xL, Bcl-w, and A1, Mcl-1 binds several BH3-only proteins with high affinity, including Bim, Puma, and tBid. However, whereas Mcl-1 also binds strongly to Noxa but not to Bad, the opposite holds for Bcl-2, Bcl-xL, and Bcl-w.16,17 Furthermore, Mcl-1 restrains Bak, but Bcl-2 does not,18,19 with the exception of rare BCL-2 allelic variants.20
Expression of Mcl-1 is widespread and overlaps with but is not identical to that of Bcl-2 and Bcl-xL.21 Gene targeting in mice has revealed that Mcl-1 is essential for pre-implantation development of the embryo and its implantation22 ; for the survival of multipotential hematopoietic stem/progenitor cells and lymphoid progenitors23 ; for the development and maintenance of B and T lymphocytes24,25 and neutrophils26 ; and for macrophage effector function.27 Mcl-1 has also been implicated in the self-renewal capacity of pluripotent and hematopoietic human stem cells.28
To clarify further the impact of overexpression of Mcl-1 on hematopoiesis and predisposition to hematopoietic malignancies, we have generated and characterized transgenic mice that express a FLAG-tagged mouse Mcl-1 cDNA in a vector bearing transcriptional regulatory sequences from the vav gene (hereafter called vavP-Mcl-1; Figure 1A). This vector drives transgene expression in multipotential hematopoietic stem and progenitor cells and in diverse hematopoietic lineages.29,30 Our results are compared with those recently reported for transgenic mice expressing a mouse Mcl-1 cDNA under the control of the H2K promoter/enhancer.31
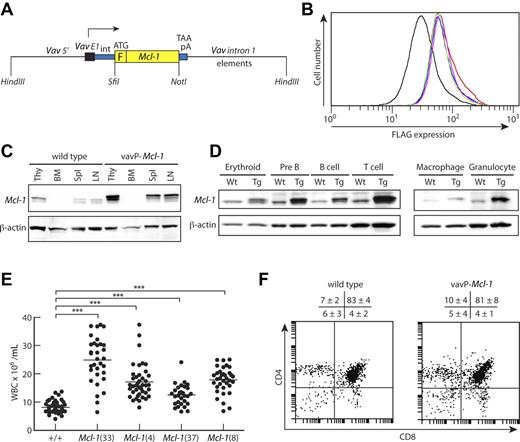
Pan-hematopoietic transgene expression in vavP-Mcl-1 transgenic mice. (A) Transgenic vector containing a mouse Mcl-1 cDNA linked to an N-terminal FLAG tag, F, flanked by promoter/enhancer elements from the vav gene.29 (B) Flow cytometric analysis of thymocytes after intracellular staining with FLAG-specific antibody to detect level of transgene expression. Each line represents one mouse. Mean fluorescence intensities are 39.6 for WT (black), 71.8 for Mcl-1(37) (green), 77.6 for Mcl-1(8) (magenta), 77.7 for Mcl-1(4) (blue), and 84.3 for Mcl-1(33) (red). (C) Western blot analysis of tissue lysates from thymus (Thy), BM, spleen (Spl), and LN from a WT (left) or vavP-Mcl-1(33) mouse (right) showing transgenic and endogenous Mcl-1 protein. (D) Western blot analysis of purified cells from WT (Wt) or Mcl-1(33) (Tg) mice: erythroid (Ter 119+ BM cells), pre-B (B220+IgM− BM cells), B (B220+ LN cells), T (Thy1+ LN cells), macrophage (Mac1+Gr1− BM cells), and granulocyte (Mac1+Gr1+ BM cells). (E) Peripheral white blood cell count for Mcl-1 transgenic mice and their WT siblings at 6 to 8 weeks of age. Bar represents mean: 8.1 × 106/mL for WT (49 mice), 24.9 for Mcl-1(33) (32 mice), 17.15 for Mcl-1(4) (41 mice), 12.5 for Mcl-1(37) (29 mice), and 17.8 for Mcl-1(8) (35 mice). All transgenic lines have significantly higher white blood cell count than WT (***P < .001). (F) Representative dot plots of stained thymocytes from WT or Mcl-1(33) transgenic mice showing percentages of CD4−CD8−, CD4+CD8+, CD4+CD8−, and CD4−CD8+ cells. Data are mean ± SD; n = 9 WT and 6 transgenic mice.
Pan-hematopoietic transgene expression in vavP-Mcl-1 transgenic mice. (A) Transgenic vector containing a mouse Mcl-1 cDNA linked to an N-terminal FLAG tag, F, flanked by promoter/enhancer elements from the vav gene.29 (B) Flow cytometric analysis of thymocytes after intracellular staining with FLAG-specific antibody to detect level of transgene expression. Each line represents one mouse. Mean fluorescence intensities are 39.6 for WT (black), 71.8 for Mcl-1(37) (green), 77.6 for Mcl-1(8) (magenta), 77.7 for Mcl-1(4) (blue), and 84.3 for Mcl-1(33) (red). (C) Western blot analysis of tissue lysates from thymus (Thy), BM, spleen (Spl), and LN from a WT (left) or vavP-Mcl-1(33) mouse (right) showing transgenic and endogenous Mcl-1 protein. (D) Western blot analysis of purified cells from WT (Wt) or Mcl-1(33) (Tg) mice: erythroid (Ter 119+ BM cells), pre-B (B220+IgM− BM cells), B (B220+ LN cells), T (Thy1+ LN cells), macrophage (Mac1+Gr1− BM cells), and granulocyte (Mac1+Gr1+ BM cells). (E) Peripheral white blood cell count for Mcl-1 transgenic mice and their WT siblings at 6 to 8 weeks of age. Bar represents mean: 8.1 × 106/mL for WT (49 mice), 24.9 for Mcl-1(33) (32 mice), 17.15 for Mcl-1(4) (41 mice), 12.5 for Mcl-1(37) (29 mice), and 17.8 for Mcl-1(8) (35 mice). All transgenic lines have significantly higher white blood cell count than WT (***P < .001). (F) Representative dot plots of stained thymocytes from WT or Mcl-1(33) transgenic mice showing percentages of CD4−CD8−, CD4+CD8+, CD4+CD8−, and CD4−CD8+ cells. Data are mean ± SD; n = 9 WT and 6 transgenic mice.
Methods
Mice
All mice used in these experiments were on a C57BL/6J background and bred at the Walter and Eliza Hall Institute (WEHI). Experiments with mice were approved by the Animal Ethics Committee. A mouse Mcl-1 cDNA, encoding residues 2 to 331, was inserted into the vavP transgenic vector previously developed in our laboratory.29 To facilitate screening, the insert encoded a FLAG epitope at the N-terminus of Mcl-1 (Figure 1A). A silent mutation was introduced at Glu 275 of Mcl-1, GAA to GAG, which eliminated a HindIII restriction site. The transgene was excised by digestion with HindIII, purified, and introduced into the inbred C57BL/6J genome by pronuclear microinjection as described.30 Transgenic pups were identified by polymerase chain reaction on tail DNA, using primers specific to the SV40 pA region, and by screening white blood cells in peripheral blood with anti-FLAG antibody by flow cytometry. Four founder transgenic mice were selected and bred with C57BL/6J mice to establish strains Mcl-1 (33), Mcl-1(4), Mcl-1 (8), and Mcl-1 (37).
To generate vavP-Mcl-1/Eμ-Myc bitransgenic offspring, vavP-mcl-1 transgenic females were mated with Eμ-Myc transgenic males. For fetal liver reconstitution experiments, E13.5 embryos from timed matings of Eμ-Myc males and vavP-Mcl-1 females (both expressing the Ly5.2 cell surface marker) were harvested and tails isolated for genotyping. Fetal livers were dispersed into single-cell suspensions and viable cell number determined by hemocytometer and trypan blue exclusion assay. A total of 2 × 106 cells were injected into the tail vein of lethally irradiated (2 × 5.5 Gy, 3 hours apart) C57BL/6J Ly5.1 recipients.
To generate Bim+/− VavP-Mcl-1(33) mice, male VavP-Mcl-1(33) mice were bred with female Bim+/− or Bim−/− mice (strain B6del/33932 ). In an attempt to generate Bim−/− VavP-Mcl-1(33) mice, male bim+/− VavP-mcl-1(33) mice were bred with female Bim+/− or Bim−/− mice; but because of birthing difficulties of Bim−/− females, too few Bim−/− VavP-Mcl-1(33) offspring were obtained to provide meaningful results.
Mouse survival analysis was carried out using GraphPad Prism (Version 5.0a) and significance determined using log-rank (Mantel-Cox) test. Moribund mice were autopsied and organs collected for histology and immunophenotyping. Soft tissue and sternum were collected into Bouin fixative or 10% formalin, respectively, before embedding in paraffin wax and staining with hematoxylin and eosin. Blood smears were stained with May-Grunwald-Giemsa.
For tumor treatment studies, 1 × 106 tumor cells were transplanted into C57BL/6J recipients and mice treated when tumors became palpable (∼ 14 days after inoculation) as previously described.33
Flow cytometric analysis of intracellular FLAG expression
Thymocytes were washed in phosphate-buffered saline (PBS) and fixed in 2% paraformaldehyde in PBS for 20 minutes on ice before washing twice with PBS. Cells were then permeabilized with fluorescence-activated cell sorter (FACS) buffer (balanced salt solution containing Mg2+ and Ca2+, supplemented with 2% fetal bovine serum) containing 0.1% saponin (permeabilization buffer) by vortexing and incubating on ice for 20 minutes. After addition of 10 μL of biotinylated rat monoclonal FLAG antibody (clone 9H118 ) and vortexing, the cells were incubated at 4°C overnight and washed twice with permeabilization buffer. After addition of 5 μL of Steptavidin-Cy5 antibody (Southern Biotechnology) in 50 μL of permeabilization buffer and vortexing, the cells were incubated at 4°C in the dark for 1 hour and washed once with permeabilization buffer and once in FACS buffer before analysis by flow cytometry using an LSR1 machine (BD Biosciences).
Immunoblotting
Western blots were carried out by standard procedures using protein extracts prepared from single-cell suspensions. Blots were probed using the following antibodies: Mcl-1 (clone 19C4-15; WEHI mAb laboratory), β-actin (clone AC-74; Sigma-Aldrich), Bim (clone 3C5; Alexis Laboratories), Bcl-2 (clone 3F11; BD Biosciences PharMingen), p53 (clone CM5; Novocastra), and ARF (p19ARF exon 2; Rockland).
Hematopoietic cell analysis
Peripheral blood was analyzed using the ADVIA hematology analyzer (Bayer) or by FACS analysis after treatment with buffer containing 0.168M ammonium chloride to deplete red blood cells. Single-cell suspensions were prepared from thymus, lymph nodes (axillary, brachial, inguinal), bone marrow (BM; one femur), and spleen and viable cells counted using a hemocytometer and trypan blue exclusion or enumerated on a Casey Counter (Scharfe). Cell composition was determined by staining with surface marker-specific antibodies and FACS analysis (LSR1, BD Biosciences). Stained single-cell suspensions were sorted using a MoFlo (Cytomation) high-speed sorter. Monoclonal antibodies used, labeled with fluorescein isothiocyanate, phycoerythrin, or allophycocyanin, included the following: RB6-8C5, anti-Gr1; MI/70, anti-Mac1; YTA3.2.1, anti-CD4; 53.6.7.2, anti-CD8; Ter119, anti-erythroid marker; ID3, anti-CD19; RA3-6B2, anti-CD45R-B220; 5.1, anti-IgM; 11-26C, anti-IgD; 145-2C11, anti-CD3; E13.161.7, anti-Sca-1; 57, anti-CD43; T329.1, anti-Thy1; A20.1, anti-Ly5.1 and 5.405.15.2, anti-Ly5.2. Cell culture and survival assays were performed as described.34 Statistical significance was determined using the Student t test (2-tailed, assuming equal variance). Proliferation analysis of cells using carboxyfluorescein diacetate succinimidyl ester labeling and in vitro time course cell counting was carried out as described.35 For spleen colony-forming cell analysis, BM cells were harvested from healthy 6- to 8-week-old mice and resuspended in PBS at 5 × 106 cells/mL. Cells were either left unirradiated or irradiated with 1.25 Gy γ-IR immediately before tail vein injection of 7.5 to 15 × 104 cells into lethally irradiated (2 × 5.5 Gy, 3 hours apart) C57BL/6J mice. Spleens were harvested 12 days later, fixed in Carnoy fixative (60% ethanol, 30% chloroform, 10% acetic acid) and macroscopic colonies counted.
Results
The VavP-Mcl-1 transgene is expressed in multiple hematopoietic lineages
Four founder VavP-Mcl-1 transgenic mice were bred to establish independent lines designated Mcl-1(33), Mcl-1(4), Mcl-1(37), and Mcl-1(8). Flow cytometric analysis of thymocytes using intracellular staining with a FLAG-specific antibody established that the transgene was expressed in all 4 lines, with the highest level in Mcl-1(33) (Figure 1B). Transgenic Mcl-1 protein was readily detected by Western blot analysis in the thymus, spleen, and lymph nodes (LNs), at levels appreciably greater than endogenous protein (Figure 1C and data not shown). All major populations of B and T lymphoid cells expressed transgenic Mcl-1, as did erythroid, macrophage, and granulocyte populations (Figure 1D and data not shown). This pattern of transgene expression mirrored that previously documented for vavP-BCL-2 mice.36
Impact of Mcl-1 transgene on hematopoiesis
We noted that healthy young mice of all 4 lines frequently displayed elevated white blood cell counts, the highest being for Mcl-1(33) mice and lowest for Mcl-1(37) mice (Figure 1E), in line with the relative levels of transgene expression (Figure 1B). To determine which cellular compartments were affected, we analyzed the composition of the peripheral blood, spleen, LNs, BM, and thymus from healthy young (6-8 weeks old) male Mcl-1(33) and Mcl-1(4) transgenic mice and their wild-type (WT; C57BL/6J) littermates (Table 1).
Hematopoietic composition of young Mcl-1 transgenic mice
| Organ/cell type . | C57BL/6J . | Mcl-1(33) . | Mcl-1(4) . |
|---|---|---|---|
| Peripheral blood | 7.9 ± 3.5 | 23.6 ± 7.6* | 17.2 ± 3.6* |
| CD4+CD8− | 1.4 ± 0.7 | 3.2 ± 1.3† | 3.2 ± 1.4‡ |
| CD4−CD8+ | 0.9 ± 0.5 | 2.8 ± 0.7* | 2.1 ± 0.5† |
| B220+ IgM/IgD− | 0.6 ± 0.8 | 1.1 ± 2.1 | 0.8 ± 1.4 |
| B220+ IgM/IgD+ | 3.7 ± 2.0 | 13.7 ± 5.6* | 8.5 ± 3.0† |
| Mac1+ | 0.5 ± 0.3 | 1.5 ± 0.9‡ | 1.5 ± 1.1‡ |
| Mac1+ Gr1+ | 0.5 ± 0.4 | 0.8 ± 0.3 | 1.0 ± 0.6 |
| Spleen | 107.4 ± 40.8 | 236.9 ± 63.7* | 206.9 ± 102‡ |
| CD4+CD8− | 14.1 ± 3.4 | 42.5 ± 14.0* | 35.3 ± 16.6† |
| CD4−CD8+ | 7.0 ± 3.4 | 20.6 ± 8.3* | 17.0 ± 8.3† |
| B220+ IgM/IgD− | 1.6 ± 0.9 | 5.2 ± 2.4† | 4.5 ± 3.0‡ |
| B220+ IgM/IgD+ | 37.7 ± 17.5 | 124.1 ± 45.3* | 102.0 ± 77.1‡ |
| Mac1+ | 2.8 ± 1.8 | 5.1 ± 2.7 | 6.2 ± 3.9‡ |
| Mac1+Gr1+ | 2.0 ± 1.9 | 3.4 ± 1.6 | 3.6 ± 1.3 |
| Ter-119+ | 29.8 ± 25.9 | 40.4 ± 12 | 32.9 ± 23.5 |
| LN | 12.4 ± 5.1 | 25.8 ± 7.5† | 20 ± 8.7 |
| CD4+CD8− | 4.4 ± 1.7 | 9.7 ± 3.1† | 7.9 ± 3.0‡ |
| CD4−CD8+ | 3.0 ± 1.1 | 6.5 ± 2.0†‡ | 5.3 ± 2.6 |
| B220+ IgM/IgD | 0.14 ± 0.05 | 0.30 ± 0.17‡ | 0.29 ± 0.16 |
| B220+ IgM/IgD+ | 4.0 ± 1.4 | 8.2 ± 2.3† | 4.9 ± 2.1 |
| BM | 25.0 ± 5.4 | 26.0 ± 7.3 | 27.7 ± 10.6 |
| Ter 119+ | 10.8 ± 3.9 | 6.0 ± 4.7 | 12.0 ± 6.5 |
| Thy1+ | 1.7 ± 1.7 | 1.5 ± 0.6 | 1.3 ± 0.5 |
| B220+ IgM/IgD | 3.5 ± 2.2 | 5.6 ± 2.4 | 4.2 ± 2.0 |
| B220+ IgM/IgD+ | 2.0 ± 1.3 | 4.9 ± 2.1† | 3.4 ± 1.7 |
| Mac1+ | 1.5 ± 1.7 | 2.5 ± 1.4 | 2.0 ± 1.9 |
| Mac1+Gr1+ | 5.5 ± 4.9 | 5.2 ± 3.2 | 4.5 ± 2.4 |
| Thymus | 170.6 ± 76.4 | 1509 ± 70.7 | 193.9 ± 55.0 |
| CD4−CD8− | 9.6 ± 5.5 | 7.2 ± 3.9 | 9.6 ± 5.3 |
| CD4+CD8+ | 141.6 ± 64.8 | 123.6 ± 62.1 | 163.0 ± 49.0 |
| CD4+CD8− | 12.7 ± 5.3 | 14.2 ± 6.3 | 13.4 ± 3.5 |
| CD4−CD8+ | 6.7 ± 4.1 | 5.9 ± 3.0 | 7.9 ± 2.6 |
| Organ/cell type . | C57BL/6J . | Mcl-1(33) . | Mcl-1(4) . |
|---|---|---|---|
| Peripheral blood | 7.9 ± 3.5 | 23.6 ± 7.6* | 17.2 ± 3.6* |
| CD4+CD8− | 1.4 ± 0.7 | 3.2 ± 1.3† | 3.2 ± 1.4‡ |
| CD4−CD8+ | 0.9 ± 0.5 | 2.8 ± 0.7* | 2.1 ± 0.5† |
| B220+ IgM/IgD− | 0.6 ± 0.8 | 1.1 ± 2.1 | 0.8 ± 1.4 |
| B220+ IgM/IgD+ | 3.7 ± 2.0 | 13.7 ± 5.6* | 8.5 ± 3.0† |
| Mac1+ | 0.5 ± 0.3 | 1.5 ± 0.9‡ | 1.5 ± 1.1‡ |
| Mac1+ Gr1+ | 0.5 ± 0.4 | 0.8 ± 0.3 | 1.0 ± 0.6 |
| Spleen | 107.4 ± 40.8 | 236.9 ± 63.7* | 206.9 ± 102‡ |
| CD4+CD8− | 14.1 ± 3.4 | 42.5 ± 14.0* | 35.3 ± 16.6† |
| CD4−CD8+ | 7.0 ± 3.4 | 20.6 ± 8.3* | 17.0 ± 8.3† |
| B220+ IgM/IgD− | 1.6 ± 0.9 | 5.2 ± 2.4† | 4.5 ± 3.0‡ |
| B220+ IgM/IgD+ | 37.7 ± 17.5 | 124.1 ± 45.3* | 102.0 ± 77.1‡ |
| Mac1+ | 2.8 ± 1.8 | 5.1 ± 2.7 | 6.2 ± 3.9‡ |
| Mac1+Gr1+ | 2.0 ± 1.9 | 3.4 ± 1.6 | 3.6 ± 1.3 |
| Ter-119+ | 29.8 ± 25.9 | 40.4 ± 12 | 32.9 ± 23.5 |
| LN | 12.4 ± 5.1 | 25.8 ± 7.5† | 20 ± 8.7 |
| CD4+CD8− | 4.4 ± 1.7 | 9.7 ± 3.1† | 7.9 ± 3.0‡ |
| CD4−CD8+ | 3.0 ± 1.1 | 6.5 ± 2.0†‡ | 5.3 ± 2.6 |
| B220+ IgM/IgD | 0.14 ± 0.05 | 0.30 ± 0.17‡ | 0.29 ± 0.16 |
| B220+ IgM/IgD+ | 4.0 ± 1.4 | 8.2 ± 2.3† | 4.9 ± 2.1 |
| BM | 25.0 ± 5.4 | 26.0 ± 7.3 | 27.7 ± 10.6 |
| Ter 119+ | 10.8 ± 3.9 | 6.0 ± 4.7 | 12.0 ± 6.5 |
| Thy1+ | 1.7 ± 1.7 | 1.5 ± 0.6 | 1.3 ± 0.5 |
| B220+ IgM/IgD | 3.5 ± 2.2 | 5.6 ± 2.4 | 4.2 ± 2.0 |
| B220+ IgM/IgD+ | 2.0 ± 1.3 | 4.9 ± 2.1† | 3.4 ± 1.7 |
| Mac1+ | 1.5 ± 1.7 | 2.5 ± 1.4 | 2.0 ± 1.9 |
| Mac1+Gr1+ | 5.5 ± 4.9 | 5.2 ± 3.2 | 4.5 ± 2.4 |
| Thymus | 170.6 ± 76.4 | 1509 ± 70.7 | 193.9 ± 55.0 |
| CD4−CD8− | 9.6 ± 5.5 | 7.2 ± 3.9 | 9.6 ± 5.3 |
| CD4+CD8+ | 141.6 ± 64.8 | 123.6 ± 62.1 | 163.0 ± 49.0 |
| CD4+CD8− | 12.7 ± 5.3 | 14.2 ± 6.3 | 13.4 ± 3.5 |
| CD4−CD8+ | 6.7 ± 4.1 | 5.9 ± 3.0 | 7.9 ± 2.6 |
Nucleated cells × 106, except peripheral blood cells (× 106/mL). Nucleated cells (mean ± SD) from 6- to 8-week-old male mice; n = 4 to 9 mice per genotype.
Significantly different from WT (P ≤ .001, Student t test).
Significantly different from WT (P ≤ .01, Student t test).
Significantly different from WT (P ≤ .05, Student t test).
The 2- to 3-fold increase in peripheral blood leukocytes was largely the result of an increase in mature B cells (B220+IgM/IgD+) and mature T cells (both CD4+ and CD8+). Monocytes (Mac1+Gr1−) were also elevated in the blood, but granulocytes (Mac1+Gr1+) were nearly normal, as were red blood cells (supplemental Figure 1A, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Interestingly, platelet counts were near normal (supplemental Figure 1B), in contrast to the 2-fold decrease seen in VavP-BCL-2 transgenic mice.36
The spleen was enlarged and total cellularity elevated approximately 2-fold (Table 1) because of approximately 3-fold increase in B lymphoid cells, CD4+ T cells, and CD8+ T cells, as well as an approximately 2-fold increase in macrophages (Mac1+Gr1−). LNs were also enlarged because of an increase in CD4+ T cells, CD8+ T cells, and B cells (only in Mcl-1(33) mice).
In contrast, the BM appeared nearly normal (Table 1), apart from an approximately 2.5-fold increase in recirculating sIg+ B cells in Mcl-1(33) mice. The frequency of marrow megakaryocytes was also normal (supplemental Figure 1C).
Despite having high expression of the transgene (Figure 1B-C), the cellularity and composition of the thymus were normal (Table 1; Figure 1F). This is another intriguing difference from VavP-BCL-2 transgenic mice, which exhibit a marked decrease in the proportion and total number of immature CD4+CD8+ thymocytes.36
Enhanced survival capacity of lymphoid cells from VavP-Mcl-1 mice
To gauge the impact of overexpression of Mcl-1 on the survival capacity of hematopoietic cell types, in vitro tests were performed. We focused largely (but not exclusively) on the Mcl-1(33) mice, in which the impact of the transgene appeared greatest. For comparison, cells from age-matched nontransgenic (+/+) littermates and VavP-BCL-2 mice were analyzed in parallel.
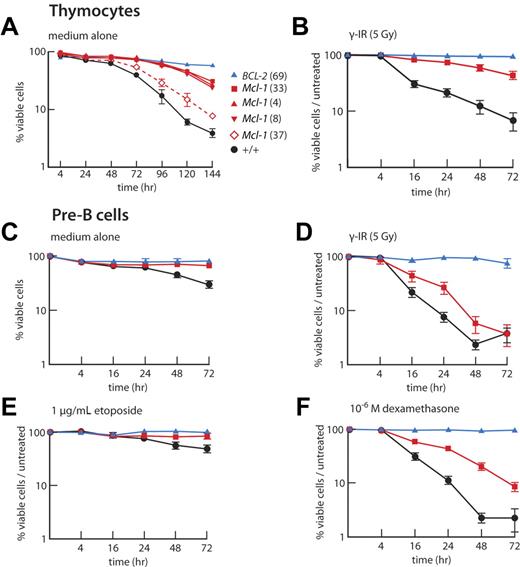
When cultured in the absence of cytokines, thymocytes from VavP-mcl-1 transgenic mice, particularly those from Mcl-1(33), Mcl-1(4), and Mcl-1(8) mice, survived better than their WT counterparts (Figure 2A). Furthermore, like VavP-BCL-2 transgenic cells, thymocytes from VavP-Mcl-1 transgenic mice were refractory to γ-irradiation (Figure 2B). The pre-B cells (B220+IgM− BM cells), B cells (B220+ LN cells) and peripheral T cells (Thy1+ LN cells) from VavP-Mcl-1 transgenic mice also showed enhanced survival in culture and were more resistant to cytotoxic agents than those from WT littermates (Figure 2C-F; supplemental Figures 2–3). Furthermore, B cells resisted apoptosis induced by anti-IgM antibody F(ab′)2 fragments, which cross-link the B-cell antigen receptor and induce apoptosis in wild-type B cells37 (Figure 3A).
Elevated Mcl-1 protects thymocytes and pre-B cells against apoptosis in vitro. (A) Thymocytes from Mcl-1 transgenic mice are resistant to cytokine deprivation. Thymocytes from WT, VavP-BCL-2 (69), and 4 independent strains of VavP-Mcl-1 mice (n = 2 for each) were cultured in vitro for 6 days without cytokines and viability assayed by flow cytometry at the indicated intervals. Data are mean ± SD. (B) Immature CD4+CD8+ thymocytes from Mcl-1(33) transgenic mice have increased resistance to apoptosis induced by γ-irradiation (5 Gy). (C-F) Pre-B cells from Mcl-1(33) transgenic mice have increased resistance to apoptosis. Pre-B cells (B220+ IgM−) sorted from BM of individual mice were cultured in medium alone (C) or in medium after being subjected to γ-irradiation (5 Gy) (D) or in medium containing etoposide (1 μg/mL) (E) or dexamethasone (10−6) (F). Viability (proportion of cells negative for propidium iodide uptake and annexin V surface staining) was determined by flow cytometry. Values have been normalized to viability in control (untreated) cultures to show stimuli-specific apoptosis. WT (black, n = 5-9 mice); Mcl-1(33) transgenic (red, n = 5-9), BCL-2(69) transgenic (blue, n = 2-5). (B-F) Values are mean ± SEM.
Elevated Mcl-1 protects thymocytes and pre-B cells against apoptosis in vitro. (A) Thymocytes from Mcl-1 transgenic mice are resistant to cytokine deprivation. Thymocytes from WT, VavP-BCL-2 (69), and 4 independent strains of VavP-Mcl-1 mice (n = 2 for each) were cultured in vitro for 6 days without cytokines and viability assayed by flow cytometry at the indicated intervals. Data are mean ± SD. (B) Immature CD4+CD8+ thymocytes from Mcl-1(33) transgenic mice have increased resistance to apoptosis induced by γ-irradiation (5 Gy). (C-F) Pre-B cells from Mcl-1(33) transgenic mice have increased resistance to apoptosis. Pre-B cells (B220+ IgM−) sorted from BM of individual mice were cultured in medium alone (C) or in medium after being subjected to γ-irradiation (5 Gy) (D) or in medium containing etoposide (1 μg/mL) (E) or dexamethasone (10−6) (F). Viability (proportion of cells negative for propidium iodide uptake and annexin V surface staining) was determined by flow cytometry. Values have been normalized to viability in control (untreated) cultures to show stimuli-specific apoptosis. WT (black, n = 5-9 mice); Mcl-1(33) transgenic (red, n = 5-9), BCL-2(69) transgenic (blue, n = 2-5). (B-F) Values are mean ± SEM.
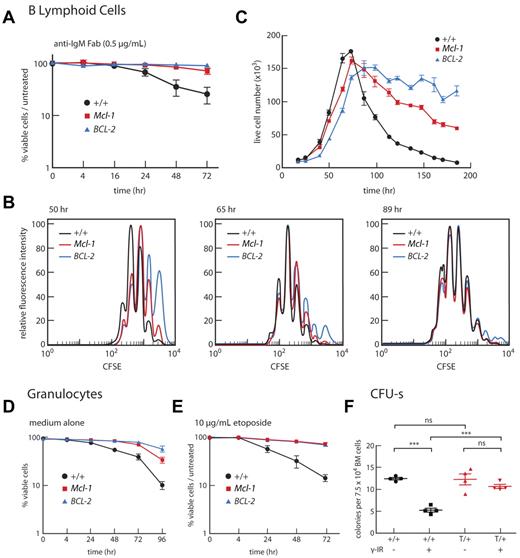
Impact of overexpression of Mcl-1 on B lymphoid, myeloid cells, and stem cells. (A-C) Mcl-1 enhances survival of B cells. (A) B220+ cells isolated from LNs of individual mice were incubated in vitro with anti-IgM F(ab′)2 (0.5 μg/mL), and viability was determined over 72 hours relative to cells in untreated cultures. WT mice (black, n = 3); Mcl-1(33) mice (red, n = 4 mice); BCL-2(69) mice (blue, n = 4). Data are mean ± SEM. (B-C) B220+ cells were isolated from spleens of WT, Mcl-1(33), and Bcl-2 mice, labeled with carboxyfluorescein diacetate succinimidyl ester and incubated with CpG. Fluorescence intensity (B) and viable cell number (C) were monitored over time. Data are representative of 3 independent experiments performed for a single mouse of each genotype. Supplemental figure 3 contains further analyses performed on B cells. (D-E) Mcl-1 enhances survival of granulocytes. Granulocytes (Mac1+Gr1+) sorted from BM of individual mice were cultured in vitro for 4 days in medium alone (D) or medium containing 10 μg/mL etoposide (E). WT mice (black, n = 5-7); Mcl-1(33) mice (red, n = 4-6); BCL-2(69) mice (blue, n = 3 or 4). Data are mean ± SEM. (F) Mcl-1 enhances resistance of multipotential stem cells to γ-irradiation. BM cells were subjected to 1.5 Gy γ-irradiation or mock-treated before transplantation (7.5-15 × 104 cells/mouse) into lethally irradiated recipients (2 × 5.5 Gy, 3 hours apart). Spleen colonies were counted on day 12, and results are plotted as mean spleen colony-forming cells (CFU-s) for triplicate recipient mice per 7.5 × 104 transplanted cells. Bars represent mean ± SEM. ***P < .001 (Student t test). ns indicates not significant. For each genotype, BM cells from 4 donor mice were each transplanted into 6 recipients (3 receiving unirradiated cells and 3 receiving irradiated cells). WT donor mice (+/+, black); Mcl-1(33) donor mice (T/+, red).
Impact of overexpression of Mcl-1 on B lymphoid, myeloid cells, and stem cells. (A-C) Mcl-1 enhances survival of B cells. (A) B220+ cells isolated from LNs of individual mice were incubated in vitro with anti-IgM F(ab′)2 (0.5 μg/mL), and viability was determined over 72 hours relative to cells in untreated cultures. WT mice (black, n = 3); Mcl-1(33) mice (red, n = 4 mice); BCL-2(69) mice (blue, n = 4). Data are mean ± SEM. (B-C) B220+ cells were isolated from spleens of WT, Mcl-1(33), and Bcl-2 mice, labeled with carboxyfluorescein diacetate succinimidyl ester and incubated with CpG. Fluorescence intensity (B) and viable cell number (C) were monitored over time. Data are representative of 3 independent experiments performed for a single mouse of each genotype. Supplemental figure 3 contains further analyses performed on B cells. (D-E) Mcl-1 enhances survival of granulocytes. Granulocytes (Mac1+Gr1+) sorted from BM of individual mice were cultured in vitro for 4 days in medium alone (D) or medium containing 10 μg/mL etoposide (E). WT mice (black, n = 5-7); Mcl-1(33) mice (red, n = 4-6); BCL-2(69) mice (blue, n = 3 or 4). Data are mean ± SEM. (F) Mcl-1 enhances resistance of multipotential stem cells to γ-irradiation. BM cells were subjected to 1.5 Gy γ-irradiation or mock-treated before transplantation (7.5-15 × 104 cells/mouse) into lethally irradiated recipients (2 × 5.5 Gy, 3 hours apart). Spleen colonies were counted on day 12, and results are plotted as mean spleen colony-forming cells (CFU-s) for triplicate recipient mice per 7.5 × 104 transplanted cells. Bars represent mean ± SEM. ***P < .001 (Student t test). ns indicates not significant. For each genotype, BM cells from 4 donor mice were each transplanted into 6 recipients (3 receiving unirradiated cells and 3 receiving irradiated cells). WT donor mice (+/+, black); Mcl-1(33) donor mice (T/+, red).
When carboxyfluorescein diacetate succinimidyl ester–labeled B cells were exposed to the T cell-independent proliferative stimulus CpG, a Toll-like receptor agonist, VavP-Mcl-1 B cells took longer, on average, to enter division than WT B cells, as previously reported for cells overexpressing Bcl-2 and other prosurvival members38 (and references therein), but their subsequent progression through the cell cycle proceeded normally (Figure 3B). Similar results were observed in response to stimulation with lipopolysaccharide or, mimicking T cell-dependent stimulation, an anti-CD40 mAb with IL-4 (data not shown). VavP-Mcl-1 transgenic B cells reached a similar maximum division number as WT B cells35 in response to in vitro stimulation with CpG, but their subsequent death rate was slower, although not as slow as that of B cells from VavP-BCL-2 mice (Figure 3C).
Taken together, these data indicate that the increased lymphoid cellularity in VavP-Mcl-1 transgenic mice is the result of enhanced cell survival rather than enhanced proliferation.
Enhanced survival capacity of myeloid cells from VavP-Mcl-1 mice
Although Mcl-1 overexpression only slightly perturbed homeostasis within the myeloid compartment (Table 1), it did enhance the resistance of at least some types of myeloid cells to stress. Granulocytes (Mac1+Gr1+) isolated from the BM of VavP-Mcl-1 mice remained viable for longer than granulocytes from WT mice when cultured without cytokines (Figure 3D), and they were also more resistant to etoposide (Figure 3E). Importantly, elevated Mcl-1 conferred significant radioprotection on BM stem cells capable of forming spleen colonies in lethally irradiated recipients (Figure 3F).
VavP-Mcl-1 transgenic mice die of late-onset hematopoietic tumors but do not develop autoimmunity
Cohorts of VavP-Mcl-1 mice were monitored until they were 20 months of age. In most mice, the size and cellular composition of the spleen and LNs remained relatively stable for at least 12 months (compare Table 1 with supplemental Table 1), although the spleen follicles had germinal centers, some of which were very large, almost approaching the appearance of a follicular lymphoma. In addition, large focal accumulations of lymphoid cells developed around portal areas in the liver (supplemental Figure 4C), with infiltrates also in the lungs (supplemental Figure 4D) and kidney (not shown). No excess mitotic activity was apparent in these lesions, which were also detectable, albeit smaller, in some normal-aged mice.
Unlike VavP-BCL-2 mice,39 there was no morbidity resulting from autoimmune kidney disease. The kidneys of aging mice were normal in color and texture, and no abnormalities were apparent on histologic examination. Furthermore, serum antinuclear antibodies were no higher than in aged WT mice in tests using fixed Hep-2 cells (not shown).
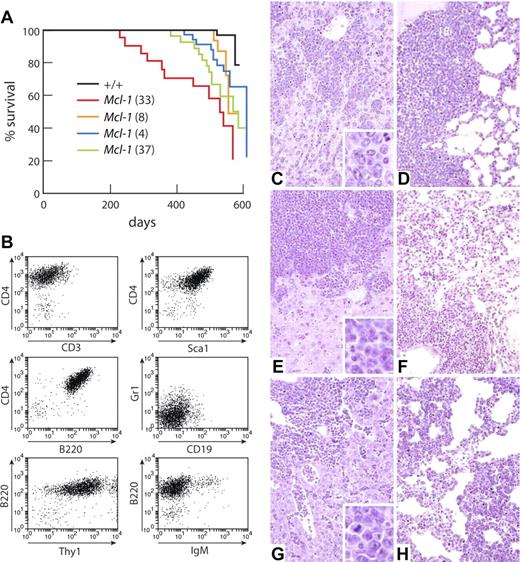
The VavP-Mcl-1 mice were, however, predisposed to lymphoma development. Most mice had died of tumors by 20 months, an age at which almost all WT littermates were still healthy (Figure 4A). Overall, tumor onset was slow although more rapid for Mcl-1(33) mice (median onset, 531 days) in keeping with their higher transgene expression (Figure 1B) and greater leukocyte accumulation (Figure 1E; Table 1). The long latency implies that additional mutations are required for Mcl-1–overexpressing cells to become malignant.
VavP-Mcl-1 mice die of lymphoma. (A) Kaplan-Meier survival curve of tumor-related deaths in cohorts of the following mice: WT (black, n = 54); Mcl-1(33) (red, n = 23), median survival, 531 days; Mcl-1(8) (green, n = 29), median survival, 571 days; Mcl-1(37) (orange, n = 18), median survival, 556 days; and Mcl-1(4) (blue, n = 38), median survival, 612 days. (B) Immunophenotype of representative stem/progenitor cell tumor (from transgenic mouse Mcl-1(33) #90). (C-D) Representative histology from VavP-Mcl-1 mouse dying of primitive/progenitor cell tumor showing infiltration of (C) liver and (D) lung. (E-F) Representative histology from VavP-Mcl-1 mouse dying of pre-B cell tumor showing infiltration of (E) liver and (F) lung. (G-H) Representative histology from VavP-Mcl-1/Eμ-Myc mouse dying of B-cell tumor showing infiltration of (G) liver and (H) lung. Sections were stained with hematoxylin and eosin. Original magnification ×20 in main panels. Sections were photographed on Kodak Ektachrome 64T film using a Zeiss Axiophot microscope and camera with a Zeiss 20×/0.60 NA objective lens. The scanned images were processed using Adobe Photoshop software.
VavP-Mcl-1 mice die of lymphoma. (A) Kaplan-Meier survival curve of tumor-related deaths in cohorts of the following mice: WT (black, n = 54); Mcl-1(33) (red, n = 23), median survival, 531 days; Mcl-1(8) (green, n = 29), median survival, 571 days; Mcl-1(37) (orange, n = 18), median survival, 556 days; and Mcl-1(4) (blue, n = 38), median survival, 612 days. (B) Immunophenotype of representative stem/progenitor cell tumor (from transgenic mouse Mcl-1(33) #90). (C-D) Representative histology from VavP-Mcl-1 mouse dying of primitive/progenitor cell tumor showing infiltration of (C) liver and (D) lung. (E-F) Representative histology from VavP-Mcl-1 mouse dying of pre-B cell tumor showing infiltration of (E) liver and (F) lung. (G-H) Representative histology from VavP-Mcl-1/Eμ-Myc mouse dying of B-cell tumor showing infiltration of (G) liver and (H) lung. Sections were stained with hematoxylin and eosin. Original magnification ×20 in main panels. Sections were photographed on Kodak Ektachrome 64T film using a Zeiss Axiophot microscope and camera with a Zeiss 20×/0.60 NA objective lens. The scanned images were processed using Adobe Photoshop software.
Autopsy of tumor-bearing mice revealed disseminated disease involving the spleen and LNs, particularly the mesenteric LN and Peyer patches. White blood cell counts were less than 50 × 106/mL in most tumor-bearing mice: 8 of 13 Mcl-1(33) and 8 of 9 Mcl-1(4) mice. Histologic examination indicated that most tumors had invaded not only the spleen and LNs but also liver (Figure 4C,E), lung (Figure 4D,F), kidney, and marrow (not shown). The cells were large, with abundant cytoplasm, and were tightly packed in uniform infiltrating populations, approximating the appearance of human large cell lymphoma. “Starry sky” macrophages were prominent.
In view of the importance of Mcl-1 for the survival of stem and progenitor cells,23 we were intrigued to find that 36% of the lymphomas (11 of 30 tumors, being 5 of 10 Mcl-1(33), 3 of 10 Mcl-1(4), 3 of 6 Mcl-1(8), and 0 of 4 Mcl-1(37)) had a phenotype resembling that of a multipotential stem or progenitor cell, being positive for both Sca-1 and B220 as well as CD4 and/or Thy1 and/or Gr1 (Figure 4B; supplemental Table 2). In addition, there were pre-B (12 of 30), B (3 of 30), T (1 of 30), and myeloid (3 of 30) tumors. Of the tumors tested, which included all phenotypes, 14 of 22 tumors—6 of 8 Mcl-1(33), 4 of 7 Mcl-1(4), 2 of 3 Mcl-1(8), and 2 of 4 Mcl-1(37)—were transplantable into nonirradiated C57BL/6J mice, where they recapitulated the original disease, infiltrating multiple organs. The transplantable tumors were aggressive, with mice becoming moribund on average 32 days after inoculation. Western blot analysis of 9 transplanted tumors from the 4 Mcl-1 lines confirmed that each expressed the transgene, at a level comparable with that detected in spleen cell lysates of premalignant Mcl-1(33) mice (not shown).
Bim restrains the impact of Mcl-1 overexpression
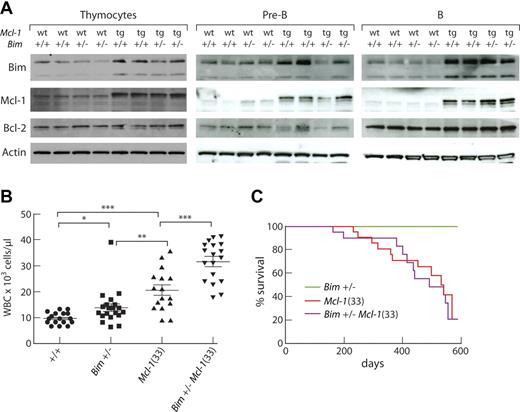
The BH3-only protein Bim is a major regulator of homeostasis in the lymphoid system.40 To determine whether Bim is an important target for the transforming function of Mcl-1, we crossed Mcl-1(33) and Bim−/− mice and monitored their progeny. Of note, Bim was present at higher levels in the thymocytes, pre-B cells, and B cells of Mcl-1 transgenic mice than their C57BL/6J littermates, which implies that higher levels of Mcl-1 are permissive for greater accumulation of Bim (Figure 5A). Because the level of Bim did not diminish with Bim heterozygosity, in either WT or transgenic mice (Figure 5A), there must be quite a dynamic range for regulating Bim expression, whether by transcriptional or posttranslational mechanisms or both. By 4 weeks of age, Bim+/−Mcl-1 transgenic mice had significantly higher white cell counts than their Bim+/− or Mcl-1 transgenic littermates (Figure 5B), resulting from a further increase in circulating lymphocytes (not shown). Nevertheless, the Bim+/−Mcl-1 mice did not die of tumors at an earlier age, the median onset of morbidity being 491 days (Figure 5C). Two of 10 tumors analyzed had the same primitive phenotype as those that develop in Bim+/+Mcl-1(33) mice and, with the exception of one T lymphoma, the remainder were pre-B lymphomas (supplemental Table 3). The difficulty of breeding significant numbers of Bim−/− VavP-Mcl-1 mice (“Mice”) unfortunately thwarted our attempt to ascertain the impact of homozygous loss of Bim.
Bim restrains the impact of Mcl-1 overexpression on hematopoietic homeostasis. (A) Western blot analysis of Bim expression in thymocytes, pre-B cells (B220+ IgM− IgD− CD43− BM cells), and B cells (B220+ LN cells) from 6- to 8-week-old WT, Bim+/−, Mcl-1(33), and Bim+/−Mcl-1(33) mice; each lane represents an individual mouse and results are typical of 2 experiments. (B) Peripheral white blood cell counts of 4-week-old mice. Points represent individual mice. Bars represent mean ± SEM. *P < .05, **P < .01, ***P < .001 (Student t test). (C) Kaplan-Meier survival curve of tumor-related deaths in cohorts of Bim+/− mice (olive, n = 8); Mcl-1(33) mice (red, n = 23), median survival, 531 days; and Bim+/−Mcl-1(33) mice (magenta n = 20), median survival, 491 days.
Bim restrains the impact of Mcl-1 overexpression on hematopoietic homeostasis. (A) Western blot analysis of Bim expression in thymocytes, pre-B cells (B220+ IgM− IgD− CD43− BM cells), and B cells (B220+ LN cells) from 6- to 8-week-old WT, Bim+/−, Mcl-1(33), and Bim+/−Mcl-1(33) mice; each lane represents an individual mouse and results are typical of 2 experiments. (B) Peripheral white blood cell counts of 4-week-old mice. Points represent individual mice. Bars represent mean ± SEM. *P < .05, **P < .01, ***P < .001 (Student t test). (C) Kaplan-Meier survival curve of tumor-related deaths in cohorts of Bim+/− mice (olive, n = 8); Mcl-1(33) mice (red, n = 23), median survival, 531 days; and Bim+/−Mcl-1(33) mice (magenta n = 20), median survival, 491 days.
Elevated Mcl-1 accelerates Myc-driven lymphomagenesis
Inhibition of apoptosis accelerates Myc-driven lymphomagenesis, as first shown by overexpressing Bcl-2 in lymphoid cells in Eμ-Myc transgenic mice.41 When VavP-Mcl-1 transgenic mice were crossed with Eμ-Myc mice, all bitransgenic offspring died very rapidly of tumors, the median age of survival being only 30.5 days versus 94 days for Eμ-Myc littermates (Figure 6A). Most of these tumors were B lymphomas (CD19+B220+IgM+; Figure 6B; supplemental Table 4). At autopsy, the enlargement of the spleen and LNs in the Mcl-1/Myc mice relative to their body weight was comparable with that of moribund Myc littermates, but the bitransgenic mice had much higher white blood cell counts (mean of 477 × 106/mL compared with a mean of 65 × 106/mL). The histopathology of the bitransgenic tumors (Figure 4G,H) was comparable with that of the Myc tumors. Both were more aggressive than Mcl-1 tumors; the cells were larger, more mitotic cells were apparent, and leukemic infiltration of the heart and marrow was more common (4 of 4 Mcl-1/Myc and 3 of 3 Myc mice versus 1 of 12 Mcl-1 mice). In addition, the leukemic cells infiltrated and thickened the alvelolar walls in the lungs, instead of accumulating in solid masses as in Mcl-1 transgenic mice (compare Figure 4H with Figure 4D,F).
Mcl-1 synergizes with Myc in lymphomagenesis. (A) Kaplan-Meier plot of tumor-related deaths in Eμ-Myc/Mcl-1(33) mice (red, n = 6), median survival, 30.5 days and Eμ-Myc transgenic mice (black, n = 34), median survival, 94 days. Survival curves are significantly different (P < .0001). (B) Dot plot showing immunophenotype of a representative B-cell tumor from an Eμ-Myc/Mcl-1(33) mouse. (C) Graph of percentage pre-B cells (B220+ CD19+ IgM− IgD−) in E18 blood; genotypes as indicated. Blood was isolated from the jugular vein of E18 embryos and red cells depleted before staining with cell surface markers for flow cytometry. **P<.01 ; ***P<.001. (D) Dot plot examples of E18 white blood cell analysis. Numbers at bottom of quadrants indicate mean percentage pre-B cells (B220+ CD19+ IgM− IgD−) ± SEM (n = 8 WT; 3 VavP-Mcl-1(33); 17 Eμ-Myc; 11 VavP-Mcl-1/Eμ-Myc). (E) Kaplan-Meier plot of tumor-related deaths for lethally irradiated C57BL/6J mice reconstituted with 2 × 106 fetal liver cells (1-3 recipients per fetal liver) from Eμ-Myc/Mcl-1(33) mice (red, n = 5), 12 recipients, median survival, 41 days and Eμ-Myc mice (black, n = 7), 16 recipients. Survival curves are significantly different (P < .0001). (F) Dot plot showing immunophenotype of a representative stem/progenitor cell tumor arising after transplantation of Eμ-Myc/Mcl-1(33) fetal liver cells.
Mcl-1 synergizes with Myc in lymphomagenesis. (A) Kaplan-Meier plot of tumor-related deaths in Eμ-Myc/Mcl-1(33) mice (red, n = 6), median survival, 30.5 days and Eμ-Myc transgenic mice (black, n = 34), median survival, 94 days. Survival curves are significantly different (P < .0001). (B) Dot plot showing immunophenotype of a representative B-cell tumor from an Eμ-Myc/Mcl-1(33) mouse. (C) Graph of percentage pre-B cells (B220+ CD19+ IgM− IgD−) in E18 blood; genotypes as indicated. Blood was isolated from the jugular vein of E18 embryos and red cells depleted before staining with cell surface markers for flow cytometry. **P<.01 ; ***P<.001. (D) Dot plot examples of E18 white blood cell analysis. Numbers at bottom of quadrants indicate mean percentage pre-B cells (B220+ CD19+ IgM− IgD−) ± SEM (n = 8 WT; 3 VavP-Mcl-1(33); 17 Eμ-Myc; 11 VavP-Mcl-1/Eμ-Myc). (E) Kaplan-Meier plot of tumor-related deaths for lethally irradiated C57BL/6J mice reconstituted with 2 × 106 fetal liver cells (1-3 recipients per fetal liver) from Eμ-Myc/Mcl-1(33) mice (red, n = 5), 12 recipients, median survival, 41 days and Eμ-Myc mice (black, n = 7), 16 recipients. Survival curves are significantly different (P < .0001). (F) Dot plot showing immunophenotype of a representative stem/progenitor cell tumor arising after transplantation of Eμ-Myc/Mcl-1(33) fetal liver cells.
Remarkably, however, the proportion of VavP-mcl-1/Eμ-Myc mice found at weaning was considerably lower than expected. Of 88 mice, obtained from 11 different mating pairs, only 6 (7%) were doubly transgenic, considerably less than the expected 22 (25%). To investigate whether bitransgenic mice were dying during development, timed matings were set up and embryos genotyped at E13 and E18. Bitransgenic offspring were found at near expected Mendelian frequency at both these stages (7 of 40, 17.5% at E13; and 13 of 46, 28% at E18), suggesting that the premature deaths were occurring just before birth or shortly thereafter. Subsequent analysis of newborn pups indicated that most deaths occurred within a few hours of birth. The bitransgenic E18 embryos had a greatly elevated proportion of pre-B cells (B220+CD19+Ig−) in their blood compared with the other embryos (29.7% ± 3.3% versus 14.3% ± 1.3% for Eμ-Myc, 4.6% ± 1.5% for VavP-Mcl-1 and 6.7% ± 1.6% for WT embryos; Figure 6C-D), and this may well have been responsible for their early demise, although histologic examination failed to detect significant foci of lymphoma cells in the liver, lungs, kidney, or thymus (not shown).
Transplantation tests were performed by injecting 1 to 5 μL of blood from E18 embryos into nonirradiated WT mice. Of 13 VavP-Mcl-1/ Eμ-Myc E18 embryos tested, 11 have already given rise to tumors (median, 89 days); and notably, 9 of 9 analyzed had the stem/progenitor cell phenotype (CD4+B220+). In contrast, none of the mice transplanted with blood cells from Eμ-Myc embryos (n = 12) has died of tumors.
Mcl-1 promotes transformation of transplanted hematopoietic stem cells
As another approach to determining the impact of overexpression of Mcl-1 on Myc- driven lymphomagenesis, fetal liver cells from E13 VavP-Mcl-1/Eμ-Myc and Eμ-Myc Ly5.2 C57BL/6J embryos were transplanted into lethally irradiated Ly5.1 C57BL/6J recipients. Strikingly, every mouse transplanted with Mcl-1/Myc fetal liver cells developed terminal lymphomas within 51 days (median, 41 days; Figure 6E), whereas only 3 of those transplanted with Eμ-Myc fetal liver cells has become ill (6 months thus far; previous data suggest that only 50% will die by 12 months42 ). Furthermore, each of the 12 tumors that developed from 6 independent Mcl-1/ Myc E13 fetal livers had a primitive stem/progenitor cell phenotype (Figure 6F; supplemental Table 4), whereas those arising from transplanted Myc fetal liver cells were B lymphoid tumors. Each of the 4 Mcl-1/Myc tumors tested was transplantable into nonirradiated recipient mice.
Mcl-1 overexpression in lymphomas increases resistance to in vivo treatment with cyclophosphamide
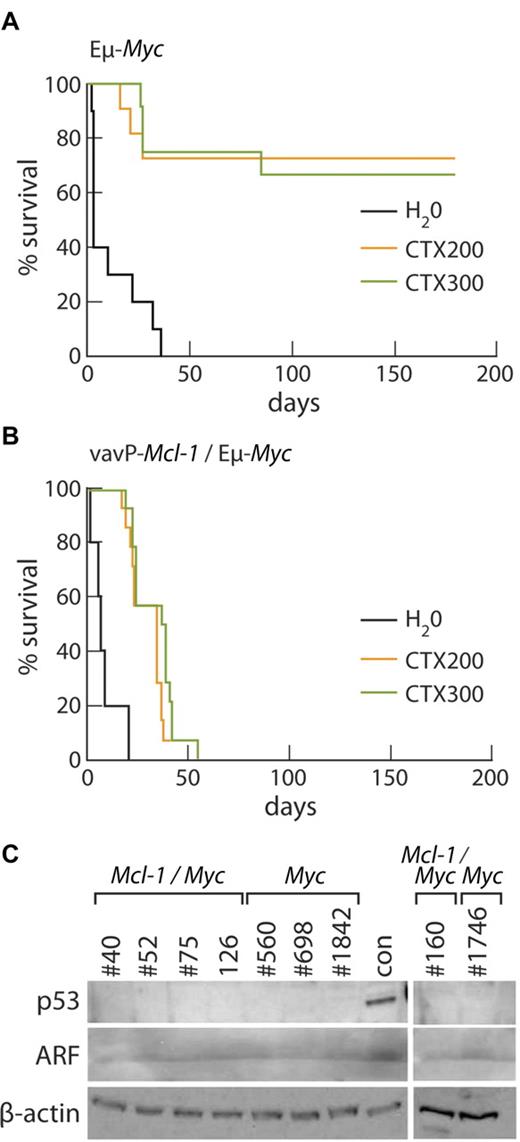
To test the impact of overexpression of Mcl-1 on chemosensitivity, tumor cells from 5 primary Mcl-1/Myc and 4 Myc B lymphoid tumors were transplanted into multiple nonirradiated C57BL/6J recipients; and once the lymphomas became palpable, the tumor-bearing mice were treated with a single high dose of cyclophosphamide (200 or 300 mg/kg) or vehicle alone (water). Survival data are presented as Kaplan-Meier plots (Figure 7A-B). The Mcl-1/Myc tumors showed a uniformly poor response to cyclophosphamide (Figure 7B), none of the treated mice surviving beyond 55 days, whereas most of the mice transplanted with Myc lymphomas treated with cyclophosphamide survived for at least 180 days (Figure 7A). The median survival of treated Mcl-1/Myc tumor-bearing mice was significantly less than that of treated Myc tumor-bearing mice: P = .001 at 200 mg/kg; P = .0002 at 300 mg/kg. Lymphomas arising in Eμ-Myc mice frequently contain mutations within the p53/ARF pathway, which increase their chemoresistance.43,44 None of the Mcl-1/Myc tumors appeared to have p53/ARF mutations, as evidenced by the finding that they did not express high levels of p53 or ARF proteins, and neither did the Myc tumors chosen for comparison (Figure 7C).
Mcl-1 confers resistance to cyclophosphamide therapy in vivo. (A) Kaplan-Meier survival curves of Eμ-Myc tumor-bearing mice treated with water (black, n = 10), median survival, 3 days; 200 mg/kg cyclophosphamide (orange, n = 11); or 300 mg/kg cyclophosphamide (green, n = 12). Data from 4 independent Eμ-Myc tumors (1 pre-B, #1746 and 3 B-cell tumors, #698, #560, and #1842), each of which was transplanted into a total of 7 of 10 recipients. (B) Kaplan-Meier survival curves of Mcl-1(33)/Eμ-Myc tumor-bearing mice treated with water (black, n = 10), median survival, 7 days; 200 mg/kg cyclophosphamide (orange, n = 14), median survival, 35 days; and 300 mg/kg cyclophosphamide (green, n = 14), median survival, 38 days. Data from 5 independent Mcl-1/Eμ-Myc tumors (1 pre-B cell, #160 and 4 B-cell tumors, #40, #52, #75, and #126), each of which was transplanted into a total of 8 recipients. The median survival of treated Mcl-1/Myc tumor-bearing mice was significantly less than that of treated Myc tumor-bearing mice: P = .001 at 200 mg/kg; P = .0002 at 300 mg/kg. (C) Western blot analysis showed that, unlike the control tumor (con) having a mutation affecting the p53-ARF pathway, none of the Mcl-1/Myc or Myc tumors used in treatment studies shown in panels A and B overexpresses p53 or ARF protein.
Mcl-1 confers resistance to cyclophosphamide therapy in vivo. (A) Kaplan-Meier survival curves of Eμ-Myc tumor-bearing mice treated with water (black, n = 10), median survival, 3 days; 200 mg/kg cyclophosphamide (orange, n = 11); or 300 mg/kg cyclophosphamide (green, n = 12). Data from 4 independent Eμ-Myc tumors (1 pre-B, #1746 and 3 B-cell tumors, #698, #560, and #1842), each of which was transplanted into a total of 7 of 10 recipients. (B) Kaplan-Meier survival curves of Mcl-1(33)/Eμ-Myc tumor-bearing mice treated with water (black, n = 10), median survival, 7 days; 200 mg/kg cyclophosphamide (orange, n = 14), median survival, 35 days; and 300 mg/kg cyclophosphamide (green, n = 14), median survival, 38 days. Data from 5 independent Mcl-1/Eμ-Myc tumors (1 pre-B cell, #160 and 4 B-cell tumors, #40, #52, #75, and #126), each of which was transplanted into a total of 8 recipients. The median survival of treated Mcl-1/Myc tumor-bearing mice was significantly less than that of treated Myc tumor-bearing mice: P = .001 at 200 mg/kg; P = .0002 at 300 mg/kg. (C) Western blot analysis showed that, unlike the control tumor (con) having a mutation affecting the p53-ARF pathway, none of the Mcl-1/Myc or Myc tumors used in treatment studies shown in panels A and B overexpresses p53 or ARF protein.
Discussion
Mcl-1 is the most divergent of the antiapoptotic members of the Bcl-2 family and, unlike Bcl-2, can interact with the BH3-only protein Noxa16,17 and sequester proapoptotic Bak.18,19 We were keen, therefore, to compare the impact of overexpression of Mcl-1 and Bcl-2 during hematopoiesis. Accordingly, we generated VavP-Mcl-1 transgenic mice and compared their phenotype with that previously described for VavP-BCL-2 mice.36,39
Hematopoiesis was significantly perturbed in the VavP-Mcl-1 transgenic mice, particularly in the mature lymphoid compartments (both B and T; Table 1). Young mice developed splenomegaly and lymphadenopathy, with B lymphoid cells, CD4+ T cells, and CD8+ T cells elevated 2- to 3-fold. These changes are similar to those reported previously for young mice expressing a human MCL-1 minigene2 and similar, albeit less marked, than those found in VavP-BCL-2 mice.36 Interestingly, however, the VavP-Mcl-1 mice did not exhibit the deficit in platelets or CD4+CD8+ thymocytes characteristic of VavP-BCL-2 mice.36 The increased lymphoid cellularity in VavP-Mcl-1 transgenic mice is the result of enhanced survival rather than enhanced proliferation, as also concluded for B and T lymphoid cells overexpressing Bcl-2.45,46
Both immature and mature lymphocytes (T and B) from VavP-Mcl-1 mice were more robust than their normal counterparts when challenged in vitro with a variety of cytotoxic insults (Figure 2; supplemental Figures 2, 3). The BH3-only protein Bim, a proapoptotic member of the wider Bcl-2 family, appears to restrain the impact of Mcl-1 overexpression because mice lacking one Bim allele developed higher white cell counts (Figure 5B). Nevertheless, Bim haploinsufficiency did not accelerate tumor onset in VavP-Mcl-1 mice (Figure 5C), as it did in the context of Eμ-Myc-driven lymphomagenesis.47
Granulocytes from VavP-Mcl-1 transgenic mice showed increased resistance in vitro to cytokine deprivation and the DNA-damaging agent etoposide, and their spleen colony-forming stem cells were more resistant to γ-irradiation (Figure 3). Nevertheless, as in VavP-BCL-2 mice,36 myelopoiesis was not grossly perturbed, indicating that other mechanisms can override inhibition of the mitochondrial apoptosis pathway to ensure myeloid homeostasis.
The pathologic consequences of overexpression of Mcl-1 during hematopoiesis differed markedly from that found previously for Bcl-2. Whereas VavP-BCL-2 mice had high levels of autoantibodies and many developed autoimmune kidney disease,39 the level of autoantibodies in VavP-Mcl-1 mice was no higher than in WT mice, and none has developed the systemic lupus erythematosus-like autoimmune kidney syndrome (not shown).
Like VavP-BCL-2 mice, VavP-Mcl-1 mice are susceptible to lymphoma development. However, strikingly, whereas VavP-BCL-2 mice developed follicular lymphoma,39 VavP-Mcl-1 mice frequently died of a lymphoma with a phenotype resembling a hematopoietic stem/progenitor cell, being simultaneously positive for cell surface antigens characteristic of B lymphoid, T lymphoid, and/or myeloid lineages (Figure 4). Other tumor types arising in VavP-Mcl-1 mice included pre-B, B, and, less frequently, myeloid tumors (supplemental Table 2). This tumor spectrum differs from that reported for transgenic mice expressing an MCL-1 minigene, which primarily developed clonal B-cell lymphomas, including follicular lymphoma and diffuse large-cell lymphoma.3 Differences in the regulatory sequences driving the 2 Mcl-1 transgenes presumably contribute to their distinctive impact. The differential outcome for the VavP-driven transgenes, however, implies a functional difference between Bcl-2 and Mcl-1, perhaps reflective of cell type-specific differences in the stability of these prosurvival proteins and/or differences in their binding pattern to proapoptotic family members. Notably, as a single genetic change, overexpression of Mcl-1 appears to render stem and progenitor cells more susceptible to transformation than overexpression of Bcl-2.
Inhibition of apoptosis accelerates Myc-driven lymphomagenesis, as was first shown by overexpressing Bcl-2 in lymphoid cells in Eμ-Myc transgenic mice.41 Myc-driven lymphomagenesis is also accelerated by overexpression of Mcl-1. All bitransgenic VavP-Mcl-1/Eμ-Myc offspring that survived past weaning died within 5 weeks of age (median, 30.5 days) because of aggressive disseminated B- or pre-B cell lymphoma accompanied by leukemia (Figure 6A). Most, however, died around birth, their frequency dropping from approximately 25% at E18 to only approximately 7% at weaning. The E18 embryos appeared normal histologically but had abnormally high levels of pre-B cells in their blood (Figure 6C-D), and this may well have contributed to the perinatal lethality. Nevertheless, transplantation of E18 blood did not result in pre-B (or B) cell tumors. Instead, strikingly, the outcome was always a stem/progenitor cell tumor. Presumably malignant stem cells were already present in the blood of the bitransgenic embryos, albeit at levels too low to be readily detected by flow cytometry. These findings indicate that the progression of pre-B and B cells to full malignancy was dependent on the acquisition of further synergistic mutation(s) and raise the possibility that, for primitive, stem-like hematopoietic cells, no further mutation was necessary.
Our results differ from those reported in another recent study in which an H2K-Mcl-1 transgene provoked early onset pre-B lymphomas in Eμ-Myc mice,31 and we assume the difference is primarily because of the timing and/or level of Mcl-1 expression achieved.
Our conclusion that Myc plus Mcl-1 is a highly potent oncogenic combination for stem/progenitor cells was further substantiated in a hematopoietic reconstitution setting: Every lethally irradiated mouse transplanted with VavP-Mcl-1/Eμ-Myc fetal liver cells died within 7 weeks of a stem/progenitor cell tumor (Figure 6E-F).
Stem/progenitor cell tumors were also the invariable outcome in Eμ-Myc mice coexpressing Eμ-driven Bcl-2,41 Eμ-driven Bcl-xL,48 and vavP-driven BCL-2 (K.J.C. and S.C., unpublished data, April 2010), although, intriguingly, perinatal lethality was not a feature of any of these other crosses. Indeed, to our knowledge, the only other example of such a dramatic acceleration of Eμ-Myc-driven lymphomagenesis has been with overexpression of the oncoprotein Pim-1, a ser/thr kinase, which provoked disseminated transplantable pre-B ALL in utero.49
Mcl-1 overexpression rendered B lymphoid tumors highly resistant to treatment in vivo with cyclophosphamide, an agent in common clinical use (Figure 7). Furthermore, the resistance was as great as we have previously determined in vivo for tumors from Eμ-BCL-2/ Eμ-Myc33 and VavP-BCL-2/Eμ-Myc tumors (K.J.C. and S.C., unpublished data, April 2010). In contrast, a recent short-term in vitro study comparing tumor cell lines derived from H2K-Mcl-1/Eμ-Myc mice and tet-BCL-2/Eμ-Myc mice concluded that Mcl-1-dependent leukemia cells are more sensitive to chemotherapy than Bcl-2-dependent counterparts.31 The apparent discrepancy may indicate that a lower level of Mcl-1 expression was achieved with the H2K-Mcl-1 than the VavP-Mcl-1 transgene. However, at least 2 of the 3 BCL-2/Eμ-Myc cell lines used in that study had extremely high p19ARF levels,31 indicative of lesions in the p53/ARF pathway that would further enhance resistance and therefore set a high standard of drug resistance with which to compare the effect of Mcl-1 overexpression.
In recent years, Bcl-2 has been center stage in studies of the impact of antiapoptotic proteins in tumorigenesis. Our results suggest, however, that Mcl-1 may be even more potent. Certainly, elevated expression of Mcl-1 is associated with poor prognosis and drug resistance in a wide variety of human tumors,4-9 and MCL-1 amplification is common in diverse malignancies.10 The mice and tumors developed in this study should facilitate preclinical studies of new therapeutic approaches to tackling Mcl-1-overexpressing malignancies.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank their colleagues A. Strasser, A. Ng, P. Hodgkin, L. O'Reilly, J. Adams, and D. Huang for useful discussions; L. O'Reilly, A. Strasser, and D. Huang for reagents; N. Anstee and J. Blyth for technical assistance; B. Helbert and C. Young for genotyping; J. Corbin for Advia blood readings; and K. McKenzie and G. Siciliano for excellent mouse husbandry and WEHI FACS and histology services.
This work was supported by postdoctoral fellowships from EMBO and the Human Frontier in Science Program (K.J.C.), National Health and Medical Research Council (Australia; program grant 461221), Leukemia & Lymphoma Society (SCOR 7015-02), National Cancer Institute (CA43540), and the Walter and Eliza Hall Institute from the National Health and Medical Research Council, Victorian Government, and Australian Cancer Research Foundation (infrastructure grants).
National Institutes of Health
Authorship
Contribution: K.J.C. and S.C. conceived the studies, planned experiments, analyzed data, and wrote the manuscript; K.J.C., C.J.V., and M.L.T. performed experiments; M.L.B. produced the primary vavP-Mcl-1 mice by pronuclear microinjection; D.M. analyzed histopathology; P.B. provided reagents and advice; and C.L.S. advised on experimental protocols and data analysis.
Conflict-of-interest disclosure: S.C., K.J.C., C.J.V., C.L.S., and P.B. are engaged in a research collaboration with Genentech and Abbott to develop novel cancer therapeutics but have not themselves received research funding or financial incentives. The remaining authors declare no competing financial interests.
Correspondence: Suzanne Cory, Molecular Genetics of Cancer Division, Walter & Eliza Hall Institute of Medical Research, 1G Royal Parade, Parkville 3052, Victoria, Australia; e-mail: cory@wehi.edu.au.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal