Abstract
Immature dendritic cells (DCs) specialize in antigen capture and maintain a highly dynamic pool of intracellular major histocompatibility complex class II (MHCII) that continuously recycles from peptide loading compartments to the plasma membrane and back again. This process facilitates sampling of environmental antigens for presentation to T helper cells. Here, we show that a signaling pathway mediated by the DC immunoreceptor tyrosine-based activation motif (ITAM)–containing adaptors (DAP12 and FcRγ) and Vav family guanine nucleotide exchange factors controls the half-life of surface peptide-MHCII (pMHCII) complexes and is critical for CD4 T-cell triggering in vitro. Strikingly, mice with disrupted DC ITAMs show defective T helper cell priming in vivo and are protected from experimental autoimmune encephalitis. Mechanistically, we show that deficiency in ITAM signaling results in increased pMHCII internalization, impaired recycling, and an accumulation of ubiquitinated MHCII species that are prematurely degraded in lysosomes. We propose a novel mechanism for control of T helper cell priming.
Introduction
Dendritic cells (DCs) possess a unique capacity to sample their environment and present exogenous antigens on major histocompatibility complex class II (MHCII) for subsequent priming of antigen-specific naive T lymphocytes.1-3 In the absence of maturation stimuli or danger signals, immature DCs continuously sample their environment by constitutive macropinocytosis and phagocytosis,4-6 while maintaining a dynamic intracellular pool of MHCII for peptide loading.7 Although immature DCs perform a critical role in immune surveillance and are competent to activate naive T cells,8,9 peptide-MHCII (pMHCII) complexes are labile as a result of rapid recycling and degradation.7,10,11 Upon maturation, DCs down-regulate antigen uptake and pMHCII recycling, leading to the accumulation of specific pMHCII complexes on the plasma membrane, thus promoting stable interactions with antigen-specific T cells.7,10,12,13
Despite intense study, the exact molecular mechanisms regulating MHCII trafficking remain incompletely understood. Although the MHCIIβ chain contains a conserved dileucine motif that promotes clathrin-mediated endocytosis, this motif may not be strictly required for internalization.14 Recently, internalization and sorting of MHCII were shown to be regulated by the MARCH ubiquitin ligases, which polyubiquitinate MHCIIβ chains.15-19 After ubiquitination, it is thought that MHCII internalization may require association with ubiquitin-binding clathrin adaptors that promote endocytosis from the plasma membrane and targeting to luminal vesicles within multivesicular bodies, a fate that culminates in lysosomal degradation of MHCII.13 However, it remains unclear at which intracellular locations MHCII is ubiquitinated/deubiquitinated and precisely how this directs MHCII trafficking. In an alternative model, MHCII is thought to be internalized in a clathrin- and dynamin-independent manner in lipid microdomains, perhaps by Arf6-mediated endocytosis.20-22 While these 2 models are not mutually exclusive, little is known about the signal transduction pathways that regulate pMHCII trafficking and antigen presentation.
Recent work demonstrated that immunoreceptor tyrosine-based activation motif (ITAM) signaling downstream of several adhesion receptors, including integrins, is essential for effector functions in neutrophils and DCs.23-25 In this context, integrin-mediated adhesion can lead to the activation of Src family kinases, which phosphorylate ITAMs in the adaptor molecules DAP12 and FcRγ26,27 that serve as docking sites to recruit the tyrosine kinase Syk through its tandem SH2 domains.23,28-30 In turn, Syk phosphorylates several substrates orchestrating the assembly of a signaling complex in which SLP-7631 and Vav proteins32 are essential components for initiating cellular responses associated with inflammation.24,25,33 However, ITAM signaling in DCs can also inhibit inflammatory responses.34,35 For example, DAP12 and FcRγ deficiency was shown to augment in vitro maturation of DCs stimulated with Toll-like receptor ligands and enhance presentation of exogenous antigen on MHCII for activation of CD4 T cells,29 even though DAP12 deficiency appears to at least partially protect mice from central nervous system inflammation in experimental autoimmune encephalomyelitis (EAE).36 Thus, the precise role of ITAM signaling in DCs during T-cell priming and MHCII presentation remains unclear.
In this context, recent studies in DCs have demonstrated that active alkalization of the phagosome by the nicotinamide adenine dinucleotide phosphate oxidase NOX2, which itself depends on ITAM signaling, is required for cross-presentation of particulate antigens.23,24,37 Thus, the electrogenic activity of NOX2 is thought to delay the induction of lysosomal proteases, so that internalized particulate antigens are rescued from rapid degradation and remain available for loading onto MHCI after export into the cytoplasm and proteasomal processing. Such alkalization is transient and is followed by phagosome acidification, activation of lysosomal proteases, and generation of peptides loaded onto MHCII molecules. Hence, in this model, MHCI- and MHCII-restricted presentation of particulate antigens occurs sequentially, with cross-presentation taking place early at more neutral pH, followed by loading of MHCII at more acidic pH. Previously, we have shown that DCs lacking essential components of the ITAM signaling apparatus fail to undergo oxidative burst and cross-present particulate antigens on MHCI for activation of CD8 T cells.24 However, a potential involvement of this signaling pathway in MHCII antigen presentation remains to be determined.
Here, we identify a previously unknown function for ITAM signaling in DCs, which is independent of NOX2 and is critically required for efficient CD4 T-cell priming in vivo and in vitro. We propose a novel model of the regulatory mechanism of MHCII trafficking in immature DCs and demonstrate that ITAM signaling in DCs is critical for efficient priming of antigen-specific and autoimmune CD4 T cell by promoting salvage of pMHCII through a recycling pathway.
Methods
Mice
VavNULL mice have been previously described.38 DF mice (DAP12−/−FcRγ−/−) were provided by M. Colonna (Washington University). OT2 mice were a gift from H. Virgin (Washington University). WT B6 mice were purchased from The Jackson Laboratory. All animal procedures were performed in accordance with institutional guidelines and approved by the Animal Studies Committee at Washington University School of Medicine.
Antigen presentation assays
Bone marrow–derived DCs were cultured in granulocyte macrophage colony-stimulating factor previously described24 and were used on days 5-7 of culture. CD4-positive OT2 T cells were purified from spleens and lymph nodes by negative selection with MACS beads (Miltenyi Biotec) as per the manufacturer's suggestion. Purified T cells were then labeled with carboxyfluorescein succinimidyl ester (CFSE, Vybrant CFDA SE cell tracer kit; Invitrogen) as directed by the manufacturer. T cells (200 × 103) were mixed with DCs (50 × 103) in round-bottom 96-well plates with the indicated doses of OT2 peptide (a gift from Dr Paul Allen, Washington University) or ovalbumin (OVA)–coated latex beads (see Graham et al24 for protocol) for 3 days of culture. Alternatively, DCs were pulsed with OT2 peptide (100nM) in serum-free media for 2 hours at 37°C, washed thoroughly, and cultured at the indicated numbers with OT2 T cells (200 × 103) for 3 days. At the end of culture, T cells were stained with anti–Vα2-allophycocyanin (APC; clone B20.1; eBioscience), anti–CD4-APC (clone GK1.5; Becton Dickinson), and analyzed on a FACSCalibur flow cytometer (Becton Dickinson) with FlowJo software V8.8.1 (TreeStar). For analysis of activation marker expression, T cells (without CFSE label) and DCs were cocultured with antigen for 18 hours before staining with anti–CD25-fluorescein isothiocyanate (FITC, clone PC61.5; eBioscience) and anti–CD69-PE (clone H1.2F3; Becton Dickinson).
T-cell hybridoma activation
The B11 T-cell hybridoma specific for β-galactosidase (amino acids 429-441) peptide presented by I-Ab was a generous gift from Paul Allen (Washington University). Bone marrow–derived DCs were pulsed with β-galactosidase peptide in serum free medium for 4 hours at 37°C and then washed thoroughly. DCs were then either fixed immediately (0 chase) or cultured for 24 hours before fixation (24-hour chase). Fixation consisted of 0.008% glutaraldehyde at room temperature for 3 minutes followed by quenching and washing in PBS supplemented with glycine. Fixed DCs were then distributed at the indicated densities into 96-well round-bottom plates with B11 cells (105 cells/well). After overnight culture, supernatants were collected for determination of IL-2 production by enzyme-linked immunosorbent assay. Plates (Maxisorp; Nunc) were coated overnight at 4°C with capture antibody (1 μg/mL clone JES6-1A12; Biolegend) in phosphate-buffered saline (PBS), 0.1M NaHCO3. Plates were washed thoroughly in water and blocked (PBS, 0.5% bovine serum albumin [BSA], 0.1% Tween-20) for 1 hour. After washing, culture supernatants were added to plates and incubated at room temperature for 2 hours. Plates were washed and biotin-anti-interleukin-2 (IL-2, clone JES6-5H4; Biolegend) was added at 0.5 μg/mL in blocking buffer for 1 hour. Subsequently, excess antibody was removed by washing and streptavidin–horseradish peroxidase (HRP; Southern Biotechnology) diluted 1:10 000 was added to the plates for 30 minutes. After final washing, plates were developed with 1-Step Ultra TMB substrate (Thermo Scientific) as recommended by manufacturer and analyzed with a automated plate reader (TriStar LB 941; Berthold Technologies) equipped with a 450-nm filter.
OVA immunizations
B6 and DF mice (6- to 8-weeks old) were immunized with 10nM OVA323-339 peptide emulsified with complete Freund adjuvant (CFA; Sigma-Aldrich). Mice were injected subcutaneously with 50 μL of prepared emulsion into the hind footpad. At 7 days after footpad injection, the popliteal draining lymph nodes were harvested and disrupted into single cell suspensions in Dulbecco modified Eagle medium (DMEM) supplemented with 10% fetal calf serum (Atlanta Biologicals), 2mM l-glutamine, 1mM sodium pyruvate, 100 U/mL penicillin and streptomycin, 1 mL/100 mL media of a 100× concentrated nonessential amino acid solution (all from Invitrogen), and 50μM 2-mercaptoethanol (Sigma-Aldrich).
Proliferation assays
Cells were cultured in Microtest U-bottom 96-well tissue culture plates from Becton Dickinson Labware at a concentration of 2 × 105 cells/well and stimulated in triplicate with the indicated doses of myelin oligodendrocyte glycoprotein (MOG) amino acids 35-55 or OVA323-339 peptides. As a positive control, cells were stimulated with Concanavalin A (2.5 μg/mL). During the last 18 hours of the 3-day culture period, 0.5-1 μCi 3H-thymidine was added to each well. At the end of culture, cells were harvested with Micro96 Harvester (Molecular Device) on glass fiber filter (Wallac) and then dried and counted with MicroBeta Trilux 1450 LSC & Luminescence Counter Wallac (PerkinElmer) using dedicated software (Wallac 1450 MicroBeta for Windows Workstation, Version 4.01.014).
ELISPOT assays
For ELISPOT analysis, MultiScreen-IP sterile 96-well filtration plates (with 0.45-μm hydrophobic high protein binding Immobilon-P membrane) were purchased from Millipore Corporation. Plates were initially prewet with 70% ethanol, washed with PBS, then coated with primary (capture) anti–mouse IL-2 or interferon-γ (IFN-γ) antibody (both from Becton Dickinson) at a final concentration of 5 μg/mL in PBS for incubation overnight at 4°C. Cells (from spleens or draining popliteal lymph nodes) were cultured in triplicate at a concentration of 2 × 105/well with MOG or OVA323-339 peptides at the indicated doses. Cells were incubated for 20 hours at 37°C with 5% CO2 concentration. After overnight culture, plates were extensively washed with distilled water and blocked with 1% BSA (from Fisher Scientific) in PBS for 1 hour at room temperature. Subsequently, the secondary (detection) biotinylated anti–mouse IL-2 or IFN-γ antibody (at a final concentration of 2 μg/mL in 1% BSA/PBS) together with streptavidin–Alkaline phosphatase (AKP) from Becton Dickinson (1000× diluted in 1% BSA/PBS) was applied and left for 1 hour at room temperature. Plates were extensively washed with distilled water, and Sigma Fast BCIP/NBT (Sigma-Aldrich) was added as per manufacturer's recommendations. Plates were then left to dry overnight at room temperature, and the number of spots was calculated with an ELISPOT reader provided by Cellular Technology Ltd using dedicated Immunospot software Version 5.0.9 for ELISPOT analysis.
Experimental autoimmune encephalitis
Mice were immunized on day 0 by subcutaneous injection with 50 μg of MOG emulsified in incomplete Freund adjuvant containing 50 μg of Mycobacterium tuberculosis (strain H37RA). Intravenous administration of 300 ng of pertussis toxin (List Biological Laboratories) occurred on days 0 and 3. Mice were subsequently evaluated daily and graded for development of EAE. Clinical scores of 1-5 were assigned as follows: grade 1, tail weakness; grade 2, hind limb weakness sufficient to impair righting; grade 3, one limb plegic; grade 4, hind limb paralysis; and grade 5, moribund. Spleens were harvested from immunized mice on days 21-25 after immunization, and single-cell suspensions were made after red blood cell lysis. Proliferation assays were set up in 96-well flat-bottom plates with 2.5 × 105 cells/well and 20 μg/mL MOG peptide in DMEM supplemented with 10% FBS. Tritiated thymidine at 0.5 μCi/well was added to all wells 18 hours before harvest and analyzed as described above in proliferation assays.
Metabolic labeling of MHCII
Bone marrow–derived DCs were starved in Met/Cys-free media at 37°C for 30 minutes before pulsing with 0.5 mCi [35S] labeling mix (Perkin Elmer) for 30 minutes. For each sample, 5 × 106 cells were washed and chased for the indicated time points then lysed for 10 minutes on ice in 1 mL of lysis buffer (0.5% NP-40, 50mM Tris-HCl, 5mM MgCl2, supplemented with complete protease inhibitors [Roche], pH 7.4). Lysates were precleared with protein A/G sepharose (Pierce Biotechnology) and MHCII was immunuprecipitated with 4 μg anti-MHCII (clone Y3P; a gift from Emil Unanue, Washington University) rotating at 4°C for 2 hours. Subsequently, samples were washed thoroughly in lysis buffer and eluted in Laemmli sample buffer (2% sodium dodecyl sulfate [SDS], 2.5% 2-mercaptoethanol) at room temperature or by boiling for 5 minutes. Samples were then resolved by polyacrylamide gel electrophoresis (PAGE) on a 12% gel. The gels were fixed, impregnated with enhancing solution (Enhance; Perkin Elmer), dried, and exposed to film (Kodak Biomax MR).3 After developing films, densitometric measurement of band intensity (in units of mean integrated pixel density) was performed using ImageJ software (National Institutes of Health). The band intensity of pMHCII at 9 hours chase was normalized to that of 3 hours chase to express the extent of pMHCII degradation more than time.
MHCII surface retention
Dendritic cells were plated in 24-well plates at 1 million cells/mL and treated with or without Brefeldin A (GolgiPlug protein transport inhibitor at 1:1000; BD Biosciences). At the indicated time points (0, 3, 6, or 9 hours), cells were stained with CD11c-APC (BD Biosciences), I-Ab-FITC (BD Biosciences), or immunoglobulin G-FITC isotype control for analysis by fluorescence-activated cell sorting (FACS). CD11c positive cells were then analyzed for MHCII expression. The mean fluorescence intensity of MHCII at the given time point was then calculated and expressed as a percent of the mean fluorescence intensity at time = 0.
MHCII ubiquitination
Dendritic cells (5 × 106 per sample) were lysed for 10 minutes on ice in 1 mL lysis buffer (0.5% NP-40, 50mM Tris-HCl, 5mM MgCl2, 20mM N-ethylmaleimide, supplemented with complete protease inhibitors [Roche], pH 7.4). Lysates were precleared in protein A/G sepharose (Pierce Biotechnology) and immunoprecipitated with 3 μg of anti-MHCII (clone M5/114) by rotating at 4°C overnight. Samples were thoroughly washed in lysis buffer, eluted in Laemmli sample buffer by boiling, resolved by PAGE on a 10% gel, and transferred to polyvinylidene fluoride membranes (Millipore). Membranes were blotted with antibodies directed toward ubiquitin (clone P4D1; Santa Cruz Biotechnology) or MHCII β chain (clone KL295; ATCC). Primary antibodies were detected with HRP-coupled anti-mouse antibodies (Zymed) and developed using ECL (GE Healthcare) as recommended by the manufacturer.
MHCII recycling and internalization
Analysis of MHCII recycling was performed essentially as previously described.39 In summary, DCs were surface-biotinylated (50 × 106 cells/mL in PBS, pH 8.0) with Sulfo-NHS-SS-Biotin (Pierce Biotechnology) at a concentration of 0.5 mg/mL for 30 minutes on ice. Cells were washed with PBS 10mM glycine and chased for 20 minutes at 37°C in DMEM, at which point cells were stripped by 2 15-minute treatments on ice with MESNA buffer (100mM mercaptoethanesulphonic acid [Sigma-Aldrich], 50mM Tris, 100mM NaCl, 1mM EDTA [ethylenediaminetetraacetic acid], 0.2% BSA, pH 8.6). Cells were chased again at 37°C for the indicated time points before repeating the stripping procedure. Cells were then lysed for 5 minutes on ice in 1 mL of lysis buffer (0.5% NP-40, 50mM Tris-HCl, 5mM MgCl2, supplemented with complete protease inhibitors [Roche], pH 7.4). Lysates were mixed with protein A/G sepharose (Pierce Biotechnology) and immunoprecipitated with 2 μg of anti-MHCII (clone M5/114) by rotating at 4°C overnight. Samples were thoroughly washed in lysis buffer, eluted in nonreducing 2× Laemmli sample buffer, resolved by PAGE on a 10% gel, and transferred to polyvinylidene fluoride membranes. Membranes were then blotted with streptavidin-HRP and developed using ECL as recommended by the manufacturer. After developing the blots, densitometric measurment of band intensity (in units of mean integrated pixel density) was performed using ImageJ software. The relative amount of recycling MHCII that reemerged on the plasma membrane was determined as follows: 1 − (ITx/IT0), where ITx = band intensity at x minutes of chase, and IT0 = band intensity at 0 minutes chase. The amount of internalized MHCII relative to total surface MHCII was determined as follows: 100 × ([IT20 − IT0]/ITotal), where IT20 = band intensity at 20 minutes of chase, IT0 = band intensity at 0 minutes of chase, and ITotal = band intensity of total surface MHCII.
Statistical analysis
Data are expressed throughout as mean ± SD. datasets derived from the indicated genotypes were compared using the 2-tailed unpaired Student t test. Differences were considered statistically significant when P < .05.
Results
Requirement for ITAM signaling in presentation of particulate and soluble antigen by DCs
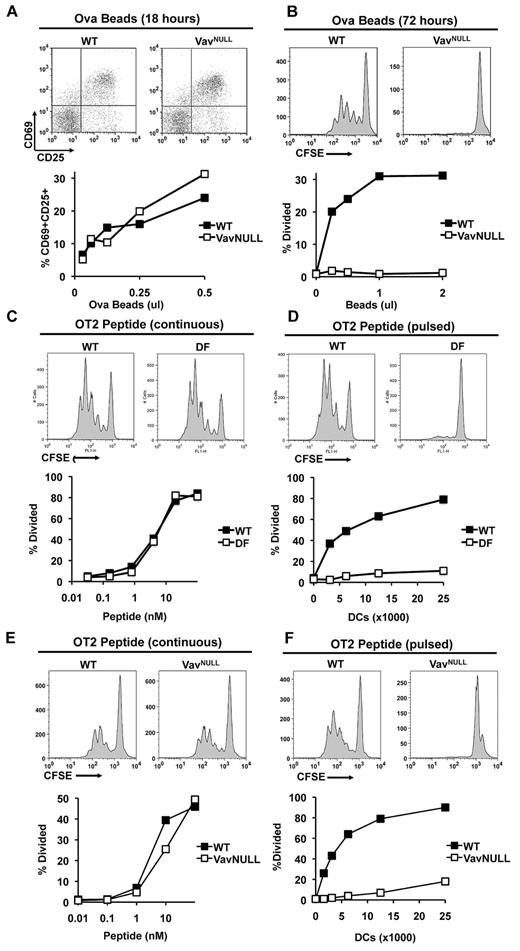
Several recent studies indicated that the ITAM-containing activating adaptors, DAP12 and FcRγ, can mediate both activating and inhibitory signals in myeloid cells (reviewed in Hamerman and Lanier34 and Turnbull and Colonna35 ). In this context, the ITAM-associated signal transduction complex including Syk, SLP-76, and Vav has recently emerged as a central regulatory pathway in myeloid cells.23,24 However, a potential requirement for the ITAM signaling module in MHCII presentation by DCs has not been conclusively addressed to date. To approach this issue, we used DCs from mice in which ITAM signaling was disrupted by deletion of DAP12 and FcRγ (DF) or deletion of all 3 Vav proteins (VavNULL). Wild-type (WT) and mutant (VavNULL or DF) DCs were incubated with OVA covalently attached to latex beads, and DCs were then cocultured with freshly isolated MHCII-restricted naive lymph node OT2 T cells. T-cell activation was analyzed by surface marker expression, and T-cell proliferation was analyzed by CFSE dye-dilution assays. In these experiments, we found that both WT and VavNULL DCs readily induced expression of activation markers, CD69 and CD25, on OT2 T cells (Figure 1A). Strikingly, however, only WT DCs induced a robust proliferative response of OT2 T cells, whereas VavNULL DCs appeared to lack this ability (Figure 1B). Similar to VavNULL, DF DCs also failed to induce OT2 T-cell proliferation (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Importantly, defective proliferation of OT2 T cells did not appear to result from diminished antigen uptake or diminished expression of MHCII and/or costimulatory receptors, as our analyses showed that surface MHCII was slightly elevated, while expression of B7.1 and B7.2 and bead internalization by VavNULL and DF DCs were similar to WT24,29 (supplemental Figure 2). In addition, coculture experiments showed that antigen-loaded WT DCs were capable of inducing OT2 proliferation in the presence or absence of antigen-loaded, or unloaded, DF or VavNULL DCs (supplemental Figure 3 and data not shown) indicating that their defects were intrinsic and did not affect OT2 T cells in trans. We also measured cytokine production in culture supernatants from these antigen presentation assays, which showed that production of IL-10 and IL-12 by VavNULL and DF DCs was similar to WT (supplemental Figures 4-5). Moreover, we found no differences in viability of VavNULL or DF DCs, compared with WT (supplemental Figure 6), and no evidence for the acquisition of a tolerogenic phenotype as evaluated by transcriptional profiling (data not shown). Thus, taken together, these data indicate an unexpected requirement for the intact ITAM signaling apparatus in DCs to induce CD4 T-cell proliferative responses to particulate antigens.
ITAM signaling is required for antigen presentation by DCs. WT and VavNULL DCs (50 000) were cultured with the indicated doses of OVA-coated latex beads and purified OT2 T cells. T cells were analyzed for expression of the activation markers CD69 and CD25 by FACS after 18 hours of stimulation (A), or analyzed for proliferation by CFSE dye dilution after 72 hours of stimulation (B). (C) WT and DF DCs (50 000) were cultured with the indicated doses of OVA peptide (continuous peptide) and purified CFSE-labeled OT2 T cells. Proliferation was determined by FACS for CFSE dye dilution on day 3 of culture. (D) Alternatively, WT and DF DCs were pulsed with a saturating dose of OVA peptide (100nM) for 2 hours and washed thoroughly (pulsed peptide). Subsequently, the indicated numbers of pulsed DCs were cultured with OT2 T cells for analysis of T-cell proliferation. Similarly, WT and VavNULL DCs were cultured with continuous peptide (E) or pulsed peptide (F) and analyzed for their ability to induce OT2 T-cell proliferation as described above. Data shown are representative of at least 5 independent experiments.
ITAM signaling is required for antigen presentation by DCs. WT and VavNULL DCs (50 000) were cultured with the indicated doses of OVA-coated latex beads and purified OT2 T cells. T cells were analyzed for expression of the activation markers CD69 and CD25 by FACS after 18 hours of stimulation (A), or analyzed for proliferation by CFSE dye dilution after 72 hours of stimulation (B). (C) WT and DF DCs (50 000) were cultured with the indicated doses of OVA peptide (continuous peptide) and purified CFSE-labeled OT2 T cells. Proliferation was determined by FACS for CFSE dye dilution on day 3 of culture. (D) Alternatively, WT and DF DCs were pulsed with a saturating dose of OVA peptide (100nM) for 2 hours and washed thoroughly (pulsed peptide). Subsequently, the indicated numbers of pulsed DCs were cultured with OT2 T cells for analysis of T-cell proliferation. Similarly, WT and VavNULL DCs were cultured with continuous peptide (E) or pulsed peptide (F) and analyzed for their ability to induce OT2 T-cell proliferation as described above. Data shown are representative of at least 5 independent experiments.
To determine whether ITAM-deficient DCs could induce OT2 T-cell proliferation in response to exogenously supplied antigenic peptides, which do not require processing by a DC and can be passively loaded onto MHCII, WT and DF DCs were incubated with varying doses of continuously supplied OVA323-339 peptide and their ability to induce OT2 T-cell proliferation was assessed using CFSE dilution assays. In these experiments, both WT and DF DCs readily induced proliferation of OT2 T cells (Figure 1C). However, when DCs were first pulsed with the OVA peptide for 2 hours and then washed to remove excess peptide before coculture with OT2 T cells, only WT, but not DF or VavNULL DCs, could induce OT2 T-cell proliferation (Figure 1D-F). These results suggest that in immature DCs, ITAM signaling is critically required for the maintenance and/or stability of surface pMHCII complexes.
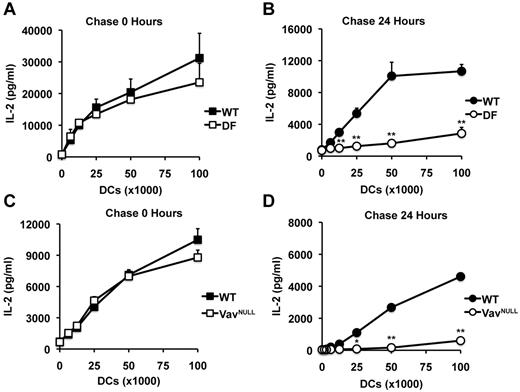
To directly test this scenario, we next used the B11 T-cell hybridoma specific for β-gal peptide presented by I-Ab, which produces IL-2 in a manner proportional to the concentration of specific pMHCII complexes, independently of costimulatory and cytokine signals. In these experiments, WT, DF, and VavNULL DCs were first pulsed with a saturating dose of β-gal peptide, the excess peptide was washed away, and DCs were fixed and incubated with B11 cells. Under these conditions, WT, DF, and VavNULL DCs induced similar levels of IL-2 production by B11 after 24 hours in culture (Figure 2A,C). Strikingly, however, when DCs were first pulsed with peptide, but then washed and chased in peptide-free media for 24 hours before fixation, we observed profound defects in antigen presentation by DF and VavNULL DCs, compared with WT (Figure 2B,D). Thus, in the absence of ITAM signals, specific pMHCII complexes expressed on the surface of immature DCs appear unstable. These results indicate that ITAM signals are critical for the ability of immature DCs to maintain surface pMHCII complexes for efficient induction of CD4 T-cell proliferation.
ITAM signaling is required for maintaining peptide-MHCII complexes on the surface of DCs. To assess the presence of pMHCII complexes on the surface of DCs, we used the B11 T-cell hybridoma specific for β-gal peptide presented by I-Ab. In these experiments, WT and DF DCs were pulsed with a saturating dose of peptide (10μM), washed, and immediately fixed (A) or chased for 24 hours before fixation (B). After fixation, DCs were cultured overnight with B11 cells, after which point, culture supernatants were analyzed for IL-2 by enzyme-linked immunosorbent assay. Similarly, WT and VavNULL DCs were pulsed with peptide and immediately fixed (C) or chased for 24 hours before fixation (D) and then used to stimulate B11 cells as described above. Data represent mean IL-2 production ± SD for triplicate samples and are representative of 5 independent experiments; *P < .05, **P < .01.
ITAM signaling is required for maintaining peptide-MHCII complexes on the surface of DCs. To assess the presence of pMHCII complexes on the surface of DCs, we used the B11 T-cell hybridoma specific for β-gal peptide presented by I-Ab. In these experiments, WT and DF DCs were pulsed with a saturating dose of peptide (10μM), washed, and immediately fixed (A) or chased for 24 hours before fixation (B). After fixation, DCs were cultured overnight with B11 cells, after which point, culture supernatants were analyzed for IL-2 by enzyme-linked immunosorbent assay. Similarly, WT and VavNULL DCs were pulsed with peptide and immediately fixed (C) or chased for 24 hours before fixation (D) and then used to stimulate B11 cells as described above. Data represent mean IL-2 production ± SD for triplicate samples and are representative of 5 independent experiments; *P < .05, **P < .01.
Requirement for ITAM signaling in DCs for priming of conventional and autoimmune CD4 T cells in vivo
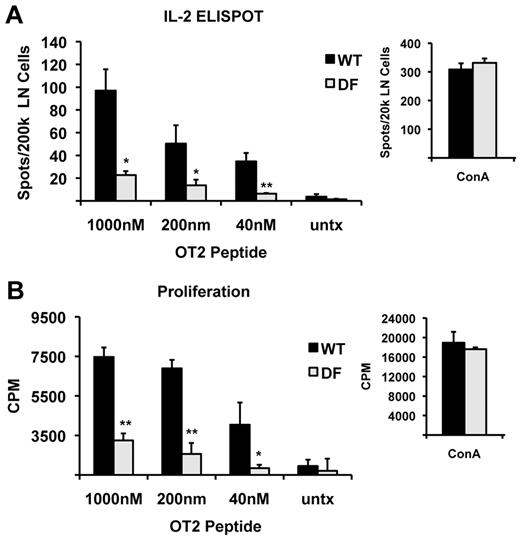
Given these results, and previously published data indicating that DAP12 deficiency attenuates rather than augments progression of EAE, a murine model of multiple sclerosis that requires CD4 T cell–dependent Th1 and Th17 responses,40-42 we hypothesized that the ITAM adaptors may be involved as positive regulators of DC function in antigen presentation in vivo. To test this hypothesis, we first determined the requirement for DAP12 and FcRγ during in vivo priming of CD4 T-cell responses with a conventional antigen. Importantly, T-lineage cells developing in DF mice appear phenotypically normal and do not show any obvious developmental or functional abnormalities (data not shown). WT and DF mice were immunized with the MHCII/I-Ab-restricted OVA peptide using a standard protocol, and the antigen-specific T-cell response was determined by in vitro restimulation of draining lymph node cells 7 days later (Figure 3). Strikingly, while WT mice generated a robust response to OVA immunization, DF mice showed a reduced response as indicated by diminished frequency of IL-2 producing T cells visualized by ELISPOT assays (Figure 3A) and decreased T-cell proliferation in [3H]-thymidine incorporation assays (Figure 3B). As expected, both DF and WT T cells responded vigorously to stimulation with ConA (Figure 3A-B), indicating that the attenuation of antigen-specific response in DF mice is not due to defects intrinsic to T cells. Furthermore, expression of MHCII and B7.2 in lymph node DCs was similar in DF and WT mice treated with CFA (supplemental Figure 7). We interpret these results as suggesting that the ITAM-containing adaptors, DAP12 and FcRγ, are critically required for efficient priming of CD4 T cells in vivo.
ITAM signaling is required for T-cell priming in vivo. WT and DF mice were immunized in the footpad with OVA peptide in CFA, and 7 days later, the draining popliteal lymph nodes were harvested. Lymph node cells were restimulated in vitro with the indicated doses of OVA peptide (continuous peptide) and analyzed 1 day later for the frequency of antigen-specific T cells by ELISPOT (A) and 3 days later for proliferation by thymidine incorporation (B). Data shown are the mean ± SD of triplicate samples. At least 4 mice per group were analyzed; *P < .05, **P < .01.
ITAM signaling is required for T-cell priming in vivo. WT and DF mice were immunized in the footpad with OVA peptide in CFA, and 7 days later, the draining popliteal lymph nodes were harvested. Lymph node cells were restimulated in vitro with the indicated doses of OVA peptide (continuous peptide) and analyzed 1 day later for the frequency of antigen-specific T cells by ELISPOT (A) and 3 days later for proliferation by thymidine incorporation (B). Data shown are the mean ± SD of triplicate samples. At least 4 mice per group were analyzed; *P < .05, **P < .01.
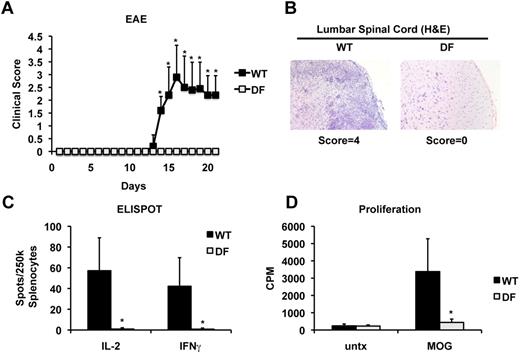
Given these results, we decided to determine whether DAP12 and FcRγ are also required for efficient generation of encephalitogenic CD4 T cells in EAE. In this regard, previous studies showed protective effects of the loss of DAP12 expression in EAE,36 however susceptibility of mice lacking both DAP12 and FcRγ has not been tested. To address this, DF and WT mice were immunized with myelin oligodendrocyte glycoprotein peptide 35-55 (MOG35-55) and analyzed for development of clinical signs of the disease. As expected, WT mice developed initial signs of disease associated with an ascending paralysis starting at day 14 postimmunization and progressing through day 21 (Figure 4A). In contrast, DF mice appeared completely resistant to EAE induction and remained free of any clinical signs of disease throughout the duration of the experiment (Figure 4A). At peak disease, WT mice exhibited notable infiltration of mononuclear cells into the lumbar spinal cord, but such central nervous system-infiltrates were virtually absent in DF mice (Figure 4B). Next, we measured antigen-specific T-cell responses in MOG35-55-immunized mice by restimulation of spleen cells in vitro. Remarkably, WT mice showed a robust response to MOG as measured by ELISPOT assays of IL-2 and IFNγ-producing T cells and by [3H]-thymidine incorporation, but the MOG-specific T-cell response was virtually undetectable in DF mice (Figure 4C-D). In contrast to DF mice, mice single-deficient in FcRγ did not exhibit any detectable defects in CD4 T-cell priming, whereas mice single-deficient in DAP12 showed a mild reduction (supplemental Figure 8). In agreement with previous studies, FcRγ-deficient mice were only marginally protected from EAE, whereas DAP12-deficient mice showed partial protection, compared with WT36,43-48 (supplemental Figure 9). Taken together, these results show that DAP12 and FcRγ play an essential but functionally redundant role during in vivo priming of both conventional antigen-specific and autoimmune (pathogenic) CD4 T cells. These results highlight the requirement for the ITAM signaling pathway in the regulation of MHCII antigen presentation in DCs.
Disruption of ITAM signaling is protective in EAE and inhibits autoreactive T-cell priming. (A) Mice were immunized with MOG35-55 peptide and pertussis toxin to induce disease. During the course of disease, mice were assigned a clinical score with grade 1, tail weakness; grade 2, hind limb weakness sufficient to impair righting; grade 3, one limb plegic; grade 4, hind limb paralysis; grade 5, moribund. Data are expressed as mean clinical score ± SD for n ≥ 5 mice per group. After the peak of disease (3 weeks), spinal chords were harvested and stained with hematoxylin and eosin (B). In addition, spleens were recovered and restimulated in vitro with MOG peptide to determine autoreactive T-cell frequencies by ELISPOT (C) and proliferation by thymidine incorporation (D). Data represent the mean ± SD for n ≥ 5 mice per group; *P < .05.
Disruption of ITAM signaling is protective in EAE and inhibits autoreactive T-cell priming. (A) Mice were immunized with MOG35-55 peptide and pertussis toxin to induce disease. During the course of disease, mice were assigned a clinical score with grade 1, tail weakness; grade 2, hind limb weakness sufficient to impair righting; grade 3, one limb plegic; grade 4, hind limb paralysis; grade 5, moribund. Data are expressed as mean clinical score ± SD for n ≥ 5 mice per group. After the peak of disease (3 weeks), spinal chords were harvested and stained with hematoxylin and eosin (B). In addition, spleens were recovered and restimulated in vitro with MOG peptide to determine autoreactive T-cell frequencies by ELISPOT (C) and proliferation by thymidine incorporation (D). Data represent the mean ± SD for n ≥ 5 mice per group; *P < .05.
ITAM signaling regulates the stability and half-life of pMHCII in immature DCs
While the mechanism of protein antigen processing and presentation through the MHCII pathway is relatively well understood at present, little is known about signaling pathways regulating pMHCII expression, internalization, and re-expression in immature DCs. In these cells, pMHCII complexes are labile and undergo rapid recycling and degradation7,10,11 which is thought to be critically important for their capacity to constitutively sample the environment for priming of antigen-specific naive T cells.8,9 Given the requirement for ITAM signals in the activation of naive CD4 T lymphocytes both in vitro and in vivo, we hypothesized that ITAM signals may be involved in the regulation of pMHCII dynamics in DCs. To address this, we first decided to determine the half-life of pMHCII complexes in WT and ITAM-deficient immature DCs. In these experiments, WT, DF, and VavNULL, DCs were pulsed with 35S-Met and -Cys, washed, and chased for 0-9 hours. Subsequently, MHCII complexes were immunoprecipitated with Y3P monoclonal antibody recognizing mature I-A complexes, and immunoprecipitates were then resolved by PAGE and analyzed by autoradiography. Immediately after pulse (0 hours chase), few newly formed MHCII molecules were immunoprecipitated, presumably because the majority of labeled MHCII molecules were associated with the invariant chain at this time point, and were not recognized by the Y3P antibody (Figure 5A-B). After a 3-hour chase, peptide-loaded MHCII complexes emerged as a single SDS-resistant band of 55 kDa, which dissociated into I-Aα and I-Aβ monomers upon boiling. Remarkably, at 9 hours, SDS-resistant pMHCII complexes decayed by only approximately 20% in WT DCs; however, in DF and VavNULL DCs, these complexes decayed by more than 50% (Figure 5A-B). Thus, even though the generation of SDS-resistant complexes in DF and VavNULL DCs at 3 hours was similar if not more robust than in WT DCs, these complexes appeared unstable in ITAM-mutants (Figure 5A-C). Importantly, the half-life of MHCI was similar in WT and VavNULL DCs, indicating that the accelerated degradation of MHCII in DF and VavNULL DCs was not the result of any generalized defect(s) in protein synthesis or degradation (supplemental Figure 10). This finding is consistent with our previous report that ITAM signaling is not required for presentation of soluble protein antigen on MHCI to CD8 T cells.24 Taken together, the data indicate that ITAM signals are required to regulate the half-life of SDS-resistant pMHCII complexes in immature DCs. Of note, this process appears to be independent of ITAM-mediated regulation of the NOX2 nicotinamide adenine dinucleotide phosphate oxidase and reactive oxygen species (ROS) production, as the half-life of pMHCII complexes in NOX2-deficient DCs was similar to WT (supplemental Figure 11).
ITAM signaling controls the half-life and surface retention of peptide-MHCII complexes. DCs were pulse-labeled with [35S] for 30 minutes, washed, and chased for the indicated time points. Cells were then lysed, and mature MHCII complexes were immunoprecipitated with Y3P antibody before resolution by PAGE and autoradiography. In WT DCs, stable peptide-MHCII complexes (αβ-peptide), which are SDS-resistant in nonboiled samples, appeared by 3 hours and were slightly reduced after a 9-hour chase. While VavNULL (A) or DF (B) DCs generated stable peptide-MHCII complexes at 3 hours, they rapidly decayed by the 9-hour time point. (C) Quantification of pMHCII decay from 3-9 hours was achieved by densitometric analysis of pMHCII bands at 9 hours normalized to 3 hours. Data represent the relative mean reduction in pixel intensity ± SD from 4 independent experiments. (D) WT and VavNULL DCs were treated with Brefeldin A to block transport of newly synthesized MHCII through the Golgi network, and surface MHCII was detected by FACS at the indicated time points to determine the rate at which MHCII was removed from the cell surface. Data represent the mean percentage ± SD of remaining surface MHCII (based on mean fluorescence intensity [MFI] at time 0) from 5 independent experiments; *P < .05, **P < .01.
ITAM signaling controls the half-life and surface retention of peptide-MHCII complexes. DCs were pulse-labeled with [35S] for 30 minutes, washed, and chased for the indicated time points. Cells were then lysed, and mature MHCII complexes were immunoprecipitated with Y3P antibody before resolution by PAGE and autoradiography. In WT DCs, stable peptide-MHCII complexes (αβ-peptide), which are SDS-resistant in nonboiled samples, appeared by 3 hours and were slightly reduced after a 9-hour chase. While VavNULL (A) or DF (B) DCs generated stable peptide-MHCII complexes at 3 hours, they rapidly decayed by the 9-hour time point. (C) Quantification of pMHCII decay from 3-9 hours was achieved by densitometric analysis of pMHCII bands at 9 hours normalized to 3 hours. Data represent the relative mean reduction in pixel intensity ± SD from 4 independent experiments. (D) WT and VavNULL DCs were treated with Brefeldin A to block transport of newly synthesized MHCII through the Golgi network, and surface MHCII was detected by FACS at the indicated time points to determine the rate at which MHCII was removed from the cell surface. Data represent the mean percentage ± SD of remaining surface MHCII (based on mean fluorescence intensity [MFI] at time 0) from 5 independent experiments; *P < .05, **P < .01.
Given the results of these metabolic labeling experiments, we decided to examine potential contribution of pMHCII internalization and reexpression rates to net surface retention in the absence of de novo synthesis. To this end, WT and VavNULL DCs were treated with Brefeldin A (GolgiPlug) to inhibit transport of newly synthesized MHCII from the Golgi to the plasma membrane, and surface MHCII levels were analyzed by FACS at 0-9 hours after treatment. Consistent with the results of the metabolic labeling studies, pMHCII complexes in WT DCs treated with Brefeldin A appeared stable and decayed from the plasma membrane with relatively slow kinetics (Figure 5D). In contrast, pMHCII complexes were lost from the cell surface of VavNULL DCs significantly more rapidly, compared with WT (Figure 5D). These results indicate that ITAM signals are critical for regulation of the half-life of SDS-resistant complexes and contribute to the retention of the pool of mature, recirculating pMHCII complexes in immature DCs.
ITAM signaling is required for efficient recycling of MHCII
In immature DCs, MHCII is thought to be internalized and sorted by a mechanism activated in response to ubiquitination signals, although this process is incompletely understood at present.15-19 To determine whether ITAM signaling regulates MHCII ubiquitination and subsequent internalization, MHCII was immunoprecipitated from WT and VavNULL DCs followed by Western blotting for ubiquitin, or total MHCIIβ chain. Strikingly, we found a significantly higher proportion of MHCII to be ubiquitinated in VavNULL DCs, compared with WT (Figure 6A). Together, these results suggest that a disruption of ITAM signaling leads to an accumulation of ubiquitinated MHCII in immature DCs, for example due to dysregulated ubiquitination and/or inefficient routing into the recycling compartment(s). In support of this view, inhibition of lysosomal proteolysis with chloroquine, which has previously been shown to enhance the stability of pMHCII complexes in immature DCs,17 led to a significant accumulation of SDS-resistant MHCII complexes in mutant DCs (Figure 6B). Collectively, these results indicate that the impairment in ITAM signaling leads to enhanced ubiquitination and lysosomal degradation of pMHCII in immature DCs. Thus, ITAM signaling appears to be required to extend the half-life of pMHCII complexes via increased trafficking through a recycling compartment(s).
Disruption of ITAM signaling in DCs leads to the accumulation of ubiquitinated MHCII species and impaired recycling. (A) DCs were lysed, and MHCII was immunoprecipitated (clone M5/114), resolved by PAGE, and Western blots for ubiquitin (clone P4D1) and MHCII β chain (clone KL295) were performed. (B) The half-life of MHCII was determined in the presence and absence of chloroquine, to inhibit lysosomal proteolysis. DCs were pulse-labeled with [35S] and chased for the indicated time points. Where indicated, chloroquine (50μM) was added 2 hours into the 9-hour chase. After chase, MHCII was immunoprecipitated, resolved by PAGE, and detected by autoradiography. (C) MHCII internalization was determined biochemically by using a surface biotinylating reagent that can be cleaved by MESNA, a cell impermeant reducing agent. DCs were first surface-biotinylated, chased for the indicated time points to allow internalization of MHCII, and treated with MESNA to remove biotin from MHCII that remained on the cell surface. Detection of biotinylated MHCII that had been internalized was achieved by immunoprecipitation with anti-MHCII antibody followed by Western blotting with streptavidin. Densitometric measurement of band intensity was used to calculate the percentage of internalized MHCII relative to total surface MHCII. Data are representative of 6 independent experiments. (D) Recycling of internalized MHCII back to the plasma membrane was determined biochemically. WT and DF DCs were surface biotinylated and chased for 20 minutes to allow internalization of recycling MHCII. Any biotinylated MHCII remaining on the surface of the cells was then removed by MESNA treatment. DCs were then chased for the indicated time points to allow internalized MHCII to reemerge on the plasma membrane, at which point biotin was removed with a second MESNA treatment. Detection of biotinylated MHCII that was trapped in intracellular compartments and protected from MESNA was achieved by immunoprecipitation with anti-MHCII antibody followed by Western blotting with streptavidin. During the course of the chase, loss of biotinylated MHCII indicates efficient recycling. Densitometric measurement of band intensity was used to calculate the relative amount of recycling MHCII that reemerged on the plasma membrane at each time point. Data are representative of 6 independent experiments; **P < .01.
Disruption of ITAM signaling in DCs leads to the accumulation of ubiquitinated MHCII species and impaired recycling. (A) DCs were lysed, and MHCII was immunoprecipitated (clone M5/114), resolved by PAGE, and Western blots for ubiquitin (clone P4D1) and MHCII β chain (clone KL295) were performed. (B) The half-life of MHCII was determined in the presence and absence of chloroquine, to inhibit lysosomal proteolysis. DCs were pulse-labeled with [35S] and chased for the indicated time points. Where indicated, chloroquine (50μM) was added 2 hours into the 9-hour chase. After chase, MHCII was immunoprecipitated, resolved by PAGE, and detected by autoradiography. (C) MHCII internalization was determined biochemically by using a surface biotinylating reagent that can be cleaved by MESNA, a cell impermeant reducing agent. DCs were first surface-biotinylated, chased for the indicated time points to allow internalization of MHCII, and treated with MESNA to remove biotin from MHCII that remained on the cell surface. Detection of biotinylated MHCII that had been internalized was achieved by immunoprecipitation with anti-MHCII antibody followed by Western blotting with streptavidin. Densitometric measurement of band intensity was used to calculate the percentage of internalized MHCII relative to total surface MHCII. Data are representative of 6 independent experiments. (D) Recycling of internalized MHCII back to the plasma membrane was determined biochemically. WT and DF DCs were surface biotinylated and chased for 20 minutes to allow internalization of recycling MHCII. Any biotinylated MHCII remaining on the surface of the cells was then removed by MESNA treatment. DCs were then chased for the indicated time points to allow internalized MHCII to reemerge on the plasma membrane, at which point biotin was removed with a second MESNA treatment. Detection of biotinylated MHCII that was trapped in intracellular compartments and protected from MESNA was achieved by immunoprecipitation with anti-MHCII antibody followed by Western blotting with streptavidin. During the course of the chase, loss of biotinylated MHCII indicates efficient recycling. Densitometric measurement of band intensity was used to calculate the relative amount of recycling MHCII that reemerged on the plasma membrane at each time point. Data are representative of 6 independent experiments; **P < .01.
Next, to directly test this notion, we analyzed the kinetics of MHCII recycling (internalization and reemergence) using an assay in which a cleavable biotin is first used to label surface MHCII and subsequently chased and cleaved to determine internalization and reemergence rates. Consistent with published studies,39 these experiments showed that in WT DCs approximately 10% of surface MHCII molecules became internalized at 20 minutes (Figure 6C). However, the internalization rate was increased approximately 2-fold in mutant cells (Figure 6C). In contrast, the rate of MHCII reemergence on the plasma membrane over the course of 60 minutes was consistently reduced in DF DCs, compared with WT (Figure 6D). Thus, taken together, these data indicate that in the absence of ITAM signaling, pMHCII complexes in immature DCs become excessively ubiquitinated and inefficiently recycled and, as a consequence, may be rerouted to the lysosomal compartment and prematurely degraded, resulting in a net loss of MHCII from the recycling pool. We propose a model in which the ITAM apparatus is critically important for the control of internalization and reemergence of surface MHCII in immature DCs.
Discussion
Considerable effort has been directed toward understanding the cell biology of MHCII presentation,1,2,13 and significant progress has been made in characterizing the role of MHCII synthesis/degradation during antigen presentation by dendritic cells.3 Recently, several microbial PAMPs and cytokines have been shown to enhance antigen presentation by up-regulating costimulatory molecules and inducing maturation of DCs, yet the mechanisms governing MHCII trafficking in immature DCs remain obscure.11,13,21 Here, we present data demonstrating that ITAM signaling in DCs controls CD4 T-cell priming by promoting salvage of MHCII through a recycling pathway. Thus, in the absence of ITAM signaling, MHCII is inefficiently recycled, instead accumulating in ubiquitinated form, and ultimately being degraded in lysosomes. The functional consequences of defective ITAM signaling in DCs are a reduced half-life of mature pMHCII complexes leading to impaired antigen presentation and activation of both conventional antigen-specific and autoimmune CD4 T cells.
Shortly after the first formal demonstration that MHCII can be recycled and reloaded,39 a breakthrough study showed that lipopolysaccharide (LPS) treatment in DCs extends the half-life of pMHCII and induces its redistribution to the plasma membrane.7 While much of the research has been focused on the mechanism and the consequences of stabilizing pMHCII in mature DCs7,16-19 our work presented here highlights the importance of the regulation of pMHCII maintenance in immature DCs. Before antigen encounter, immature DCs perform the critical task of antigen surveillance and must continuously sample their environment, maintaining a dynamic pool of MHCII for recycling and reloading. By disrupting ITAM signaling, we show here that MHCII recycling is impaired, and MHCII is prematurely degraded. Indeed, we can extend the half-life of MHCII in ITAM signaling–deficient DCs by treatment with LPS, yet this leads to only partial rescue of antigen presentation defects in vitro (supplemental Figure 12 and data not shown). Moreover, immunization of DF mice with OVA in the presence of adjuvant fails to induce a robust CD4 T-cell response. We interpret these results as an indication that, upon initial antigen encounter, DCs process the antigen quickly, but the up-regulation and stabilization of pMHCII may require more time (up to 8 hours,7 and our unpublished observations). Thus, there appears to be a window of time, between antigen encounter and DC maturation, during which robust presentation of specific antigen requires ITAM signaling for MHCII recycling and salvage.
Investigation into the role of ITAM signaling in DCs and other myeloid cells has revealed the complex nature of this pathway. DAP12 deficiency has been shown to increase mortality in mouse models of sepsis as a result of hyperinflammatory responses.49,50 On the other hand, DAP12 is required for survival and expansion of macrophages.51 Moreover, deficiency in both DAP12 and FcRγ results in profound functional defects in neutrophils.23 Thus, ITAM signaling performs critical positive and negative regulatory functions in myeloid cells.35 Most relevant to antigen presentation, a previous report demonstrated that DCs lacking DAP12 and FcRγ could be more efficient antigen-presenting cells compared with WT DCs.29 Importantly, antigenic peptide was continuously present (not pulsed) in these experiments, and very low doses of LPS or CpG were used to mature DCs. Therefore, since DF DCs respond more robustly than WT to these low dose stimuli, they also induce stronger T-cell responses.29 While WT and DF DCs respond similarly in terms of up-regulation of costimulatory molecules when higher doses of toll-like receptor ligands are used, we show here that under the conditions where DCs are pulsed with antigen, rather than antigen being continuously supplied throughout the culture, ITAM signaling is clearly required for antigen presentation. Finally, the most compelling evidence for a critical role of ITAM signaling for antigen presentation by MHCII in vivo is the observation that DF mice fail to prime CD4 T-cell responses after immunization with a conventional antigen, or an autoantigen.
Our work presented here reveals a novel regulatory mechanism for MHCII trafficking and raises 2 important issues: (1) what are the receptors that engage the ITAM-containing adaptors to initiate the signal, and (2) how do these signals converge with the vesicular trafficking machinery in DCs? While we currently do not know the identity of the receptor(s) generating ITAM signals in DCs, our data make some potentially informative predictions. Importantly, DF mice exhibit a remarkably more severe phenotype than either the DAP12 or FcRγ single knockouts with respect to defects in CD4 T-cell priming and protection from EAE36,43,48 (supplemental Figures 8-9). This observation suggests that ITAM signaling in DCs is initiated by a class of receptors that uses both DAP12 and FcRγ, such as integrins,23 or a host of distinct receptors that associate with DAP12 or FcRγ. Several receptors known to pair with DAP12 (TREM2, SIRPβ1) and FcRγ (OSCAR, Mincle) are expressed in myeloid cells, and certainly more will be identified in the future. For example, MHCII was recently reported to interact with FcRγ, and cross-linking of MHCII by specific antibodies or Lag-3 inhibits DC function.52 It is also formally possible that ITAM-associated receptors may be dispensable for the generation of ITAM signals in myeloid cells. Rather, expression of ITAM-containing adaptors and tonic tyrosine kinase activation may be sufficient to maintain ITAM signaling.53 Nevertheless, we favor a model in which interactions between DCs and the extracellular matrix or neighboring cells promotes integrin engagement to initiate ITAM signaling through DAP12 and FcRγ.
The second issue pertains to the mechanism by which ITAM signals regulate MHCII trafficking. ITAM signaling through Vav guanine nucleotide exchange factors has long been recognized as a critical regulator of Rac GTPases and actin dynamics.54 Given that endocytosis and vesicle sorting require actin and microtubule remodeling, ITAM signaling may control MHCII trafficking at the level of the cytoskeleton. Another newly identified role for Vav is its regulation of the cytoskeleton by direct interaction with dynamin.55 In this context, Vav may exert control over MHCII endocytosis by recruiting dynamin, which facilitates membrane scission/pinching in nascent endosomes.56 Alternatively, ITAM signaling may regulate the recruitment of the newly discovered ubiquitin-binding clathrin adaptors that simultaneously bind ubiquitinated transmembrane receptors like MHCII and direct the assembly of clathrin-coated pits during endocytosis. While at present we do not understand exactly how ITAM signaling regulates MHCII trafficking, considerable evidence exists to support a model in which MHCII is ubiquitinated and subsequently endocytosed in a clathrin-dependent manner.14-17 However, additional work suggests that MHCII can be endocytosed in a clathrin- and dynamin-independent manner, presumably in lipid microdomains.20 In addition, each model may be applicable in different contexts, for example in distinct DC subsets and maturation states. Therefore, it is possible that ITAM signaling regulates a ubiquitin-dependent clathrin-mediated internalization process resulting in recycling of MHCII through the H2-DM compartment. While in the absence of ITAM signaling, clathrin-mediated internalization and recycling of MHCII may be impaired, lipid microdomain-mediated endocytosis may remain intact. Thus, rerouting of MHCII in the absence of ITAM signaling could result in premature destruction.
Ligation of ITAM-associated receptors could alter expression of the MHCII ubiquitinating ligase MARCH1; however, our analyses of mRNA expression showed similar steady-state levels of MARCH1 in WT and VavNULL DCs, and similar degree of reduction upon LPS stimulation (supplemental Figure 13). Thus, signals emanating from ITAM-associated receptors do not seem to regulate mRNA levels of MARCH1. While it is possible that posttranslational regulation of MARCH1 activity may involve ITAM signaling, it is also possible that regulation of MARCH1 enzymatic activity may be achieved via indirect effects on its E2 partner and/or other components of a complex. Alternatively, MHCII regulation could involve deubiquitinating enzymes, or conceivably, could involve modulation/competition by binding of another ubiquitin homolog such as ISG15.57
Thus, although at present we do not completely understand the molecular mechanism involved, our results presented here provide new insights into the regulatory circuits governing MHCII trafficking by implicating a specific signal transduction pathway in the process. Using mice deficient in ITAM signaling in DCs, we show that this process appears critical for efficient priming of both conventional and pathogenic (autoimmune) CD4 T cells in vivo, providing a model in which the molecular machinery driving MHCII trafficking and antigen presentation can be further dissected.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr Emil Unanue, Marco Colonna, Ted Hansen, Paul Allen, and Ramnik Xavier for helpful suggestions and providing reagents. We also thank Drs Lisa Denzin and Sergio Grinstein for insightful discussions.
This work was supported by National Institutes of Health grant nos. R01AI061077 (to W.S.), R01AI073718 (to W.S.), the Leukemia & Lymphoma Society Scholar Award (to W.S.), and a Special Fellowship Award from the Leukemia & Lymphoma Society (to D.B.G.).
National Institutes of Health
Authorship
Contribution: D.B.G., H.M.A., G.B.G., L.P., J.S., Y.W., G.S.B., and G.S., performed experiments; D.B.G. and W.S. designed and supervised the research; S.G., K.F., R.B., J.A.C., A.H.C., J.H.R., and M.C. contributed new reagents and/or analytical tools; D.B.G., H.M.A., G.B.G., L.P., R.B., J.A.C., and W.S. analyzed the data; and D.B.G., H.M.A., and W.S. prepared the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Wojciech Swat, Department of Pathology and Immunology, Box 8118, Washington University School of Medicine, 660 S Euclid Ave, St Louis, MO, 63110; e-mail: swat@wustl.edu.
References
Author notes
D.B.G. and H.M.A. contributed equally to this study.

![Figure 5. ITAM signaling controls the half-life and surface retention of peptide-MHCII complexes. DCs were pulse-labeled with [35S] for 30 minutes, washed, and chased for the indicated time points. Cells were then lysed, and mature MHCII complexes were immunoprecipitated with Y3P antibody before resolution by PAGE and autoradiography. In WT DCs, stable peptide-MHCII complexes (αβ-peptide), which are SDS-resistant in nonboiled samples, appeared by 3 hours and were slightly reduced after a 9-hour chase. While VavNULL (A) or DF (B) DCs generated stable peptide-MHCII complexes at 3 hours, they rapidly decayed by the 9-hour time point. (C) Quantification of pMHCII decay from 3-9 hours was achieved by densitometric analysis of pMHCII bands at 9 hours normalized to 3 hours. Data represent the relative mean reduction in pixel intensity ± SD from 4 independent experiments. (D) WT and VavNULL DCs were treated with Brefeldin A to block transport of newly synthesized MHCII through the Golgi network, and surface MHCII was detected by FACS at the indicated time points to determine the rate at which MHCII was removed from the cell surface. Data represent the mean percentage ± SD of remaining surface MHCII (based on mean fluorescence intensity [MFI] at time 0) from 5 independent experiments; *P < .05, **P < .01.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/116/17/10.1182_blood-2009-10-250415/4/m_zh89991058870005.jpeg?Expires=1765920050&Signature=HOPJETqdkK6FIA4qi1973f11VVl2kOTORK2zQkA61XIglvpTLbsDpKk44n6c6IRfBUZhq0Uf6Ix5v9~MFaKmZ5THTPgFHl9sJm~NZ3NPz2d-c56FBilRpzfBUGXO2CvV41taSO4xCWot4uHNVwcCHF-MCa26pSiKO4vosyJAZ0kGZsCfsMNbNqMYhh3goH4tiTMuI49OifT04nXZ5zhxI-SrTo-TObPvXW6lzoVjLzr6kaUcBPYjJWeP7yzut2HRbTQB0QixOOyMKkNmOABprWHjxMbw35gWAZkhcdLi-4JIVVNc2jcRU9lgmhbhW69HY9ffkujpOZ-8mEB170LmmA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 6. Disruption of ITAM signaling in DCs leads to the accumulation of ubiquitinated MHCII species and impaired recycling. (A) DCs were lysed, and MHCII was immunoprecipitated (clone M5/114), resolved by PAGE, and Western blots for ubiquitin (clone P4D1) and MHCII β chain (clone KL295) were performed. (B) The half-life of MHCII was determined in the presence and absence of chloroquine, to inhibit lysosomal proteolysis. DCs were pulse-labeled with [35S] and chased for the indicated time points. Where indicated, chloroquine (50μM) was added 2 hours into the 9-hour chase. After chase, MHCII was immunoprecipitated, resolved by PAGE, and detected by autoradiography. (C) MHCII internalization was determined biochemically by using a surface biotinylating reagent that can be cleaved by MESNA, a cell impermeant reducing agent. DCs were first surface-biotinylated, chased for the indicated time points to allow internalization of MHCII, and treated with MESNA to remove biotin from MHCII that remained on the cell surface. Detection of biotinylated MHCII that had been internalized was achieved by immunoprecipitation with anti-MHCII antibody followed by Western blotting with streptavidin. Densitometric measurement of band intensity was used to calculate the percentage of internalized MHCII relative to total surface MHCII. Data are representative of 6 independent experiments. (D) Recycling of internalized MHCII back to the plasma membrane was determined biochemically. WT and DF DCs were surface biotinylated and chased for 20 minutes to allow internalization of recycling MHCII. Any biotinylated MHCII remaining on the surface of the cells was then removed by MESNA treatment. DCs were then chased for the indicated time points to allow internalized MHCII to reemerge on the plasma membrane, at which point biotin was removed with a second MESNA treatment. Detection of biotinylated MHCII that was trapped in intracellular compartments and protected from MESNA was achieved by immunoprecipitation with anti-MHCII antibody followed by Western blotting with streptavidin. During the course of the chase, loss of biotinylated MHCII indicates efficient recycling. Densitometric measurement of band intensity was used to calculate the relative amount of recycling MHCII that reemerged on the plasma membrane at each time point. Data are representative of 6 independent experiments; **P < .01.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/116/17/10.1182_blood-2009-10-250415/4/m_zh89991058870006.jpeg?Expires=1765920051&Signature=iPOdyXbRKIvBlOMaLsUd2iPACpXnyeuVNIZbDJ9XjW5Mc6M6PgzAWL7jBLuPoXksE9jYZV3yu599jtkXxeKP6jTmIXsDGT-0ig6TgDkzZZTSzawd2iOIJUysH3JOmC9UlXLRtqX2VeqLus~C52kKnAubnQywbWSWbNtMjLd1rbN48dlN01VYMeNd3ezMg~M6hUwvJBnMcxO5Q~jJPIGBh3gsG7Czr2lF8E95fPwdvc36hkBbqqhHMeJ3KcFRy4onTDsp4x4RoyjCOujnHuqPC-dFsklcfPW09QObB6x1jvf8xgL2jjyZYFFYePiUkyLHGDNGRVw~Usls66pARDiX1g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal