Abstract
Arsenic trioxide (As2O3) is a highly effective treatment for patients with relapsed acute promyelocytic leukemia (APL); its role as consolidation treatment for patients in first remission has not been defined. We randomized 481 patients (age ≥ 15 years) with untreated APL to either a standard induction regimen of tretinoin, cytarabine, and daunorubicin, followed by 2 courses of consolidation therapy with tretinoin plus daunorubicin, or to the same induction and consolidation regimen plus two 25-day courses of As2O3 consolidation immediately after induction. After consolidation, patients were randomly assigned to one year of maintenance therapy with either tretinoin alone or in combination with methotrexate and mercaptopurine. Ninety percent of patients on each arm achieved remission and were eligible to receive their assigned consolidation therapy. Event-free survival, the primary end point, was significantly better for patients assigned to receive As2O3 consolidation, 80% compared with 63% at 3 years (stratified log-rank test, P < .0001). Survival, a secondary end point, was better in the As2O3 arm, 86% compared with 81% at 3 years (P = .059). Disease-free survival, a secondary end point, was significantly better in the As2O3 arm, 90% compared with 70% at 3 years (P < .0001). The addition of As2O3 consolidation to standard induction and consolidation therapy significantly improves event-free and disease-free survival in adults with newly diagnosed APL. This trial was registered at clinicaltrials.gov (NCT00003934).
Introduction
Acute promyelocytic leukemia (APL) is a distinct clinical and pathologic entity that accounts for approximately 10% to 15% of acute myeloid leukemia cases. The unique features of APL have been well described1,2 and include a characteristic morphologic appearance,3 a reciprocal translocation between the long arms of chromosomes 15 and 17,4 younger age of onset,5 and severe consumptive coagulopathy with a high incidence of early fatal hemorrhage.1,6,7 Leukemia cells from the majority of patients with APL contain 2 novel fusion genes, PML-RARα and RARα-PML, which are the molecular consequences of the chromosomal translocation.8,9 The PML-RARα gene, which fuses sequences from the promyelocytic leukemia (PML) gene at chromosome band 15q22, with domains B through F of the retinoic acid receptor alpha (RAR)α gene at band 17q11-12 is the target of tretinoin (all-trans-retinoic acid or ATRA), which overcomes the dominant negative effect of the PML-RARα fusion and induces differentiation and apoptotic cell death of the malignant clone. The presence of the PML-RARα fusion transcript provides a convenient and practical “molecular marker” for diagnosing APL using a reverse transcriptase polymerase chain reaction (RT-PCR) assay.
Current treatment of APL combines ATRA with anthracycline-based chemotherapy to induce remission, followed by consolidation chemotherapy and ATRA maintenance.10-13 These approaches yield complete remission (CR) rates of approximately 90% and improved event-free survival. The white blood cell (WBC) count at presentation has been identified as the most important prognostic factor for clinical outcome.12,14
Arsenic trioxide (As2O3) was approved for treatment of patients with relapsed APL, as it effectively induces CR in the majority of these patients.15-17 The current study was designed to test the hypothesis that the early addition of arsenic trioxide as postremission therapy would improve the event-free survival (EFS) of patients with APL in first CR, and to compare the efficacy and toxicities of maintenance therapy with ATRA alone or in combination with oral methotrexate and mercaptopurine.
Methods
Study population
The North American Leukemia Intergroup Protocol C9710 opened in June 1999 and closed to accrual in March 2005. This trial was approved by all participating institutional review boards and was registered at ClinTrial.gov (NCT00003934). Eligibility for C9710 required a clinical diagnosis of APL with subsequent confirmation of PML-RARα by RT-PCR assay at 1 of 3 cooperative group central laboratories, and written informed consent. Because of the urgency of therapy in patients with APL, they could be registered before completion of RT-PCR studies, but if the RT-PCR study was negative, the patient was ineligible and removed from study. No prior therapy was allowed other than hydroxyurea or corticosteroids to control an elevated WBC count.
Patient registration and data collection were managed by the Cancer and Leukemia Group B (CALGB) Statistical Center. Data quality was ensured by careful review of data by CALGB Statistical Center staff and by the study chairperson. Statistical analyses were performed by CALGB statisticians.
Study design
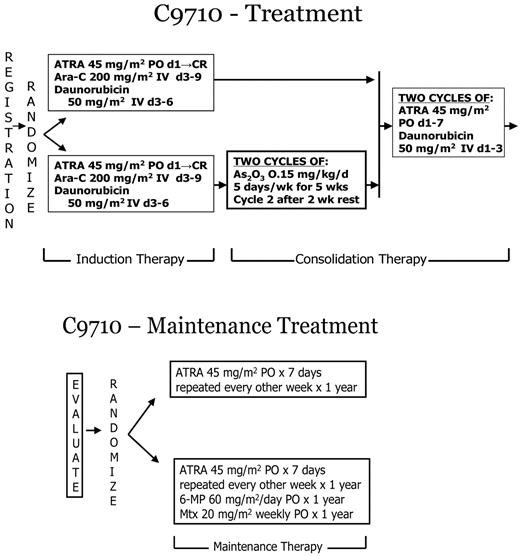
Adult patients (≥ 15 years) were randomized at study entry (stratified by age group: 15-60 years, > 60 years) to standard induction and consolidation therapy with or without two 25-day courses of As2O3 consolidation given after the standard induction and before the consolidation treatments (Figure 1). By study design children < 15 years of age were assigned to the standard non-As2O3 arm, and their results will be reported in detail separately. Remission induction therapy used oral ATRA (45 mg/m2/day, divided in twice-daily doses) beginning on day 1 and continuing until CR or day 90, cytarabine (200 mg/m2 daily as a continuous intravenous infusion for 7 days) on days 3 through 9, and daunorubicin (50 mg/m2 intravenously daily for 4 days) on days 3 through 6. Supportive care including management of coagulopathy, transfusions, and antibiotics was at the discretion of the treating physician. Study requirements included coagulation tests at least 3 times per week until normal and use of heparin was discouraged. Treatment of suspected APL differentiation syndrome was directed by the study and included dexamethasone 10 mg twice daily for 3 days and holding ATRA until symptoms resolved.
Treatment schema for C9710, a prospective randomized clinical trial evaluating As2O3 as early consolidation for adults with APL in first remission.
Treatment schema for C9710, a prospective randomized clinical trial evaluating As2O3 as early consolidation for adults with APL in first remission.
Consolidation therapy for both study arms began within 2 to 4 weeks of achievement of hematologic remission. Patients randomly assigned to the investigational arm received two 25-day courses of As2O3 (0.15 mg/kg daily intravenously over 1 hour for 5 days each week for 5 weeks) with a 2-week interval between courses. All patients received standard consolidation with 2 courses of ATRA (45 mg/m2 daily in divided doses for 7 days) plus daunorubicin (50 mg/m2 intravenously daily on the first 3 days). As2O3 was supplied under a Clinical Trials Agreement between the Division of Cancer Treatment and Diagnosis, the National Cancer Institute (NCI), and Cephalon, Inc.
Patients who remained in CR after completion of consolidation therapy were assigned by a second randomization (stratified by consolidation arm and by age group: 15-60 and > 60 years) to one year of ATRA maintenance (45 mg/m2 daily in divided doses for 7 days repeated every other week), with or without oral 6-mercaptopurine (60 mg/m2 daily) and oral methotrexate (20 mg/m2 weekly). Maintenance therapy began 2 to 4 weeks after recovery from the final course of consolidation therapy. The initial 50 patients were randomized to maintenance ATRA or observation. When preliminary results from other trials suggested a benefit from maintenance therapy, this study was amended to the randomized treatments described above.
As part of the quality assurance program of the CALGB, members of the Audit Committee visit all participating institutions at least once every 3 years to review source documents. The auditors verify compliance with federal regulations and protocol requirements, including those pertaining to eligibility, treatment, adverse events, tumor response, and outcome in a sample of protocols at each institution. Such on-site review of medical records was performed for a subgroup of 109 patients (19%) of the 582 patients under this study.
Study endpoints
The primary endpoint for the randomized comparison of the 2 consolidation arms was EFS, defined as the time from study entry to first event. An event was defined as failure to achieve a CR, relapse after achieving a CR, or death. Disease-free survival (DFS) was defined as the time from attainment of a CR to relapse or death. Overall survival was defined as the time from study entry to death. Response and relapse were defined by 1990 NCI criteria.18 Toxicity was graded using the NCI Common Toxicity Criteria version 2.X. Risk status was defined as low or intermediate for WBC count ≤ 10 × 109/L and high for WBC count > 10 × 109/L.12,14
Statistical analysis
Interim analyses were conducted semiannually by the independent CALGB Data and Safety Monitoring Board (DSMB) using the guidelines by Friedlin et al,19 and compared EFS, DFS, and survival using log-rank tests. Kaplan-Meier estimates were used for comparison of EFS, DFS, and survival at 3 years.
All analyses were based on intention-to-treat and included all patients who had a confirmed diagnosis of APL and any available data, independent of the amount of therapy received or compliance with protocol therapy and follow-up.
Results by induction/consolidation arm were released by the DSMB in November 2006, and preliminary results were released by the NCI on January 24, 2007. Statistical analyses for this report were completed on December 1, 2008. Median and maximum follow up of surviving patients are 54 months and 102 months, respectively. Relatively few events have occurred to evaluate the maintenance randomization. However, the interaction effect of induction and consolidation by maintenance was examined using a proportional hazard model on DFS (primary end point of maintenance randomization) and survival.
Results
Induction
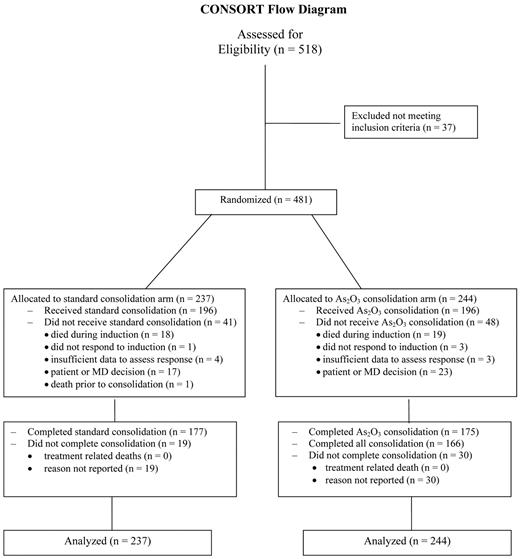
Five hundred eighteen adults (15-79 years) with untreated APL were enrolled by 5 North American cooperative groups (CALGB, Eastern Cooperative Oncology Group, Southwest Oncology Group, Children's Oncology Group, and National Cancer Institute of Canada Clinical Trials Group. Thirty-seven adults who did not have confirmation of PML-RARα by RT-PCR, were ineligible and were not included in the analyses. Patient characteristics of the 481 patients randomized to the standard arm and to the investigational As2O3 arm were very similar. (Table 1). The Consolidated Standards of Reporting Trials diagram for the trial is shown in Figure 2.
Pretreatment characteristics
| Characteristic . | No As2O3 . | As2O3 . | ||
|---|---|---|---|---|
| n . | (%) . | n . | (%) . | |
| Eligible patients | 237 | 244 | ||
| Age, y | ||||
| 15-60 | 197 | (83) | 207 | (85) |
| > 60 | 40 | (17) | 37 | (15) |
| Sex | ||||
| Male | 124 | (52) | 123 | (50) |
| Female | 113 | (48) | 121 | (50) |
| Race | ||||
| White | 196 | (83) | 196 | (80) |
| African American | 14 | (6) | 19 | (8) |
| Hispanic | 10 | (4) | 7 | (3) |
| Other | 17 | (7) | 22 | (9) |
| Performance status | ||||
| 0 | 91 | (38) | 94 | (39) |
| 1 | 102 | (43) | 89 | (36) |
| 2 | 26 | (11) | 36 | (15) |
| 3 or 4 | 11 | (5) | 14 | (6) |
| Unknown | 7 | (3) | 11 | (4) |
| Risk group* | ||||
| Low | 67 | (28) | 69 | (28) |
| Intermediate | 112 | (47) | 120 | (49) |
| High | 58 | (25) | 55 | (23) |
| Peripheral blood, median (range) | ||||
| WBC, ×109/L | 2.2 | (0.2-117.4) | 2.4 | (0.2-138.6) |
| Platelets, ×109/L | 29.5 | (1.0-221) | 30.0 | (3.0-232) |
| Hemoglobin, g/dL | 9.4 | (6.0-13.7) | 9.0 | (4.3-14.5) |
| Characteristic . | No As2O3 . | As2O3 . | ||
|---|---|---|---|---|
| n . | (%) . | n . | (%) . | |
| Eligible patients | 237 | 244 | ||
| Age, y | ||||
| 15-60 | 197 | (83) | 207 | (85) |
| > 60 | 40 | (17) | 37 | (15) |
| Sex | ||||
| Male | 124 | (52) | 123 | (50) |
| Female | 113 | (48) | 121 | (50) |
| Race | ||||
| White | 196 | (83) | 196 | (80) |
| African American | 14 | (6) | 19 | (8) |
| Hispanic | 10 | (4) | 7 | (3) |
| Other | 17 | (7) | 22 | (9) |
| Performance status | ||||
| 0 | 91 | (38) | 94 | (39) |
| 1 | 102 | (43) | 89 | (36) |
| 2 | 26 | (11) | 36 | (15) |
| 3 or 4 | 11 | (5) | 14 | (6) |
| Unknown | 7 | (3) | 11 | (4) |
| Risk group* | ||||
| Low | 67 | (28) | 69 | (28) |
| Intermediate | 112 | (47) | 120 | (49) |
| High | 58 | (25) | 55 | (23) |
| Peripheral blood, median (range) | ||||
| WBC, ×109/L | 2.2 | (0.2-117.4) | 2.4 | (0.2-138.6) |
| Platelets, ×109/L | 29.5 | (1.0-221) | 30.0 | (3.0-232) |
| Hemoglobin, g/dL | 9.4 | (6.0-13.7) | 9.0 | (4.3-14.5) |
Low risk group, WBC ≤ 10 and platelets > 40; intermediate, WBC ≤ 10 and platelets ≤ 40; and high, WBC > 10.
Consolidated Standards of Reporting Trials diagram for North American Intergroup Study C9710.
Consolidated Standards of Reporting Trials diagram for North American Intergroup Study C9710.
Induction therapy was identical for both arms and yielded similar response rates of 90%: 213 of 237 and 219 of 244, respectively. Nineteen patients (8%) on each arm died during induction. One patient on the standard arm and 3 on the As2O3 arm had persistent leukemia reported. Four and 3 patients, respectively, had insufficient data to evaluate response. The APL differentiation syndrome was reported in 177 (37%) patients and 162 received dexamethasone.
Consolidation
Of 213 responding patients on the standard arm, 196 (92%) received consolidation therapy, and 177 of 196 (90%) completed the planned consolidation therapy. Of the 219 responding patients randomized to the As2O3 arm, 196 (89%) received at least one dose of As2O3 during consolidation therapy; 175 of the 196 (89%) completed both courses of the As2O3 consolidation, and 166 of 196 (85%) completed all planned consolidation.
There were no treatment-related deaths reported during consolidation therapy. Maximum hematologic adverse events due to consolidation treatments were 16% grade 3 and 67% grade 4 on the standard arm, and 21% grade 3 and 54% grade 4 on the As2O3-containing arm. Maximum nonhematologic adverse events due to consolidation treatments were 30% grade 3 and 5% grade 4 for the standard arm, and 41% grade 3 and 5% grade 4 for the As2O3-containing arm. The greatest difference in grade 3 nonhematologic toxicity was headache at 3% in the non-As2O3 arm compared with 7% in the As2O3 arm, with smaller differences in electrolyte abnormalities (1% vs 3%) and nausea (3% vs 4%). No grade 3 or 4 cardiac toxicities due to QTc prolongation were observed on the As2O3 arm. No patients experienced the APL differentiation syndrome during consolidation.
Maintenance
One hundred sixty-five patients were randomized to maintenance with intermittent ATRA alone (83 from the As2O3 arm and 82 from the non-As2O3 arm), and 165 to intermittent ATRA plus 6-mercaptopurine and methotrexate chemotherapy (81 from the As2O3 arm, 84 from non-As2O3 arm). The initial 50 adults randomized to either ATRA or observation were not included in this analysis.
Outcomes
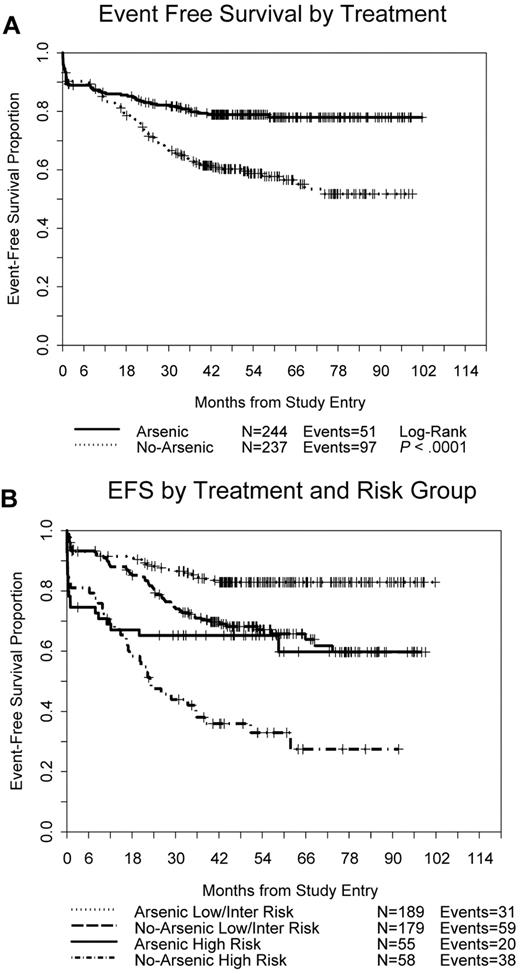
EFS was significantly better for patients randomized to receive As2O3 consolidation therapy (Figure 3A) with 51 events compared with 97 events on the non-As2O3 arm (stratified log-rank test, P < .0001); EFS at 3 years was 80% versus 63% (P < .0001). As2O3 consolidation provided significant benefit both to patients with low/intermediate risk (P = .0003) and to those with high risk APL (P = .015; Figure 3B).
Event-free survival. (A) All 481 randomized patients by treatment arm. (B) By treatment arm and APL risk group.
Event-free survival. (A) All 481 randomized patients by treatment arm. (B) By treatment arm and APL risk group.
The DFS was significantly better in patients on the As2O3 consolidation arm (Figure 4A) with 22 events compared with 72 events on the non-As2O3 arm (P < .0001); The DFS at 3 years was 90% versus 70% (P < .0001). There have been no relapses on the arsenic trioxide arm after 36 months; 1 patient died in remission at 40 months and another died in remission at 58 months both of unknown causes. Again, As2O3 consolidation provided significant benefit both to patients with low/intermediate risk (P < .0001) and to those with high risk APL (P < .0001; Figure 4B). Furthermore, As2O3 consolidation overcame the negative impact of high risk disease (Figure 4B), yielding DFS similar to that for patients in the low/intermediate risk group (P = .24). Among the 219 patients randomized to the investigational arm who achieved a remission and were therefore eligible to receive consolidation therapy, 196 received at least 1 dose of As2O3; only 7 (4%) of these patients have relapsed.
Disease-free survival. (A) All 424 patients who achieved a remission by treatment arm. (B) By treatment arm and APL risk group.
Disease-free survival. (A) All 424 patients who achieved a remission by treatment arm. (B) By treatment arm and APL risk group.
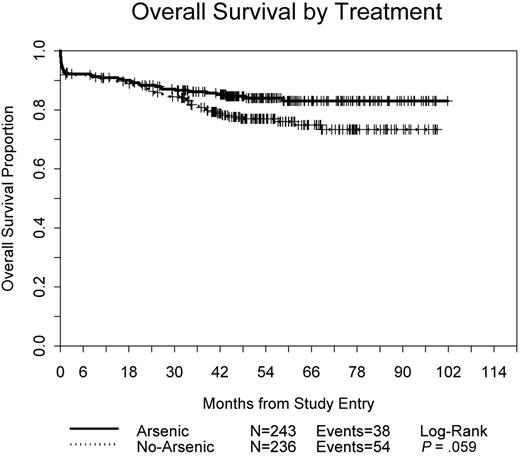
Overall survival is shown in Figure 5; there have been 38 deaths among patients assigned to receive As2O3 consolidation compared with 54 on the non-As2O3 arm (P = .059). The survival at 3 years was 86% on the As2O3 arm compared with 81% in the standard arm (P = .07).
We analyzed outcomes by risk group using the groups previously established to define risk of relapse: low (WBCs ≤ 10 × 109/L and platelets > 40 × 109/L); intermediate (WBCs ≤ 10 × 109/L and platelets ≤ 40 × 409/L); high (WBCs > 10 × 109/L).12 In general, the low and intermediate risk groups had similar outcomes while the high risk group had worse outcomes. Complete remissions were achieved in 94% of patients in the low, 93% in the intermediate, and 71% in the high risk groups (P < .0001). Death during induction occurred in 4%, 4%, and 20% in the low, intermediate, and high risk groups, respectively (P < .0001). For the entire study group, EFS (P = .24), DFS (P = .38), and survival (P = .72) were each similar between the low and intermediate risk groups, but EFS (P < .0001), DFS (P < .0001), and survival (P < .0001) were all markedly inferior for patients in the high risk group compared with the low/intermediate risk group. However, although DFS was inferior for the high risk group on the standard arm (P < .0001), DFS was similar between the low/intermediate risk group and the patients in the high risk group treated with As2O3 consolidation (P = .24; Figure 4B).
Patients in all risk groups benefited from the addition of As2O3 to the consolidation therapy. The following factors had no significant relationship with death during induction: age, performance status (PS), sex, serum creatinine, hemoglobin level, and percent CD56 expression. The following factors had no significant relationship with CR, EFS, DFS, or overall survival: age, sex, serum creatinine, hemoglobin level, and percent CD56 expression. However, there exists a significant relationship between PS and multiple outcomes: CR in both the non-As2O3 arm (P = .0088) and the As2O3 arm (P = .018); EFS in both arms (P < .0001 for non-As2O3 arm, P = .0098 for As2O3 arm); overall survival in both arms (P < .0001 for non-As2O3 arm, P = .0012 for As2O3 arm), and DFS in the non-As2O3 arm (P = .0023), but not in the As2O3 arm (P = .47).
Too few events have occurred to provide the desired power to detect differences related to maintenance therapy. Current results favor, but are not statistically significant for, ATRA plus chemotherapy compared with ATRA alone for DFS (P = .054) and overall survival (P = .11), by the stratified log-rank test. The effect due to the interaction of induction and consolidation by maintenance was not significant for DFS (P = .67) or survival (P = .97).
Discussion
This study shows that the addition of As2O3 as initial consolidation therapy for adult patients with newly diagnosed APL significantly improves EFS and DFS and enhances overall survival. These improvements are especially impressive considering our use of an intention-to-treat analysis. Of 244 patients, 48 (20%) randomized to receive As2O3 never received a single dose of this agent for consolidation therapy while on study. The outcomes for those patients who received at least one dose of As2O3 are excellent. The improvement in overall survival (P = .059) is not as dramatic as the improvements in EFS and DFS since arsenic trioxide was commercially available when this study was initiated, and it is likely that most patients on the control arm who relapsed subsequently received As2O3 therapy for their relapsed leukemia. The improvements in outcome were achieved with no additional toxicity. Arsenic trioxide consolidation therapy, although somewhat inconvenient with outpatient treatments for 5 days per week for 10 weeks, was very well tolerated. The differences in reported grade 3 nonhematologic toxicities were likely in part related to an additional 10 weeks of close monitoring during the As2O3 therapy. Our findings are consistent with prior reports that As2O3 is safe and well tolerated.17,20
The remission rate of 90% and induction death rate of 8% are similar to other studies using ATRA in combination with chemotherapy for an induction regimen.11,13,21 However, the EFS and DFS on the control arm are in general lower than those reported by others.11,12,22,23 The overall survival at 3 years of patients on the control arm (81%) was similar to those reported for 2 large trials, 85% at 3 years21 and 84% at 2 years.11
The reason for the lower EFS and DFS in our control arm is unclear. Differences in trial entry criteria, exclusions and analyses, as well as potential patient and disease differences make comparisons between separate trials conducted on different continents problematic. This trial had few limitations to protocol entry, requiring only confirmation of the diagnosis of APL and signed informed consent. Patients were treated at many sites with approximately 40% of patients registered at sites who entered ≤ 2 patients on the study; experience treating APL is recognized as a potentially important factor in outcome.13 The anthracycline in the trial was daunorubicin with a total scheduled dose of 500 mg/m2, while many European studies have used idarubicin.24,25 Importantly, this large prospective randomized trial was analyzed by intention-to-treat with no exclusions for performance status or protocol compliance. Other trials have excluded up to 9% of patients for poor clinical condition (4%-6%) or protocol violations (2%-6%).21
The addition of As2O3 to consolidation therapy clearly improves outcome with minimal additional toxicity for patients with APL. However, unanswered questions remain, such as the optimal timing for the As2O3 therapy in the overall treatment regimen, the best approach to decrease early deaths especially in patients with high risk disease (WBCs > 10 × 109/L), the role of cytarabine in APL, the need for maintenance therapy following the current improved induction and consolidation results and choice of anthracycline.
Phase 2 trials have recently demonstrated safety and activity for As2O3 as sole treatment for newly diagnosed APL26,27 or ATRA plus As2O3 as an induction regimen.28,29 The combination of ATRA, gemtuzumab ozogamicin, and As2O3 also shows promise as induction therapy29,30 and is currently being tested in the North American Leukemia Intergroup study S0535 in patients with high risk APL with a goal of decreasing early deaths, the major cause for failure among patients in the high risk group. A recent report from the European APL group comparing their sequential trials suggests that changes in supportive care have resulted in fewer early deaths in patients with high risk disease.31
Currently available data suggest that cytarabine does add benefit when given either during induction therapy23,32 or during consolidation therapy.22 This benefit may be limited to patients with high risk APL23 but these trials did not incorporate As2O3 therapy. Similarly, previous trials demonstrating benefit from 1 to 2 years of maintenance therapy10,11 did not incorporate As2O3 therapy. The current North American Leukemia Intergroup study S0521 will address this question for patients with low/intermediate risk APL; that study uses the same induction and As2O3 consolidation regimen of the C9710 study reported here, and then randomizes patients in complete molecular remission (with a negative RT-PCR assay) to maintenance therapy or to no further therapy. No prospective clinical trials have directly compared daunorubicin and idarubicin in APL; a study comparing ATRA plus either idarubicin or the combination of daunorubicin, cytarabine, and etoposide, showed no difference in response, relapse or overall survival.13,33
Our study demonstrates that As2O3 given as initial consolidation therapy is safe and improves event-free, disease-free, and overall survival for newly diagnosed patients with APL. Clinical trials currently underway will build on these results to further improve outcomes for these patients.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank the chairs of the cooperative group correlative science committees that supported the conduct of this trial, the physicians, nurses, and data coordinators, and especially the patients who participated in this study. We also thank the members of the CALGB Data and Safety Monitoring Committee, CALGB staff statistician Vera Hars, MS, data coordinator Edythe A. Parker, and protocol coordinator Michael Kelly, MA. We thank Lisa Burnett for her assistance with manuscript preparation.
This work was supported by grants from the NCI (CA31946) to the CALGB (Richard L. Schilsky, MD, Chairman) and to the CALGB Statistical Center (Stephen George, PhD, CA33601).
The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the NCI.
National Institutes of Health
Authorship
Contribution: B.L.P. was the principal investigator of the clinical trial; B.L.P., B.M., J.H.F., J.G., S. Couban, F.R.A., M.S.T., and R.A.L. participated in the conception and design of the trial; B.L.P., W.S., R.E.G., C.L.W., R.M.S., J.M.R., S. Couban, J.H.F., J.G., S. Coutre, F.R.A., M.S.T., and R.A.L. provided study materials and/or patients for the trial; B.L.P., B.M., W.S., R.E.G., C.L.W., and R.A.L. participated in analysis and interpretation of data; B.L.P., B.M., and R.A.L. wrote the manuscript; and B.L.P., B.M., W.S., R.E.G., C.L.W., R.M.S., J.M.R., S. Couban, J.H.F., J.G., S. Coutre, F.R.A., M.S.T., and R.A.L. participated in manuscript review and approval.
Conflict-of-interest disclosure: B.L.P. reports honoraria from Cephalon Oncology. R.M.S. reports participation in an advisory board for Cephalon Oncology. The remaining authors declare no competing financial interests.
A complete list of North American Leukemia Intergroup study participants is available in the supplemental Appendix (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Correspondence: Bayard L. Powell, Comprehensive Cancer Center of Wake Forest University, Medical Center Boulevard, Winston-Salem, NC 27157-1082; e-mail: bpowell@wfubmc.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal