Abstract
The molecular basis for regulation of dendritic cell (DC) development and homeostasis remains unclear. Signal regulatory protein α (SIRPα), an immunoglobulin superfamily protein that is predominantly expressed in DCs, mediates cell-cell signaling by interacting with CD47, another immunoglobulin superfamily protein. We now show that the number of CD11chigh DCs (conventional DCs, or cDCs), in particular, that of CD8−CD4+ (CD4+) cDCs, is selectively reduced in secondary lymphoid tissues of mice expressing a mutant form of SIRPα that lacks the cytoplasmic region. We also found that SIRPα is required intrinsically within cDCs or DC precursors for the homeostasis of splenic CD4+ cDCs. Differentiation of bone marrow cells from SIRPα mutant mice into DCs induced by either macrophage-granulocyte colony-stimulating factor or Flt3 ligand in vitro was not impaired. Although the accumulation of the immediate precursors of cDCs in the spleen was also not impaired, the half-life of newly generated splenic CD4+ cDCs was markedly reduced in SIRPα mutant mice. Both hematopoietic and nonhematopoietic CD47 was found to be required for the homeostasis of CD4+ cDCs and CD8−CD4−(double negative) cDCs in the spleen. SIRPα as well as its ligand, CD47, are thus important for the homeostasis of CD4+ cDCs or double negative cDCs in lymphoid tissues.
Introduction
Dendritic cells (DCs) are professional antigen-presenting cells that play 2 major roles in the immune system: initiation and modulation of the immune responses of T cells to exogenous pathogens, and maintenance of T-cell tolerance to self-components.1 The mouse spleen harbors 2 major subtypes of DCs, namely, CD11chigh conventional DCs (cDCs) and plasmacytoid DCs (pDCs), defined as CD11cint B220+ cells. The latter DCs produce type I interferons in response to viral and bacterial pathogens.1,2 The cDCs are further classified into CD4+CD8− cDCs (CD4+ cDCs), CD4−CD8− cDCs (double negative [DN] cDCs), and CD4−CD8+ cDCs (CD8+ cDCs).3,4 CD8− cDCs are present predominantly in the marginal zone of splenic lymphoid follicles as well as in marginal zone–bridging regions, whereas CD8+ cDCs are enriched in the periarteriolar lymphoid sheaths, which are populated largely by T cells, in the white pulp of the spleen.5 These cDC subtypes are in dynamic balance. The estimated half-life of mature cDCs in the spleen under steady-state conditions is only 2-3 days.6 The immediate precursors of cDCs, defined as lineage-negative CD11c+ MHC class II− SIRPαint Flt3+ cells (pre-cDCs),7-9 are widely distributed in bone marrow (BM) as well as in secondary lymphoid tissues and circulating blood, and they are thought to maintain cDCs in lymphoid tissues under steady-state conditions.
Genetic analysis of cDC development and homeostasis has revealed that many gene products regulate cDC development, with some of them selectively controlling CD8− cDCs or CD8+ cDCs.4,9 Development of CD8− cDCs is markedly suppressed in mice deficient in transcription factors, such as interferon regulatory factor 2 (IRF-2),10 IRF-4,11 RelB,12 or RBP-J.13 Moreover, the lymphotoxin-β receptor–mediated signaling pathway14 as well as tumor necrosis factor receptor–associated factor 6 (TRAF6)15 are also thought to be important for the development of CD8− cDCs. In contrast, the development of CD8+ cDCs is affected by genetic deficiency of Batf3,16 interferon consensus sequence–binding protein (ICSBP),17 or the helix-loop-helix transcription factor Id2 (inhibitor of DNA binding 2).18 However, the molecular basis for regulation of homeostasis of cDC subpopulations remains incompletely understood.
Signal regulatory protein α (SIRPα), also known as SHPS-1 or BIT, is a transmembrane protein whose extracellular region comprises 3 immunoglobulin (Ig)–like domains and whose cytoplasmic region contains immunoreceptor tyrosine-based inhibition motifs that mediate the binding and activation of the protein tyrosine phosphatases SHP-1 and SHP-2.19,20 Tyrosine phosphorylation of SIRPα is triggered by various growth factors and cytokines as well as by integrin-mediated cell adhesion to extracellular matrix proteins. SIRPα is especially abundant in DCs, macrophages, and neutrophils, being barely detectable in T or B lymphocytes.20-22 In addition, the level of SIRPα expression differs among cDC subtypes, being greater in CD8− cDCs than in CD8+ cDCs.21,23 The extracellular region of SIRPα interacts with its ligand, CD47, which is also a member of the Ig superfamily,19,20,24 with such interaction promoting the tyrosine phosphorylation of SIRPα.20,25 In contrast to the relatively restricted distribution of SIRPα, CD47 is expressed in most cell types, including a variety of hematopoietic cells.24 The interaction of SIRPα on DCs with CD47 on T cells is thought to be important for the regulation of priming by DCs of naïve T cells, which then differentiate into T helper cells, or of induction by DCs of antigen-specific cytotoxic T-cell responses.22,26 Given that the expression of SIRPα is predominant in both CD4+ cDCs and DN cDCs among cDC subtypes, SIRPα is likely important, specifically for regulation of these subtypes. Indeed, we here show that SIRPα, as well as CD47, is important for the homeostasis of CD4+ or DN cDCs in secondary lymphoid tissues.
Methods
Animals
Mice that express a mutant version of SIRPα that lacks most of the cytoplasmic region were described previously23,26 and were backcrossed to the C57BL/6 background for 5 generations. CD47-deficient (CD47 KO) mice were described previously27 and were backcrossed to the C57BL/6 background for > 10 generations. C57BL/6 mice congenic for the CD45 gene locus (B6-Ly5.1) were kindly provided by H. Nakauchi (University of Tokyo, Tokyo, Japan). Sex- and age-matched mice at 6-10 weeks of age were studied. Mice were bred and maintained at the Institute of Experimental Animal Research of Gunma University under specific pathogen-free conditions and were handled in accordance with the Animal Care Guidelines of Gunma University.
Cell preparation and flow cytometry
Cell suspensions were prepared from the spleen, peripheral lymph nodes (pLNs), thymus, and BM as described previously.26 For preparation of splenocytes or pLN cells, the spleen or pLNs were minced and then digested with collagenase (Wako) at 400 U/mL in the presence of 5mM EDTA (ethylenediaminetetraacetic acid) for 30 minutes at 37°C. The undigested fibrous material was removed by filtration through a 70-μm cell strainer (BD Falcon), and red blood cells in the filtrate were lysed with Gey's solution. The remaining cells were washed twice with phosphate-buffered saline (PBS) and then subjected to flow cytometric analysis. For preparation of a DC-enriched, low-density fraction of thymocytes, the thymus was digested as described earlier in this paragraph, and the recovered cells were suspended in 2 mL of Ca2+- and Mg2+-free Hanks balanced salt solution (HBSS; Invitrogen), containing 17% Optiprep (Axis-Shield), and then overlaid consecutively with 2 mL of 12% Optiprep in a solution containing 10mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid)-NaOH (pH 7.4), 0.88% NaCl, 1mM EDTA, and 0.5% bovine serum albumin and with 2 mL of Ca2+- and Mg2+-free HBSS. The resulting gradient was centrifuged at 700g for 15 minutes at 20°C, after which cells at the interface of the top 2 layers were collected, washed twice with PBS, and subjected to flow cytometric analysis. For preparation of BM-cell suspensions, BM cells were isolated from the femur and tibia with the use of a syringe fitted with a 23-gauge needle. Fibrous material was removed by filtration through a 70-μm cell strainer, and red blood cells in the filtrate were lysed with Gey's solution. The remaining cells were washed twice with PBS. Cells isolated from each organ were analyzed by flow cytometry with the use of a FACSCalibur, FACSCantoII, or FACSAriaII instrument (BD Biosciences), and all data were analyzed with FlowJo 8.8.4 software (TreeStar).
BM chimeras
Recipient B6-Ly5.1 mice were subjected to lethal irradiation (9.5 Gy) and then injected intravenously with 5 × 106 BM cells obtained from Ly5.2+ wild-type (WT) or SIRPα mutant (MT) mice. Conversely, recipient Ly5.2+ WT, SIRPα MT, or CD47 knockout (KO) mice were subjected to lethal irradiation and injected intravenously with 5 × 106 BM cells obtained from B6-Ly5.1 mice. For the generation of mixed chimeras, recipient B6-Ly5.1 mice were lethally irradiated and then injected intravenously with 5 × 106 mixed BM cells obtained from both B6-Ly5.1 mice and Ly5.2+ SIRPα MT mice (1:1 ratio). Then, 6-8 weeks after BM transplantation, the recipient mice were killed and splenocytes were analyzed by flow cytometry. CD47 hematopoietic chimeras were established as previously described,28 with minor modifications. In brief, recipient B6-Ly5.1 mice were subjected to sublethal irradiation (6 Gy) and then injected intravenously with 5 × 106 BM cells obtained from Ly5.2+ WT or CD47 KO mice. To prevent initial rejection of CD47 KO donor BM cells by macrophages of the recipient, we injected the recipient intravenously with liposome-entrapped dichloromethylene diphosphonate (liposome-MDPCl2),29 according to the following treatment schedule: 0.2 mg/g body weight at 2 days before and 0.1 mg/g at 3, 6, and 11 days after transplantation. Four weeks after transplantation, the recipient mice were killed and splenocytes were analyzed by flow cytometry.
Isolation and injection of pre-cDCs
Adoptive transfer of pre-cDCs was performed as described previously7 with minor modifications. In brief, BM cells derived from Ly5.2+ WT or SIRPα MT mice were incubated with a biotin-conjugated monoclonal antibody (mAb) to mouse Fms-like tyrosine kinase 3 (Flt3), and Flt3+ cells were then collected with the use of anti-biotin microbeads and magnetic-activated cell sorting (MACS). The isolated cells were stained with a fluorescein isothiocyanate (FITC)–conjugated mAb to mouse I-A; phycoerythrin (PE)–conjugated streptavidin; PE-Cy5–conjugated mAbs to mouse CD3ϵ, CD19, B220, NK1.1, and TER-119; an allophycocyanin (APC)–conjugated mAb to CD11c; and propidium iodide (PI). Pre-cDCs were sorted as PI− CD3− CD19− NK1.1− TER-119− B220− I-A− CD11c+ Flt3+ cells with the use of the FACSAriaII instrument. Purified pre-cDCs (1 × 105) were then injected intravenously into B6-Ly5.1 mice, and splenic cDCs were analyzed 8 days after the transfer.
Determination of the turnover rate of splenic cDCs
Measurement of the turnover rate of splenic cDCs was performed as described previously.6 In brief, mice were initially injected intraperitoneally with 1 mg of bromodeoxyuridine (BrdU) and were then continuously provided with BrdU (0.8 mg/mL) in sterile drinking water that was changed daily. At various times after BrdU injection, the spleen was isolated and digested with collagenase as described in the previous paragraph. Control splenocytes from mice that were not given BrdU were prepared in parallel. The cells were washed and then incubated with a PE-conjugated mAb to mouse CD8α, a PE-Cy7–conjugated mAb to mouse CD4, an APC-conjugated mAb to mouse CD11c, and an APC-Cy7–conjugated mAb to B220. The cells were washed again, fixed, permeabilized, stained with the use of a FITC BrdU Flow kit (BD Biosciences), and analyzed by 5-color flow cytometry.
Further details of the materials and methods are provided in supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Results
Selective deficiency of CD4+ cDCs in secondary lymphoid tissues of mice lacking the cytoplasmic region of SIRPα
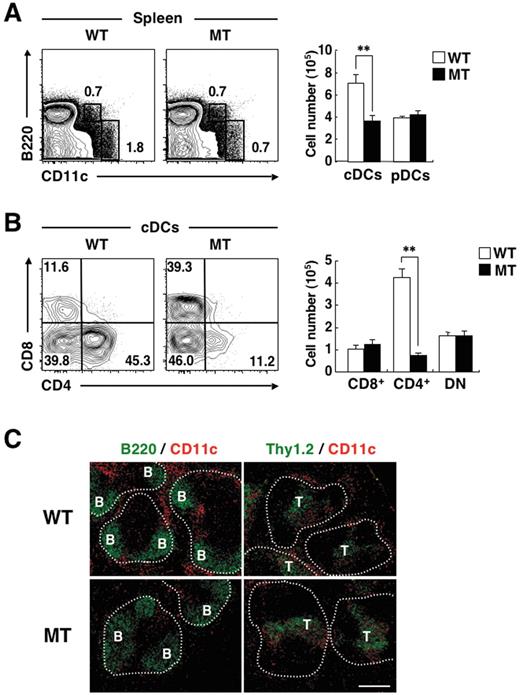
We and others have previously shown that the level of SIRPα expression on CD8− cDCs is much greater than that on CD8+ cDCs,21,23 and we confirmed this observation in the present study (supplemental Figure 1). To clarify the role of SIRPα in the regulation of CD8− cDCs, we investigated the size of cDC subpopulations in the spleen of SIRPα mutant (MT) mice that express a form of SIRPα lacking most of the cytoplasmic region.23,26 The mutant protein expressed in the transgenic mice does not undergo tyrosine phosphorylation or form a complex with SHP-1 or -2.26 Given the importance of the cytoplasmic region of SIRPα for signaling by this protein, the function of SIRPα is thought to be eliminated in the mutant mice.26 Furthermore, the cellular abundance of the mutant protein is markedly reduced, compared with that of the full-length protein in WT cells.26 We confirmed that the expression of SIRPα was markedly reduced in both CD4+ cDCs and DN cDCs in the spleen of SIRPα mutant mice, compared with that observed with WT mice (supplemental Figure 1). With the use of flow cytometric analysis, we found that the proportion as well as the absolute number of cDCs in the spleen of SIRPα MT mice were markedly decreased compared with those in WT mice, whereas the proportion and number of pDCs in the spleen were similar for WT and SIRPα MT mice (Figure 1A). In addition, among the cDC subpopulations, the proportion as well as the absolute number of CD4+ cDCs in the spleen were greatly reduced for SIRPα MT mice, whereas those of either CD8+ cDCs or DN cDCs were similar for WT and SIRPα MT mice (Figure 1B). We also examined the distribution of CD11c+ DCs in the spleen of WT or SIRPα MT mice. Immunohistofluorescence analysis revealed that staining for CD11c in the splenic marginal zones, likely corresponding to CD8− cDCs,5 was markedly reduced in SIRPα MT mice, compared with that in WT mice (Figure 1C). By contrast, staining for CD11c in the splenic periarteriolar lymphoid sheaths, which mostly reflects CD8+ cDCs, did not appear to differ between the 2 genotypes.
Selective deficiency of CD4+ cDCs in the spleen of SIRPα MT mice. (A-B) Splenocytes from WT or SIRPα MT mice were incubated with a biotin-conjugated mAb to CD4. The cells were also stained with an FITC-conjugated mAb to CD8, PE-conjugated streptavidin, an APC-conjugated mAb to CD11c, an APC-Cy7–conjugated mAb to B220, and propidium iodide (PI). The expression of CD11c and B220 on PI-negative cells (A) or that of CD4 and CD8 on CD11chigh B220– cells (cDCs; B) was analyzed by 5-color flow cytometry (left panels). The relative numbers of cDCs and CD11cint B220+ cells (pDCs; A) or those of CD4−CD8+ (CD8+ cDCs), CD4+ CD8− (CD4+ cDCs), and CD4−CD8− (DN cDCs) cells (B) are expressed as a percentage of all viable splenocytes (A) or cDCs (B) on each plot. The absolute numbers of cDCs and pDCs (A) and of CD8+ cDCs, CD4+ cDCs, and DN cDCs (B) in the spleen were also determined (right panels); data are means ± SE for 4 mice per group and are representative of 5 independent experiments. **P < .01 (Student t test). (C) Frozen sections of the spleen from WT or SIRPα MT mice were stained with an FITC-conjugated mAb to B220 (green, left panels) or to Thy1.2 (green, right panels) as well as with a biotin-conjugated mAb to CD11c and Cy3-conjugated streptavidin (red). Images were visualized with a BX-51 microscope equipped with a 10×/0.4 numeric aperture objective lens (Olympus) and a DP71 camera (Olympus), were analyzed with DP controller software (Olympus), and were processed with Adobe Photoshop CS2 software (Adobe Systems). Data are representative of 3 separate experiments. Areas of white pulp are demarcated by dotted lines. B and T represent B- and T-cell areas, respectively. Scale bar, 200 μm.
Selective deficiency of CD4+ cDCs in the spleen of SIRPα MT mice. (A-B) Splenocytes from WT or SIRPα MT mice were incubated with a biotin-conjugated mAb to CD4. The cells were also stained with an FITC-conjugated mAb to CD8, PE-conjugated streptavidin, an APC-conjugated mAb to CD11c, an APC-Cy7–conjugated mAb to B220, and propidium iodide (PI). The expression of CD11c and B220 on PI-negative cells (A) or that of CD4 and CD8 on CD11chigh B220– cells (cDCs; B) was analyzed by 5-color flow cytometry (left panels). The relative numbers of cDCs and CD11cint B220+ cells (pDCs; A) or those of CD4−CD8+ (CD8+ cDCs), CD4+ CD8− (CD4+ cDCs), and CD4−CD8− (DN cDCs) cells (B) are expressed as a percentage of all viable splenocytes (A) or cDCs (B) on each plot. The absolute numbers of cDCs and pDCs (A) and of CD8+ cDCs, CD4+ cDCs, and DN cDCs (B) in the spleen were also determined (right panels); data are means ± SE for 4 mice per group and are representative of 5 independent experiments. **P < .01 (Student t test). (C) Frozen sections of the spleen from WT or SIRPα MT mice were stained with an FITC-conjugated mAb to B220 (green, left panels) or to Thy1.2 (green, right panels) as well as with a biotin-conjugated mAb to CD11c and Cy3-conjugated streptavidin (red). Images were visualized with a BX-51 microscope equipped with a 10×/0.4 numeric aperture objective lens (Olympus) and a DP71 camera (Olympus), were analyzed with DP controller software (Olympus), and were processed with Adobe Photoshop CS2 software (Adobe Systems). Data are representative of 3 separate experiments. Areas of white pulp are demarcated by dotted lines. B and T represent B- and T-cell areas, respectively. Scale bar, 200 μm.
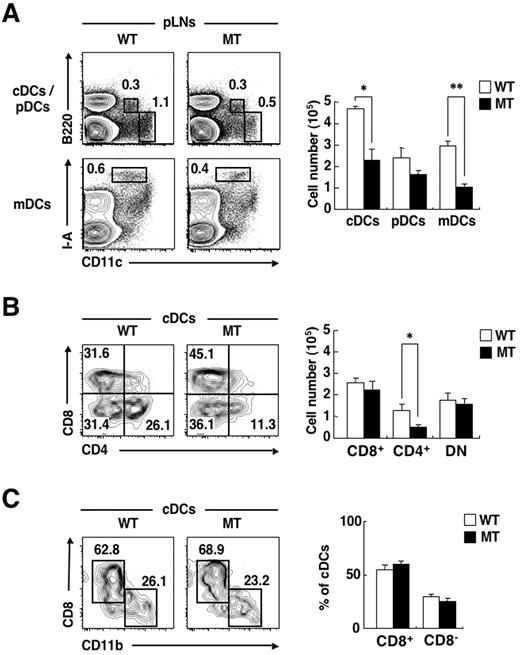
In the peripheral lymph nodes (pLNs), there exist 3 subtypes of DCs: cDCs, pDCs, and CD11cint I-Ahigh migratory DCs (mDCs), which represent Langerhans cells or dermal DCs that originally resided in the skin, but have migrated to the pLNs.30 Both CD8− cDCs and mDCs in pLNs express SIRPα at a high level.21 The proportions as well as the absolute numbers of cDCs and mDCs in pLNs were markedly reduced in SIRPα MT mice, compared with those in WT mice (Figure 2A). Among cDCs in pLNs, the proportion as well as the absolute number of CD4+ cDCs were substantially reduced in SIRPα MT mice, compared with those in WT mice (Figure 2B).
Deficiency of CD4+ cDCs and mDCs in pLNs of SIRPα MT mice. (A-B) Cells prepared from pLNs of WT or SIRPα MT mice were incubated with a biotin-conjugated mAb to CD4. The cells were also stained with PE-conjugated streptavidin, an APC-conjugated mAb to CD11c, an APC-Cy7–conjugated mAb to B220, an FITC-conjugated mAb to CD8 or to I-A, and PI. The expression of CD11c and either B220 or I-A on PI-negative cells (A) and that of CD4 and CD8 on cDCs (B) were analyzed by 5-color flow cytometry (left panels). The relative numbers of cDCs, pDCs, or CD11cint I-Ahigh mDCs are expressed as a percentage of all viable cells from pLNs in each plot (A), as are the relative numbers of CD8+, CD4+, or DN cDCs among all cDCs (B). The absolute numbers of cDCs, pDCs, and mDCs (A) or of CD8+, CD4+, or DN cDCs (B) in pLNs were also determined (right panels); data are means ± SE for 3 mice per group and are representative of 3 independent experiments. *P < .05, **P < .01 (Student t test). (C) A DC-enriched, low-density fraction of thymocytes from WT or SIRPα MT mice was incubated with a biotin-conjugated mAb to CD11b. The cells were also stained with an FITC-conjugated mAb to CD8, PE-conjugated streptavidin, an APC-conjugated mAb to CD11c, an APC-Cy7–conjugated mAb to B220, and PI. The expression of CD11b and CD8 on cDCs was analyzed by 5-color flow cytometry (left panel). The relative numbers of CD11bhigh CD8− and CD11blow CD8+ cells are shown as a percentage of all viable cDCs of the thymus on each plot. The percentage of such CD8+ or CD8− cDCs among viable cDCs was also determined (right panel); data are means ± SE for 3 mice of each group and are representative of 3 independent experiments.
Deficiency of CD4+ cDCs and mDCs in pLNs of SIRPα MT mice. (A-B) Cells prepared from pLNs of WT or SIRPα MT mice were incubated with a biotin-conjugated mAb to CD4. The cells were also stained with PE-conjugated streptavidin, an APC-conjugated mAb to CD11c, an APC-Cy7–conjugated mAb to B220, an FITC-conjugated mAb to CD8 or to I-A, and PI. The expression of CD11c and either B220 or I-A on PI-negative cells (A) and that of CD4 and CD8 on cDCs (B) were analyzed by 5-color flow cytometry (left panels). The relative numbers of cDCs, pDCs, or CD11cint I-Ahigh mDCs are expressed as a percentage of all viable cells from pLNs in each plot (A), as are the relative numbers of CD8+, CD4+, or DN cDCs among all cDCs (B). The absolute numbers of cDCs, pDCs, and mDCs (A) or of CD8+, CD4+, or DN cDCs (B) in pLNs were also determined (right panels); data are means ± SE for 3 mice per group and are representative of 3 independent experiments. *P < .05, **P < .01 (Student t test). (C) A DC-enriched, low-density fraction of thymocytes from WT or SIRPα MT mice was incubated with a biotin-conjugated mAb to CD11b. The cells were also stained with an FITC-conjugated mAb to CD8, PE-conjugated streptavidin, an APC-conjugated mAb to CD11c, an APC-Cy7–conjugated mAb to B220, and PI. The expression of CD11b and CD8 on cDCs was analyzed by 5-color flow cytometry (left panel). The relative numbers of CD11bhigh CD8− and CD11blow CD8+ cells are shown as a percentage of all viable cDCs of the thymus on each plot. The percentage of such CD8+ or CD8− cDCs among viable cDCs was also determined (right panel); data are means ± SE for 3 mice of each group and are representative of 3 independent experiments.
Two major subtypes of CD11chigh cDCs—CD8+ CD11blow cDCs and CD8−CD11bhigh cDCs—are present in the thymus.31 The expression of SIRPα, however, is relatively low in both CD8+ cDCs and CD8− cDCs in the thymus.3,21 We found that the proportions of CD8+ CD11blow cDCs or CD8−CD11bhigh cDCs in the thymus did not differ between WT and SIRPα MT mice (Figure 2C). Together, these results thus suggested that SIRPα is important for homeostatic regulation of CD4+ cDCs as well as for mDCs in secondary lymphoid organs.
Intrinsic requirement of SIRPα for cDC homeostasis in the spleen
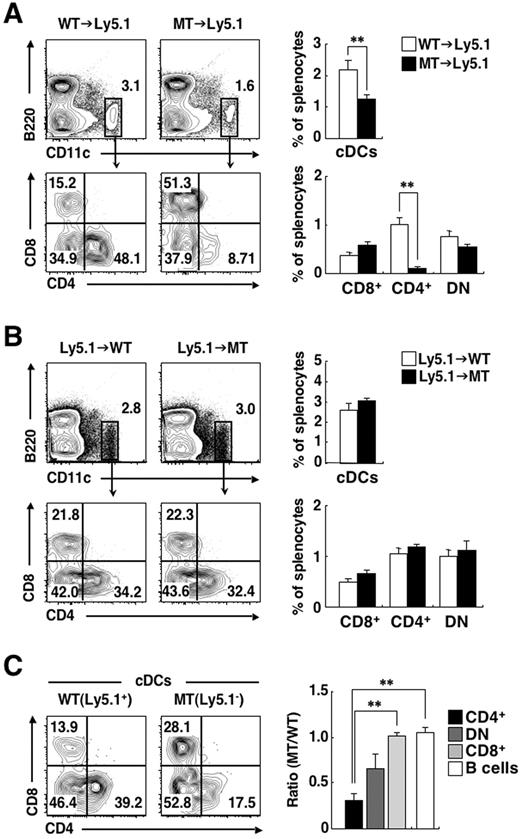
Given that splenic CD4+ cDCs express SIRPα at a high level, we next examined whether SIRPα intrinsic to CD4+ cDCs is indeed important for homeostasis of this cDC subset in the spleen. For this purpose, irradiated B6-Ly5.1 mice were reconstituted with BM cells from Ly5.2-expressing SIRPα MT (MT→Ly5.1 chimeras) or control WT (WT→Ly5.1 chimeras) mice. More than 99% of splenic CD11c+ DCs in the recipient mice did not express surface Ly5.1, suggesting that these cells were derived from the BM of donor mice (data not shown). The frequency of total cDCs as well as that of CD4+ cDCs were greatly reduced in MT→Ly5.1 chimeras, compared with those in WT→Ly5.1 chimeras (Figure 3A). In contrast, the frequency of CD8+ cDCs or that of DN cDCs did not differ between the 2 types of chimera. The extent of the decrease in the proportion of CD4+ cDCs in MT→Ly5.1 chimeras was similar to that observed in SIRPα MT mice (Figure 1B). We also reconstituted irradiated WT or SIRPα MT mice (B6-Ly5.2) with BM cells from B6-Ly5.1 WT mice. The frequencies of total cDCs or of any subtype of cDCs in the spleen were similar in the Ly5.1→MT and Ly5.1→WT chimeras (Figure 3B). These results suggested that hematopoietic SIRPα is important for the development of CD4+ cDCs in the spleen. To examine whether the SIRPα requirement is intrinsic to DCs, we reconstituted irradiated B6-Ly5.1 mice with an equal mixture of Ly5.1+ BM cells from WT mice and Ly5.2+ BM cells from SIRPα MT mice. The frequency of Ly5.2+ CD4+ cDCs, which represent cells derived from the BM of SIRPα MT donor mice, was markedly reduced, compared with that apparent for Ly5.1+ WT CD4+ cDCs (Figure 3C). The frequency of mutant DN cDCs was also reduced, compared with that of the corresponding WT cells, although this difference was not statistically significant. In contrast, the frequency of CD8+ cDCs or of B cells derived from SIRPα MT mice did not differ from that of the corresponding cells from WT mice. These results therefore suggested that SIRPα is required within cDCs or cDC precursors for normal accumulation of CD4+ cDCs in the spleen.
Intrinsic requirement of SIRPα for cDC homeostasis in the spleen. (A-B) B6-Ly5.1 mice were lethally irradiated and then reconstituted with 5 × 106 BM cells from WT or SIRPα MT mice (A). Conversely, WT or SIRPα MT mice were lethally irradiated and reconstituted with 5 × 106 BM cells from B6-Ly5.1 mice (B). Next, 6-8 weeks after transplantation, splenocytes from each chimera were incubated with a biotin-conjugated mAb to CD4. The cells were also stained with an FITC-conjugated mAb to CD8, PE-conjugated streptavidin, a PE-Cy7–conjugated mAb to Ly5.1, an APC-conjugated mAb to CD11c, an APC-Cy7–conjugated mAb to B220, and PI. The expression of CD11c and B220 on PI-negative cells or that of CD4 and CD8 on cDCs was analyzed by 6-color flow cytometry (left panels). The relative numbers of cDCs and of CD8+, CD4+, or DN cDCs are expressed as a percentage of all viable splenocytes or of cDCs, respectively, on each plot. The percentages of cDCs as well as of CD8+, CD4+, or DN cDCs among total splenocytes were also determined (right panels); data are means ± SE for a total of 7-9 mice in 3 independent experiments. **P < .01 (Student t test). (C) B6-Ly5.1 mice were lethally irradiated and then reconstituted with an equal mixture of WT (Ly5.1+) and SIRPα MT (Ly5.1−) BM cells. Then, 6-8 weeks after transplantation, splenocytes from the mixed chimeras were stained and analyzed as in (A). The expression of CD4 and CD8 on Ly5.1+ cDCs or Ly5.1− cDCs was analyzed by 6-color flow cytometry (left panel). The relative numbers of CD8+, CD4+, or DN cDCs are expressed as a percentage of Ly5.1+ cDCs or Ly5.1− cDCs on each plot. The ratios of the percentages of MT BM (Ly5.1−)–derived CD8+ cDCs, CD4+ cDCs, DN cDCs, or B cells (defined as CD11c−B220+ cells) among total Ly5.1− splenocytes to those of the corresponding cell types derived from WT BM (Ly5.1+) cells were also calculated (right panel). Data in the right panel are means ± SE for 3 mice per group and are representative of 2 independent experiments. **P < .01 (one-way analysis of variance, followed by Bonferroni test).
Intrinsic requirement of SIRPα for cDC homeostasis in the spleen. (A-B) B6-Ly5.1 mice were lethally irradiated and then reconstituted with 5 × 106 BM cells from WT or SIRPα MT mice (A). Conversely, WT or SIRPα MT mice were lethally irradiated and reconstituted with 5 × 106 BM cells from B6-Ly5.1 mice (B). Next, 6-8 weeks after transplantation, splenocytes from each chimera were incubated with a biotin-conjugated mAb to CD4. The cells were also stained with an FITC-conjugated mAb to CD8, PE-conjugated streptavidin, a PE-Cy7–conjugated mAb to Ly5.1, an APC-conjugated mAb to CD11c, an APC-Cy7–conjugated mAb to B220, and PI. The expression of CD11c and B220 on PI-negative cells or that of CD4 and CD8 on cDCs was analyzed by 6-color flow cytometry (left panels). The relative numbers of cDCs and of CD8+, CD4+, or DN cDCs are expressed as a percentage of all viable splenocytes or of cDCs, respectively, on each plot. The percentages of cDCs as well as of CD8+, CD4+, or DN cDCs among total splenocytes were also determined (right panels); data are means ± SE for a total of 7-9 mice in 3 independent experiments. **P < .01 (Student t test). (C) B6-Ly5.1 mice were lethally irradiated and then reconstituted with an equal mixture of WT (Ly5.1+) and SIRPα MT (Ly5.1−) BM cells. Then, 6-8 weeks after transplantation, splenocytes from the mixed chimeras were stained and analyzed as in (A). The expression of CD4 and CD8 on Ly5.1+ cDCs or Ly5.1− cDCs was analyzed by 6-color flow cytometry (left panel). The relative numbers of CD8+, CD4+, or DN cDCs are expressed as a percentage of Ly5.1+ cDCs or Ly5.1− cDCs on each plot. The ratios of the percentages of MT BM (Ly5.1−)–derived CD8+ cDCs, CD4+ cDCs, DN cDCs, or B cells (defined as CD11c−B220+ cells) among total Ly5.1− splenocytes to those of the corresponding cell types derived from WT BM (Ly5.1+) cells were also calculated (right panel). Data in the right panel are means ± SE for 3 mice per group and are representative of 2 independent experiments. **P < .01 (one-way analysis of variance, followed by Bonferroni test).
No impairment of in vitro differentiation of DCs from BM of SIRPα MT mice
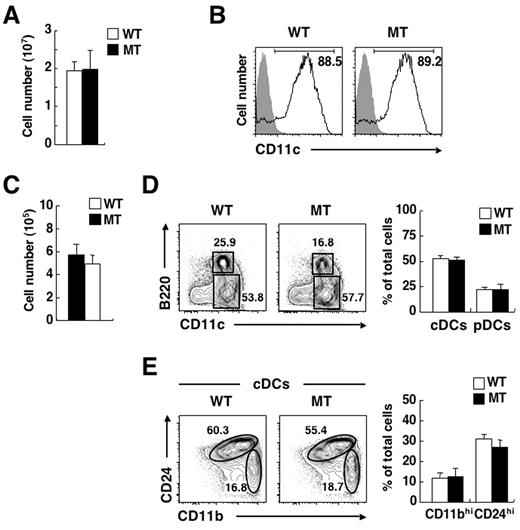
To investigate the mechanism underlying the deficiency of CD4+ cDCs in the spleen of SIRPα MT mice, we first examined the ability of BM cells of the mutant mice to differentiate into CD11c+ DCs in vitro. Culture of BM cells with granulocyte-macrophage colony-stimulating factor (GM-CSF) results in the efficient generation of CD11c+ DCs.32 Culture of BM cells from SIRPα MT mice with GM-CSF for 7 days yielded a similar number of CD11c+ DCs as did that of WT BM cells (Figure 4A), and the extent of surface expression of CD11c on these cells was also similar for the 2 genotypes (Figure 4B). Culture of BM cells with the Flt3 ligand also generates B220−CD11c+ CD24high CD11blow cells (functionally equivalent to CD8+ cDCs), B220−CD11c+ CD24low CD11bhigh cells (functionally equivalent to CD4+ or DN cDCs), as well as B220+ CD11cint pDCs in vitro.33 To evaluate further the ability of BM cells to differentiate into cDCs in vitro, we thus cultured BM cells of WT or SIRPα MT mice with the Flt3 ligand for 7 days. Culture of BM cells from SIRPα MT mice with Flt3 ligand yielded a similar number of DCs as did that of WT BM cells (Figure 4C). The proportions of B220− cDCs and B220+ pDCs generated from BM cells in the presence of the Flt3 ligand were similar for WT and SIRPα MT mice (Figure 4D). Moreover, the proportions of CD24high CD11blow cDCs and of CD24low CD11bhigh cDCs also did not differ between BM cells from WT or SIRPα MT mice (Figure 4E). Collectively, these data suggested that the differentiation of BM cells from SIRPα MT mice into CD11c+ DCs is not impaired, at least not in vitro.
No impairment of differentiation of DCs from BM of SIRPα MT mice in vitro. (A) BM cells from WT or SIRPα MT mice were cultured with GM-CSF (10 ng/mL) for 7 days in 24-well plates, after which the total number of BM-derived DCs was determined with a Burker-Turk counting chamber. Data are means ± SE from 3 independent experiments. (B) Cells cultured as in panel A were incubated with a biotin-conjugated mAb to CD11c (open traces) or control mAb (filled traces) and were also stained with PE-conjugated streptavidin and PI. The expression of CD11c on PI-negative cells was analyzed by 2-color flow cytometry. The relative number of CD11c+ cells is expressed as a percentage of all PI-negative cells on each plot. Data are representative of 3 independent experiments. (C) BM cells from WT or SIRPα MT mice were cultured with Flt3 ligand (200 ng/mL) for 7 days, after which the total number of BM-derived DCs was determined with a Burker-Turk counting chamber. Data are means ± SE from 3 independent experiments. (D-E) Cells cultured as in panel C were incubated with a biotin-conjugated mAb to CD11b and also stained with an FITC-conjugated mAb to CD24, PE-conjugated streptavidin, an APC-conjugated mAb to CD11c, an APC-Cy7–conjugated mAb to B220, and PI. The expression of CD11c and B220 on PI-negative cells (D) or that of CD11b and CD24 on cDCs (E) was analyzed by 5-color flow cytometry (left panels). The relative numbers of CD11c+ B220− cells (cDCs) and CD11cint B220+ cells (pDCs; D) or those of CD24low CD11bhigh (CD11bhi) and CD24high CD11blow (CD24hi) cDCs (E) are expressed as a percentage of all PI-negative cells (D) or of cDCs (E) on each plot. The percentages of cDCs or pDCs (D) and those of CD11bhi or CD24hi subsets (E) among PI-negative cells were also determined (right panels); data are means ± SE from 3 independent experiments.
No impairment of differentiation of DCs from BM of SIRPα MT mice in vitro. (A) BM cells from WT or SIRPα MT mice were cultured with GM-CSF (10 ng/mL) for 7 days in 24-well plates, after which the total number of BM-derived DCs was determined with a Burker-Turk counting chamber. Data are means ± SE from 3 independent experiments. (B) Cells cultured as in panel A were incubated with a biotin-conjugated mAb to CD11c (open traces) or control mAb (filled traces) and were also stained with PE-conjugated streptavidin and PI. The expression of CD11c on PI-negative cells was analyzed by 2-color flow cytometry. The relative number of CD11c+ cells is expressed as a percentage of all PI-negative cells on each plot. Data are representative of 3 independent experiments. (C) BM cells from WT or SIRPα MT mice were cultured with Flt3 ligand (200 ng/mL) for 7 days, after which the total number of BM-derived DCs was determined with a Burker-Turk counting chamber. Data are means ± SE from 3 independent experiments. (D-E) Cells cultured as in panel C were incubated with a biotin-conjugated mAb to CD11b and also stained with an FITC-conjugated mAb to CD24, PE-conjugated streptavidin, an APC-conjugated mAb to CD11c, an APC-Cy7–conjugated mAb to B220, and PI. The expression of CD11c and B220 on PI-negative cells (D) or that of CD11b and CD24 on cDCs (E) was analyzed by 5-color flow cytometry (left panels). The relative numbers of CD11c+ B220− cells (cDCs) and CD11cint B220+ cells (pDCs; D) or those of CD24low CD11bhigh (CD11bhi) and CD24high CD11blow (CD24hi) cDCs (E) are expressed as a percentage of all PI-negative cells (D) or of cDCs (E) on each plot. The percentages of cDCs or pDCs (D) and those of CD11bhi or CD24hi subsets (E) among PI-negative cells were also determined (right panels); data are means ± SE from 3 independent experiments.
No deficiency of pre-cDCs, but a reduced half-life of CD4+ cDCs in the spleen of SIRPα MT mice
To investigate further the cause of the reduced number of splenic CD4+ cDCs in SIRPα MT mice, we next determined the number of the immediate precursors of cDCs (pre-cDCs), defined as lineage-negative CD11c+ MHC class II− Flt3+ cells,7 in the spleen of the mutant animals. These pre-cDCs are widely distributed in BM as well as in secondary lymphoid tissues and circulating blood, and they are thought to give rise to all subtypes of cDCs to maintain these cells in lymphoid tissues under steady-state conditions.7 The proportion of pre-cDCs in the spleen was not decreased in SIRPα MT mice, compared with that in WT mice (Figure 5A). Given that all subtypes of cDCs arise from these pre-cDCs in the spleen, the deficiency of splenic CD4+ cDCs of SIRPα MT mice is thus unlikely to be attributable to a defect in development of pre-cDCs from BM cells or in the migration of these cells to the spleen.
No deficiency of pre-cDCs, but a reduced half-life of CD4+ cDCs in the spleen of SIRPα MT mice. (A) Splenocytes from WT or SIRPα MT mice were incubated with a biotin-conjugated mAb to Flt3 or an isotype-matched control mAb and were also stained with an FITC-conjugated mAb to I-A, PE-conjugated streptavidin, PE-Cy5–conjugated mAbs to lineage markers (CD3ϵ, CD19, B220, NK1.1, and TER-119), an APC-conjugated mAb to CD11c, and PI. The expression of CD11c and I-A on lineage- and PI-negative (Lin−PI−) cells (top left panel) or that of Flt3 on CD11c+ I-A− cells (open traces; filled traces represent staining with the isotype control; bottom left panel) was analyzed by 4-color flow cytometry. The relative numbers of CD11c+ I-A− cells or CD11c+ I-A+ cells (top left panel) and those of Flt3+ cells (pre-cDCs, bottom left panel) are expressed as a percentage of all Lin−PI− splenocytes (top left panel) or of CD11c+ I-A− cells (bottom left panel) on each plot. The percentage of pre-cDCs among total viable splenocytes was also determined (right panel); data are means ± SE from 3 separate experiments. (B) Pre-cDCs that had been sorted from BM cells of WT or SIRPα MT mice (see supplemental Figure 2) were injected intravenously into B6-Ly5.1 mice. Eight days after injection, splenocytes from recipient mice were stained as in Figure 3A. The expression of Ly5.1 on cDCs (top left panel) or that of CD4 and CD8 on Ly5.1− cDCs (derived from transferred cells of Ly5.2+ WT or MT mice; bottom left panel) was analyzed by 6-color flow cytometry. The relative numbers of Ly5.1− cells (top left panel) and of CD8+, CD4+, or DN cDCs (bottom left panel) are expressed as a percentage of all PI-negative cDCs (top left panel) or of Ly5.1− cDCs (bottom left panel) on each plot. The percentages of donor (Ly5.1−)–derived CD8+, CD4+, or DN cDCs among total cDCs were also determined (right panel); data are means ± SE from 3 independent experiments. **P < .01 (Student t test). (C) WT or SIRPα MT mice were injected intraperitoneally with 1 mg of BrdU and then continuously supplied with BrdU (0.8 mg/mL) in sterile drinking water. At various times after BrdU administration, splenocytes were isolated from the mice and stained with a PE-conjugated mAb to CD8, a PE-Cy7–conjugated mAb to CD4, an APC-conjugated mAb to CD11c, and an APC-Cy7–conjugated mAb to B220. The cells were then fixed, permeabilized, and stained with an FITC-conjugated mAb to BrdU. The percentage of BrdU-positive cells among total CD8+, CD4+, or DN cDCs in the spleen at each time point was then determined (left panels), and from these data, the half-lives of CD8+, CD4+, and DN cDCs were estimated (right panel). Data are means ± SE from 3 independent experiments. *P < .05 (Student t test).
No deficiency of pre-cDCs, but a reduced half-life of CD4+ cDCs in the spleen of SIRPα MT mice. (A) Splenocytes from WT or SIRPα MT mice were incubated with a biotin-conjugated mAb to Flt3 or an isotype-matched control mAb and were also stained with an FITC-conjugated mAb to I-A, PE-conjugated streptavidin, PE-Cy5–conjugated mAbs to lineage markers (CD3ϵ, CD19, B220, NK1.1, and TER-119), an APC-conjugated mAb to CD11c, and PI. The expression of CD11c and I-A on lineage- and PI-negative (Lin−PI−) cells (top left panel) or that of Flt3 on CD11c+ I-A− cells (open traces; filled traces represent staining with the isotype control; bottom left panel) was analyzed by 4-color flow cytometry. The relative numbers of CD11c+ I-A− cells or CD11c+ I-A+ cells (top left panel) and those of Flt3+ cells (pre-cDCs, bottom left panel) are expressed as a percentage of all Lin−PI− splenocytes (top left panel) or of CD11c+ I-A− cells (bottom left panel) on each plot. The percentage of pre-cDCs among total viable splenocytes was also determined (right panel); data are means ± SE from 3 separate experiments. (B) Pre-cDCs that had been sorted from BM cells of WT or SIRPα MT mice (see supplemental Figure 2) were injected intravenously into B6-Ly5.1 mice. Eight days after injection, splenocytes from recipient mice were stained as in Figure 3A. The expression of Ly5.1 on cDCs (top left panel) or that of CD4 and CD8 on Ly5.1− cDCs (derived from transferred cells of Ly5.2+ WT or MT mice; bottom left panel) was analyzed by 6-color flow cytometry. The relative numbers of Ly5.1− cells (top left panel) and of CD8+, CD4+, or DN cDCs (bottom left panel) are expressed as a percentage of all PI-negative cDCs (top left panel) or of Ly5.1− cDCs (bottom left panel) on each plot. The percentages of donor (Ly5.1−)–derived CD8+, CD4+, or DN cDCs among total cDCs were also determined (right panel); data are means ± SE from 3 independent experiments. **P < .01 (Student t test). (C) WT or SIRPα MT mice were injected intraperitoneally with 1 mg of BrdU and then continuously supplied with BrdU (0.8 mg/mL) in sterile drinking water. At various times after BrdU administration, splenocytes were isolated from the mice and stained with a PE-conjugated mAb to CD8, a PE-Cy7–conjugated mAb to CD4, an APC-conjugated mAb to CD11c, and an APC-Cy7–conjugated mAb to B220. The cells were then fixed, permeabilized, and stained with an FITC-conjugated mAb to BrdU. The percentage of BrdU-positive cells among total CD8+, CD4+, or DN cDCs in the spleen at each time point was then determined (left panels), and from these data, the half-lives of CD8+, CD4+, and DN cDCs were estimated (right panel). Data are means ± SE from 3 independent experiments. *P < .05 (Student t test).
To confirm further that the accumulation of CD4+ cDCs derived from pre-cDCs is indeed reduced in the spleen of SIRPα MT mice, we sorted pre-cDCs from BM of either Ly5.2+ WT or SIRPα MT mice (supplemental Figure 2) and adoptively transferred these cells to Ly5.1+ WT mice. Eight days after transfer, the frequency of Ly5.2+ CD4+ cDCs as well as that of Ly5.2+ DN cDCs derived from SIRPα MT donor mice in the recipient spleen were markedly reduced, compared with those of the corresponding cells from WT donor mice (Figure 5B). In contrast, the frequency of Ly5.2+ CD8+ cDCs derived from SIRPα MT donor mice was similar to that for the corresponding cells derived from WT mice. These data thus suggested that either the development of CD4+ cDCs from pre-cDCs or the survival of CD4+ cDCs in the spleen is impaired in SIRPα MT mice.
We therefore next examined the turnover rate of the 3 splenic cDC subpopulations by monitoring the kinetics of cell labeling with BrdU in the continuous presence of this agent.6 In the spleen of WT mice, the turnover rate of CD8+ cDCs was greater than that of the other 2 subtypes of cDCs, with the estimated half-lives of CD8+ cDCs, DN cDCs, and CD4+ cDCs being 1.5, 2.0, and 2.9 days, respectively.6 We also found that the labeling of CD8+ cDCs by BrdU in WT mice was much faster than that of CD4+ cDCs or DN cDCs (Figure 5C). However, the labeling of CD4+ cDCs was markedly faster in SIRPα MT than in WT mice. The estimated half-life of CD4+ cDCs in SIRPα MT mice was thus markedly shortened, compared with that for those in WT mice (2.0 ± 0.1 vs 3.2 ± 0.2 days; means ± SE, n = 3; P < .05; Figure 5C). By contrast, the rate of labeling with BrdU and the estimated half-life of CD8+ or DN cDCs in the spleen of SIRPα MT mice were similar to those in WT mice. These results suggested that the deficiency of CD4+ cDCs in the spleen of SIRPα MT mice is attributable, at least in part, to a reduced half-life of these cells.
Importance of both hematopoietic and nonhematopoietic CD47 for homeostasis of splenic CD4+ cDCs and DN cDCs
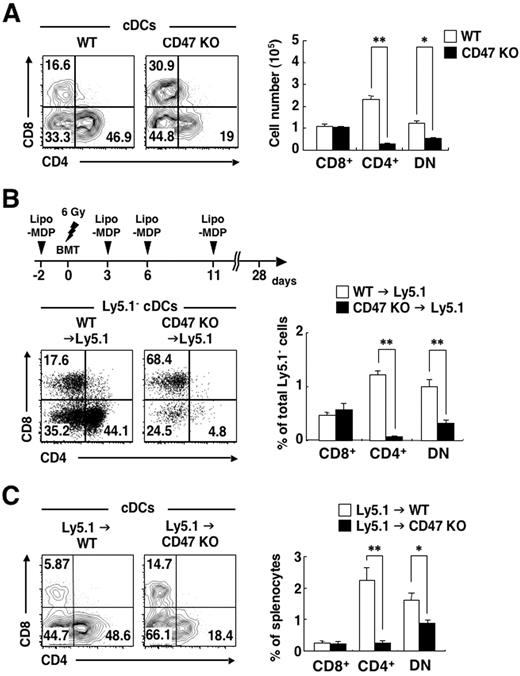
We and others have previously shown that the number of CD4+ cDCs is selectively decreased in the spleen of CD47-deficient (CD47 KO) mice on either the C57BL/6 or Balb/c background.27,34 We confirmed this observation in the present study and also found that the number of DN cDCs, but not that of CD8+ cDCs, was markedly decreased in the spleen of CD47 KO mice, compared with that in WT mice (Figure 6A). This phenotype of CD47 KO mice is thus similar to that of SIRPα ΜΤ mice, suggesting that the interaction of CD47 with SIRPα might be important for the homeostatic regulation of CD4+ cDCs. CD47 is expressed widely in both hematopoietic cells, including 3 subtypes of cDCs (supplemental Figure 3), and nonhematopoietic cells, however.20,24 We therefore next examined whether hematopoietic or nonhematopoietic CD47 is important for the accumulation of CD4+ cDCs or DN cDCs in the spleen. The transfer of BM cells from CD47 KO mice into WT recipient mice results in the rapid elimination of these cells, likely as a result of the lack of prevention by CD47-SIRPα interaction of phagocytosis by splenic macrophages.28,35 Indeed, we failed to reconstitute the BM of irradiated WT mice with BM cells from CD47 KO mice (data not shown). Intravenous injection of liposome-MDPCl2 results in the death of splenic macrophages,28,29 and such depletion of macrophages by injection of liposome-MDPCl2 before BM cell transfer resulted in successful reconstitution of irradiated WT mice with BM cells from CD47 KO mice (Figure 6B). The frequency of CD4+ cDCs as well as that of DN cDCs were markedly reduced in the spleen of CD47 KO→Ly5.1 chimeras, compared with those in WT→Ly5.1 chimeras. In contrast, the frequency of CD8+ cDCs did not differ between the 2 types of chimera (Figure 6B). Conversely, when irradiated CD47 KO mice were reconstituted with BM cells from WT mice, the frequencies of both CD4+ cDCs and DN cDCs, but not that of CD8+ DCs, in the spleen were markedly reduced, compared with those in Ly5.1→WT chimeras (Figure 6C). These data thus indicated that both hematopoietic and nonhematopoietic CD47 is important for homeostasis of both CD4+ cDCs and DN cDCs in the spleen.
Importance of both hematopoietic and nonhematopoietic CD47 for homeostasis of splenic CD4+ cDCs and DN cDCs. (A) Splenocytes from WT or CD47 KO mice were stained as in Figure 1A, and the expression of CD4 and CD8 on cDCs was analyzed by 5-color flow cytometry (left panel). The relative numbers of CD8+, CD4+, and DN cDCs are expressed as a percentage of PI-negative cDCs in each plot. The absolute numbers of CD8+, CD4+, and DN cDCs in the spleen were also determined (right panel); data are means ± SE from 3 mice per group and are representative of 3 independent experiments. *P < .05, **P < .01 (Student t test). (B) B6-Ly5.1 mice were subjected to sublethal irradiation and then reconstituted with 5 × 106 BM cells from either WT or CD47 KO mice on day 0 (BMT). MDPCl2 entrapped in liposomes (Lipo-MDP) was administrated intravenously on days −2, 3, 6, and 11, as indicated in the top panel, to prevent initial rejection of CD47 KO donor cells by recipient macrophages. Splenocytes were prepared from recipient mice on day 28 and stained as in Figure 3A. The expression of CD4 and CD8 on cDCs was analyzed by 6-color flow cytometry (bottom left panel). The relative numbers of CD8+, CD4+, and DN cDCs are expressed as a percentage of Ly5.1− cDCs on each plot. The percentages of CD8+, CD4+, and DN cDCs among total PI-negative Ly5.1− cells were also determined (right panel); data are means ± SE from 5 or 6 mice per group in 2 independent experiments. **P < .01 (Student t test). (C) WT or CD47 KO mice were subjected to lethal irradiation and reconstituted with 5 × 106 BM cells from B6-Ly5.1 mice. Eight weeks after transplantation, splenocytes were prepared from recipient mice and stained as in panel B. The expression of CD4 and CD8 on cDCs was analyzed by 6-color flow cytometry (left panel). The relative numbers of CD8+, CD4+, and DN cDCs are expressed as a percentage of total cDCs in each plot. The percentages of CD8+, CD4+, and DN cDCs among total splenocytes were also determined (right panel); data are means ± SE from 3 mice per group and are representative of 2 independent experiments. *P < .05, **P < .01 (Student t test).
Importance of both hematopoietic and nonhematopoietic CD47 for homeostasis of splenic CD4+ cDCs and DN cDCs. (A) Splenocytes from WT or CD47 KO mice were stained as in Figure 1A, and the expression of CD4 and CD8 on cDCs was analyzed by 5-color flow cytometry (left panel). The relative numbers of CD8+, CD4+, and DN cDCs are expressed as a percentage of PI-negative cDCs in each plot. The absolute numbers of CD8+, CD4+, and DN cDCs in the spleen were also determined (right panel); data are means ± SE from 3 mice per group and are representative of 3 independent experiments. *P < .05, **P < .01 (Student t test). (B) B6-Ly5.1 mice were subjected to sublethal irradiation and then reconstituted with 5 × 106 BM cells from either WT or CD47 KO mice on day 0 (BMT). MDPCl2 entrapped in liposomes (Lipo-MDP) was administrated intravenously on days −2, 3, 6, and 11, as indicated in the top panel, to prevent initial rejection of CD47 KO donor cells by recipient macrophages. Splenocytes were prepared from recipient mice on day 28 and stained as in Figure 3A. The expression of CD4 and CD8 on cDCs was analyzed by 6-color flow cytometry (bottom left panel). The relative numbers of CD8+, CD4+, and DN cDCs are expressed as a percentage of Ly5.1− cDCs on each plot. The percentages of CD8+, CD4+, and DN cDCs among total PI-negative Ly5.1− cells were also determined (right panel); data are means ± SE from 5 or 6 mice per group in 2 independent experiments. **P < .01 (Student t test). (C) WT or CD47 KO mice were subjected to lethal irradiation and reconstituted with 5 × 106 BM cells from B6-Ly5.1 mice. Eight weeks after transplantation, splenocytes were prepared from recipient mice and stained as in panel B. The expression of CD4 and CD8 on cDCs was analyzed by 6-color flow cytometry (left panel). The relative numbers of CD8+, CD4+, and DN cDCs are expressed as a percentage of total cDCs in each plot. The percentages of CD8+, CD4+, and DN cDCs among total splenocytes were also determined (right panel); data are means ± SE from 3 mice per group and are representative of 2 independent experiments. *P < .05, **P < .01 (Student t test).
Discussion
We have shown here that the number of splenic CD4+ cDCs is specifically reduced in the spleen of SIRPα MT mice. Furthermore, the decrease in the number of splenic CD4+ cDCs was also observed in hematopoietic or mixed BM chimeras, indicating that SIRPα is required in a cell-autonomous manner for homeostatic regulation of CD4+ cDCs. This conclusion is consistent with the finding that the level of SIRPα expression on CD4+ cDCs is much greater than that on CD8+ cDCs. The numbers of CD4+ cDCs and mDCs in pLNs, both of which express SIRPα at a high level,21 were also markedly reduced, whereas the number of SIRPαlow cDCs in the thymus was not, in SIRPα MT mice. We further investigated the cause of the reduction in the number of CD4+ cDCs in the spleen of SIRPα MT mice. We found that neither the differentiation of DCs from BM cells in vitro nor the accumulation of pre-cDCs in the spleen was impaired for SIRPα MT mice, suggesting that SIRPα is not important for differentiation of pre-cDCs from BM cells or for migration of pre-cDCs to the spleen. Indeed, either the LN homing capacity of, or chemotactic migration of BMDCs from, SIRPα MT mice was not impaired (H.I., Y.S., T.M., unpublished data, December 2008)). In contrast, the accumulation of CD4+ cDCs as well as that of DN cDCs derived from pre-cDCs that had been adoptively transferred from SIRPα MT mice were markedly reduced in extent in the spleen of recipient WT mice. Finally, we showed that the half-life of CD4+ cDCs was markedly shortened in the spleen of SIRPα MT mice. Together, our results thus indicate that SIRPα is important for the survival of CD4+ cDCs in the spleen.
The molecular mechanism underlying regulation by SIRPα of cDC survival remains only partially elucidated. Both CD40 and receptor activator of NF-κB (RANK), both of which are expressed on DCs, are thought to promote DC survival through activation of NF-κB and up-regulation of the antiapoptotic protein, Bcl-xL.36-39 However, promotion of the survival of DCs by a mAb to CD40 or by RANK ligand was similar for cells isolated from the BM of either SIRPα MT or WT mice (Y.S., T.M., unpublished data, July 2009), suggesting that the deficiency of splenic CD4+ cDCs in SIRPα MT mice is unlikely attributable to impairment of the CD40- or RANK-mediated signaling pathways. In contrast, the expression of SIRPα on CD4+ cDCs or their progenitors may have consequences on their phagocytosis and, consequently, antigen-presentation function. Diminished presentation of self- or foreign antigens by SIRPα-deficient DCs might lead to diminished interaction with naive and memory T cells. Given that activated T cells promote the survival of DCs by CD40L or RANKL,36-39 less “survival signals” from T cells in SIRPα MT mice may also contribute to the reduction in the survival of CD4+ cDCs. The Notch–RBP-J signaling pathway is also thought to be important for the homeostasis of CD8− cDCs through the promotion of cell survival,13 but it is unclear whether SIRPα-mediated signaling interacts with Notch–RBP-J signaling. In contrast, SHP-2, a protein tyrosine phosphatase that binds to the immunoreceptor tyrosine–based inhibition motifs in the cytoplasmic region of SIRPα, is implicated in the activation of mitogen-activated protein kinase or NF-κB in response to growth factors or cytokines.40,41 Indeed, forced expression of either SIRPα or SHP-2 enhanced the prosurvival action of brain-derived neurotrophic factor in cultured neurons.42,43 Such effects also require the activities of mitogen-activated protein kinase and phosphatidylinositol 3-kinase, signaling molecules that are important for the promotion of cell survival. The binding of SHP-2 to the tyrosine-phosphorylated cytoplasmic region of SIRPα is thought to increase the catalytic activity of this protein tyrosine phosphatase.19,20 SIRPα MT mice express a version of SIRPα that lacks the cytoplasmic region of this protein. The action of SHP-2 downstream of SIRPα might be thus responsible for the promotion of the survival of CD4+ cDCs in the spleen.
Similar to SIRPα MT mice, CD47 KO mice manifested a marked decrease in the number of both CD4+ cDCs and DN cDCs, but not in that of CD8+ cDCs, in the spleen. Moreover, the accumulation of pre-cDCs in the spleen was also not impaired, but rather increased in CD47 KO mice (supplemental Figure 4). Examination of the kinetics of cDC labeling with BrdU previously showed that the labeling of CD4+ cDCs at 48 hours was markedly increased in the spleen of CD47 KO mice, compared with that in WT mice,34 indicating that the turnover rate of CD4+ cDCs in the spleen of CD47 KO mice is increased, as observed with SIRPα MT mice in the present study. Given that CD47 is a ligand for SIRPα, the similarity of these CD47 KO mouse phenotypes to those of SIRPα MT mice suggests that interaction of CD47 with SIRPα is important for homeostatic regulation of CD4+ cDCs. In contrast to SIRPα, however, our results with BM chimeras suggest that both hematopoietic and nonhematopoietic CD47 is important for the homeostasis of both CD4+ cDCs and DN cDCs in the spleen. With regard to the role of hematopoietic CD47, cis-interaction of CD47 with SIRPα within cDCs or trans-interaction of CD47 on other hematopoietic cell types (such as T or B cells) with SIRPα on DCs might be important for the regulation of CD4+ cDCs in the spleen. However, it seems unlikely that CD47 on cDCs directly regulates CD4+ and DN cDC homeostasis, given that CD47 is expressed in all cDC subsets (supplemental Figure 3), whereas only CD4+ cDCs and DN cDCs (but not CD8+ cDCs) are deficient in the spleen of CD47 KO mice.
With regard to the role of nonhematopoietic CD47, trans-interaction of CD47 on nonhematopoietic cells, such as stromal or endothelial cells, with SIRPα on cDCs might be important for the regulation of CD4+ cDCs in the spleen. Indeed, CD47 is expressed in splenic and BM stromal cells44 (Y.S., T.M., unpublished data, October 2009) as well as in ECs.24 Stromal cells in BM or secondary lymphoid organs are implicated in the regulation of the proliferation, maturation, and survival of DCs.45-47 Both soluble factors, such as interleukin-7,48 produced by stromal cells as well as cell-to-cell contact with stromal cells,49 are thought to be important for such regulation. Indeed, the Notch ligands Delta-1 and Jagged-1 expressed on BM stromal cells regulate DC differentiation by interacting with Notch on DC precursors.50 CD47-SIRPα signaling is thus another important contributor to the regulation of cDC homeostasis by stromal cell–DC interaction. Ligation of SIRPα by CD47 is thought to promote the tyrosine phosphorylation of the cytoplasmic region of SIRPα.20 We therefore propose that either cis- or trans-interaction of CD47 with SIRPα on cDCs promotes CD4+ cDCs survival in secondary lymphoid organs by inducing the tyrosine phosphorylation of SIRPα. In contrast, ligation of CD47 by SIRPα might activate the signaling downstream of CD47 and change the microenvironment of developing DCs, thus leading to enhanced survival of SIRPα+ cDCs. CD47 KO mice manifested a decrease in the number of DN cDCs, but SIRPα MT mice did not, suggesting that the regulation by CD47 of DN cDCs is independent of SIRPα. Thus, further investigation will be necessary to clarify the precise mechanism by which SIRPα and CD47 regulate the homeostasis of cDCs in secondary lymphoid tissues.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank C. F. Lagenaur for the mAb to SIRPα; K. Okumura for the mAb to CD16/32; H. Nakauchi for B6-Ly5.1 congenic mice; D. Hashimoto for technical suggestions; T. Ohteki and N. Onai for suggestions and discussion; as well as Y. Hayashi, Y. Niwayama-Kusakari, E. Urano, and R. Koitabashi for their technical assistance.
This work was supported by a Grant-in-Aid for Scientific Research on Priority Areas Cancer, a Grant-in-Aid for Scientific Research (B), a Grant-in-Aid for Young Scientists (B), and a grant of the Global COE Program from the Ministry of Education, Culture, Sports, Science, and Technology of Japan; a grant from the Swedish Research Council (31X-14286) and the Faculty of Medicine, Umeå University; and a Young Researcher Award from Umeå University.
Authorship
Contribution: Y.S. designed and performed the experiments, analyzed the data, and wrote the manuscript; H.I., T.K., Y. Kanazawa, M.S.-H., and H.K. performed experiments; H. Ohnishi, Y.M., H. Okazawa, Y. Kaneko, and Y.N. contributed conceptually to the project; P.-A.O. generated and provided CD47 knockout mice; M.N. prepared liposome-MDPCl2; and T.M. supervised the entire project and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Takashi Matozaki, Laboratory of Biosignal Sciences, Institute for Molecular and Cellular Regulation, Gunma University, 3-39-15 Showa-Machi, Maebashi, Gunma 371-8512, Japan; e-mail: matozaki@showa.gunma-u.ac.jp or matozaki@med.kobe-u.ac.jp.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal