In this issue of Blood, Sabado and colleagues report on the dynamics of DCs during the early phases of primary HIV infection, showing that changes in DC numbers and function which may play pivotal roles in the immunopathogenesis of chronic HIV infection, can in fact be observed even during the early stages of the disease.
Dendritic cells (DCs) can be regarded as gatekeepers for the immune system. They provide the initial sparkle to ignite immune reactions against pathogens, but also shape and regulate the activity of effector immune cells and preserve the vital equilibrium between efficient immune responses and potentially catastrophic outbursts of uncontrolled immune reactions.1 The dysregulation of DC function during HIV infection, as well as their direct interaction with the virus, are central to both the spread of the infection and the progression of the immunodeficiency. Myeloid DCs (mDCs) that accumulate at the site of infection can be productively infected and directly allow infection of CD4 T cells through cell-mediated virus transfer.2 Plasmacytoid DCs (pDCs) are activated to produce chemokines that attract CD4 T cells to the site of infection, providing a fertile ground for the systemic dissemination of HIV.3 Besides the events that favor the initial spread of the infection, alterations of mDC and pDC activity are also thought to play leading roles in inducing and sustaining the chronic immune activation that characterizes HIV infection and drives its immunopathogenesis.4
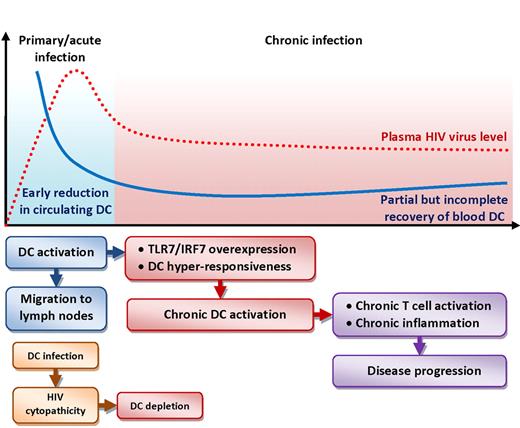
Schematic representation of the changes in blood DC numbers (blue line, both pDCs and mDCs) over time compared with HIV plasma viral load (dotted red line) during the acute (light blue background) and chronic (red background) phases of infection. The flowchart below the graph illustrates cellular and molecular events that may affect DC dynamics and their consequences on HIV disease. Activation of DCs during acute infection leads to their migration to lymphoid tissues, resulting in the reduction of circulating DCs. HIV-mediated cytopathic effect may contribute to DC depletion. Activated DCs are hyperresponsive to further stimulation due to overexpression of Toll-like receptor 7 (TLR7) and interferon regulatory factor 7 (IRF7). Chronic activation of DCs supports pathogenic T-cell activation and chronic inflammation.
Schematic representation of the changes in blood DC numbers (blue line, both pDCs and mDCs) over time compared with HIV plasma viral load (dotted red line) during the acute (light blue background) and chronic (red background) phases of infection. The flowchart below the graph illustrates cellular and molecular events that may affect DC dynamics and their consequences on HIV disease. Activation of DCs during acute infection leads to their migration to lymphoid tissues, resulting in the reduction of circulating DCs. HIV-mediated cytopathic effect may contribute to DC depletion. Activated DCs are hyperresponsive to further stimulation due to overexpression of Toll-like receptor 7 (TLR7) and interferon regulatory factor 7 (IRF7). Chronic activation of DCs supports pathogenic T-cell activation and chronic inflammation.
This report shows that the depletion of both mDCs and pDCs from the circulation already occurs during the early phases of HIV infection, likely before or around the time when viremia peaks during acute infection (patients in Fiebig stages I-II).5 The fact that both DC types retain the ability to up-regulate the chemokine receptor CCR7, functionally associated with migration to lymph nodes, suggests a preferential redistribution from blood to lymphoid organs,5 where HIV replication is more active and where DCs exert their immune functions. The reduction in circulating DCs is not transient or limited to the early phases of infection, but is protracted throughout the chronic phase and only partially recovered by antiretroviral therapy.5 One of the most interesting pieces of information presented is that the changes in DC numbers are evident even in patients identified as long-term nonprogressors and elite controllers, who maintain an asymptomatic status with often undetectable viremia and apparent control of the infection for extended periods of time.5 Thus, the migration or depletion of DCs from the circulation may not only be irreversible, but may also lie beneath the immune-mediated control of the infection, potentially undermining the immunologic balance even in apparently immunocompetent patients.
The finding that, despite their reduction in number during primary HIV infection, both mDCs and pDCs retain the ability to stimulate T-cell responses—at least as alloantigens—and conserve or increase their responsiveness to direct stimulation with HIV or TLR7/8 ligands is noteworthy.5 This is substantiated by the authors' finding that the expression of molecules involved in the signaling cascade of DC activation, such as TLR7 and IRF7, is increased in DCs from patients with primary HIV infection.5 These observations confirm that DCs can react—or overreact—to stimuli and elicit T-cell activation, preserving their role as master regulators of immune responses. This is a central issue in HIV pathogenesis. Thus, activated mDCs and pDCs that have transferred to lymphoid tissues may directly contribute to triggering chronic T-cell activation and exhaustion, as well as release inflammatory mediators that sustain pathogenic immune activation. Consistent with this view, the authors report the increased expression of interferon-stimulated genes in total leukocytes as well as remarkable differences in the gene expression profiles of both DC types from primary HIV-infected patients compared with controls.5
The classic view of HIV disease as a chronic and relatively slow progressing condition has been reviewed in recent years, and increasing evidence suggests that the virologic and immunologic events that occur during primary HIV infection irremediably damage the immune system, which is left incapable of recovery and condemned to gradually fail. Rapid and irreversible loss of CD4 T lymphocytes from the gut mucosa occurs during acute HIV infection of humans and simian immunodeficiency virus (SIV) infection of macaques, leading to disruption of the mucosal barrier and microbial translocation, a chain of events that triggers and promotes pathogenic immune activation during the course of the disease.6 Furthermore, recent evidence showsthat the up-regulation of interferon-stimulated genes is transient and limited to the acute phase in nonpathogenic SIV infection of natural host nonhuman primates. In contrast, the burst of these responses does not cease after the peak of viremia in pathogenic SIV infection of macaques, and correlates with chronic immune activation and disease progression.7,8 The current study places the dysregulation of pDCs and mDCs in the same perspective, suggesting that the generalized activation of these cells represents one of the early immunologic events in response to HIV infection, which imprints permanent consequences on the immune system and may pose the basis for immunopathogenic events that characterize the whole course of the disease (see figure).
High-quality research often raises more questions than the answers it provides. The study by Sabado and colleagues forces us to reflect on which of the events leading to and following DC activation during primary HIV infection are part of what could be considered a “normal” immune response, and at which point these mechanisms are distorted by HIV in a pathogenic manner. Even more important are the implications that these findings may have for the development of prophylactic and therapeutic interventions. Can the rapid dysregulation of DCs during acute HIV infection negatively impact the efficacy of a vaccine-induced immune response? Can immunotherapy reverse the early changes in DC dynamics that seem to persist even when viral replication is spontaneously of pharmacologically controlled? Restoring, at least in part, the anatomic distribution and the resting status of DCs may represent a key step toward the recovery of immune responses in HIV-infected patients.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■
REFERENCES
Wellcome Trust

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal