Abstract
The identification of the genes necessary for human T-cell leukemia virus (HTLV-1) persistence in humans may provide targets for therapeutic approaches. We demonstrate that ablation of the HTLV-1 genes encoding p12, p30, or the HBZ protein, does not affect viral infectivity in rabbits and in this species, only the absence of HBZ is associated with a consistent reduction in virus levels. We observed reversion of the HTLV-1 mutants to the HTLV-1 wild-type genotype in none of the inoculated rabbits. In contrast, in macaques, the absence of HBZ was associated with reversion of the mutant virus to the wild-type genotype in 3 of the 4 animals within weeks from infection. Similarly, reversion to the wild type was observed in 2 of the 4 macaque inoculated with the p30 mutant. The 4 macaques exposed to the p12 knock remained seronegative, and only 2 animals were positive at a single time point for viral DNA in tissues. Interestingly, we found that the p12 and the p30 mutants were also severely impaired in their ability to replicate in human dendritic cells. These data suggest that infection of dendritic cells may be required for the establishment and maintenance of HTLV-1 infection in primate species.
Introduction
Human T-cell leukemia virus 1 (HTLV-1) is the causative agent of adult T-cell leukemia/lymphoma, and HTLV-1–associated myelopathy/tropical spastic paraparesis.1,2 Like other complex retroviruses, the HTLV-1 genome encodes structural, enzymatic, regulatory, and other nonstructural proteins.3,4 The Tax and Rex proteins encoded by open reading frames (orfs) IV and III, respectively, are positive regulators of viral expression.5 The orf-I encodes the p12 and p8 proteins, whereas orf-II encodes the p13 and p30 proteins.3-5 An antisense viral mRNA from the 3′ end of the virus encodes HTLV basic leucine zipper factor (HBZ).6,7
The orf-I product consists of 2 proteins that have different functions. The 12-kD precursor protein (p12), resides in the endoplasmic reticulum (ER)/Golgi,8-10 binds the heavy chain of major histocompatibility complex (MHC) class I in the ER, and reroutes it for degradation into the proteosome. p12 decreases surface expression of MHC-I,8 mediates the down modulation of the intercellular adhesion molecule 1 (ICAM-1) and ICAM-2, and inhibits the killing of CD4+ HTLV-1–infected cells by natural killer cells. The orf-I protein products also increases lymphocyte function antigen-1 clustering,11,12 interacts with the ER resident proteins calnexin and calreticulin,10 and promotes Ca+ release.13,14 The ER resident p12 protein interacts with the interleukin-2 receptor (IL-2R) γ and β chains,15 increases signal transducer and activator of transcription 5 activation,16 and increases IL-2 production.16,17 Thus, the ER-associated functions of p12 appear to facilitate the proliferation of infected T cells and protect them from immune recognition in the immune-competent host. Removal of a noncanonical ER retention signal located within the first 30 amino acids of p12 yields the p8 protein that localizes to the cell surface and is recruited to the immunologic synapse on T-cell receptor ligation.18,19 In contrast to p12, the p8 protein induces T-cell anergy by down-regulating proximal T-cell receptor signaling.18
The p30 protein is a nuclear resident protein that binds to and retains the Tax/Rex messenger inside the nucleus,20 governs the switch between virus replication and latency,21 and enhances cell survival by altering cell cycle regulation.22 The p30 protein also contains serine-rich domains with distant homology to transcriptional activators such as Oct-1, Oct-2, Pit-1, and POU-M13 and, depending on the dose, differentially activates or inhibits transcription from the cyclic adenosine monophosphate response element binding responsive elements and Tax-responsive elements.5,6,23 The p30 protein stabilizes the transcriptional interaction of c-Myc with the 60 kDa (Tat)–interacting protein of HIV type 1 transcriptional interaction24 and alters the expression of cellular genes.25,26
The HBZ protein is encoded by an antisense transcript that starts from the 3′ long terminal repeat and overlaps with the ORFs of p12, p13, and p30.7 The HBZ expression has complex effects on T-cell proliferation6,27,28 ; its expression is regulated by Tax29 and correlates with the severity of HTLV-1–associated myelopathy/tropical spastic paraparesis.30 HBZ also suppresses Tax-mediated viral transcription by competing with Tax for the recruitment of several transcription factors to the viral promoter.31,32 HBZ affects cellular gene expression by sequestering Jun B into nuclear bodies33 and by suppressing the nuclear factorκB pathway.28 HBZ also increases the transcription of the human telomerase reverse transcriptase.34
The concerted expression of all these viral genes is probably important for viral persistence in the infected host. Prior studies in the rabbit model have shown that proviruses, in which p12 or p30 expression was ablated by the introduction of DNA fragments deletions or insertions, were severely impaired in their infectivity.35-38 However, because the mutations introduced in those molecular clones also introduced significant changes in HBZ, whose absence alone decreases viral levels in the infected rabbits,39 the relative contribution of HBZ, p30II, and p12I to viral persistence remains unknown. In this study, we ablated one gene at a time in a HTLV-1 molecular clone and found that neither p12 nor p30 is required for efficient infectivity of rabbits and that only the absence of HBZ was associated with lower virus levels in blood and tissues in this animal species. Unexpectedly, the p12, p30, and, to a lesser extent, HBZ, were found to be essential for infection of macaques. To address the mechanism underlying this phenomenon, we evaluated the infectivity of these mutant viruses in primary human dendritic cells (DCs) and found that the lack of DCs infectivity in vitro paralleled the inability of the mutant viruses to infect macaques in vivo. Our data underscore the importance of these genes in HTLV-1 infectivity and suggest the relevance of the macaque model for HTLV-1 infection of humans.
Methods
HTLV-1 mutagenesis and cell line characterization
Mutations to ablate p12, p30, and HBZ were introduced in the ClaI/SalI cassette from the HTLV-1 molecular clone pBST40 that encompasses the orf-I and the orf-II. The molecular clone pACH41 was cleaved at the ClaI/SalI to generate the backbone for the construction of all viral mutants. The pBST ClaI/SalI cassette was ligated to the pACH backbone to obtain the wild-type HTLV-1 clone that carries a glycine at position 29 of p12 (29G). Because the molecular clone pACH encodes a serine at position 29 (V.W.V., unpublished observation), we also mutated the glycine to a serine (29S) and cloned both wild-type viruses into the pH6neo plasmid, containing the neomycin/geneticin resistance gene (kind gift of Patrick Green, Department of Veterinary Biosciences, Ohio State University), creating the molecular clones named 29G and 29S (Figure 1A). Mutations to ablate p12, p30, and HBZ were introduced into the pBST cassette. To generate the p30 knockout (KO) mutant, we replaced the leucine in position 3 (CTA) of p30 with a termination codon (TAA). This mutation introduced an amino acid change from a serine to tyrosine at position 153 of HBZ (Table 1). The HBZ KO was obtained by replacing the arginine (CGA) at position 11 with the termination codon (TGA). This mutation did not cause any amino acid change in p30 (Table 1). The generation of the 12 KO clone has been described earlier,18 and in this mutant the first methionine (ATG) was mutated to a valine (GTG). This mutation does not affect p30, but changes histidine 151 to glutamine in HBZ (Table 1). The mutants were generated with the use of complementary mutant site-specific primers, the Phusion enzyme, and mastermix (Finnzymes; New England Biolabs), where the reaction in the thermocycler was followed by DpnI digestion before transformation of One Shot Max Efficiency DH5α (Invitrogen).
The following DNA primers were used to generate the mutant clone: p30ko forward, 5′-CCTGCATTTTTTCTTTCCTAGCATAATGGTGTTTCGCCTTAAAAGCCCCT-3′; p30ko reverse, 5′-AGGGGCTTTTAAGGCGAAACACCATTATGCTAGGAAAGAAAAAATGCAG-3′; pHBZko forward, 5′-GGCATGGAACAGGCAAACATCAAAACAGCCCTAC-3′; pHBZko reverse, 5′-AGCCTGTTCCATGCCCGGAGGACCT-3′; p12ko forward, 5′-TCTTCCTAGCACTCTGCTGTTTCGCCTT-3′; p12ko reverse, 5′-AAGGCGAAACAGCAGAGTGCTAGGAAGA-3′; p29S-forward 5′-TGCTTTCTCCGGGCGACGTCAGCAGCCTTCTTCTC-3′; and p29S-reverse, 5′-GCGGAGAAGAAGGCTGCTGACGTCGCC-3′.
The mutant clones were inserted into the pH6neo vector, and all the resulting clones were verified by DNA sequencing of the ClaI/SalI fragment inserted in the provirus. 729-6 human lymphoblastoid B cells (5 × 106) were electroporated with 5 μg of 29Gneo, 29Sneo, 30KO neo, 12KOneo, or HBZKOneo with the use of AMAXA (Amaxa Biosystems) according to the manufacturer's guidelines. Infected cells were selected by culture in neomycin as previously described.39 Analysis of intracellular p24 was performed as previously described,20 using the appropriate antibodies overnight at 4° followed by an anti–rabbit or anti–mouse immunoglobulin G/horseradish peroxidase–conjugated donkey antibody (Santa Cruz Biotechnology). Blots were developed with the use of either West-Pico or West-Dura chemiluminescent detection system (Pierce Chemical). The Anti-Tax (Tab172) antibody was kindly provided by Dr J. N. Brady (National Cancer Institute), and the anti-HTLV p24 antibody (43101) was purchased from Advanced BioScience Laboratories Inc. The anti–β-tubulin (TUB 2.1) antibodies were purchased from Sigma.
Animal inoculation
Six groups of 5 animals each of specific pathogen-free New Zealand White female rabbits (Harlan), were inoculated by the lateral ear vein with 1.0 × 108 of the uninfected 729-6 B-cell line, as a control, or with 1.0 × 108 of the cell lines expressing the 29S, 29G, 12KO, 30KO, or HBZKO viruses. A few hours before inoculation, cells were γ irradiated (50 Gy [5000 rad]) to prevent outgrowth of the cell inocula in vivo while allowing virus transmission. The rabbits were evaluated for signs of clinical disease, bled at the time indicated, and humanely killed at 20-25 weeks after inoculation. The macaques used in this study were colony-bred Indian Rhesus macaques obtained from Covance Research Products. The animals were housed and handled in accordance with the standards of the Association for the Assessment and Accreditation of Laboratory Animal Care International. The care and use of the animals were in compliance with all relevant institutional (National Institutes of Health) guidelines. All macaques were seronegative and DNA polymerase chain reaction (PCR) negative for simian T-cell lymphotropic virus I and simian immunodeficiency virus sequences at the initiation of the study. Three groups of 4 animals each were used for each viral mutant, and an additional group of 2 animals was used as control. The inocula, given intravenously through the cephalic vein, consisted of 1 × 108 irradiated 729 B cells either uninfected (2 animals) or infected with the12KO30KO or HBZKO viruses (4 macaques per group).
Tissue collection from animals and HTLV-1–specific T-cell responses in macaques
Peripheral blood mononuclear cells (PBMCs) were separated from whole blood by density gradient centrifugation (Ficoll). Lymph nodes and appendixes were homogenized and passed through a 100-μm cell strainer, then separated by density gradient centrifugation. Jejunal biopsies were treated with 1mM ultrapure dithiothreitol (Invitrogen) for 25 minutes in Hanks Buffered Saline Solution (Gibco Invitrogen) without calcium chloride, magnesium chloride, and magnesium sulfate. HTLV-1–specific immune responses in PBMCs were measured with 1 μg/mL each of the antibodies anti-CD28 and anti-CD49d and 1 μg/mL HTLV-1:Gag pool peptides, Tax pool peptides in 1 mL of RPMI 1640 medium supplemented with 10% fetal bovine serum. The negative control (anti-CD28, anti-CD49d) and positive control (staphylococcal enterotoxin B, 1 μg/mL) were included. The cultures were incubated for 1 hour followed by an additional 4 hours in the presence of the secretion inhibitor monensin (0.5 μL/mL; BD PharMingen) and brefeldin A (10 μg/mL; Sigma). Cells were washed and stained with anti–human CD3, anti–human CD4, and anti–humanCD8 (BD PharMingen) and permeabilized with the use of fluorescence-activated cell sorting perm/wash buffer (BD Biosciences). After this treatment, the cells were stained with antibodies to interferon-γ, tumor necrosis factor-α (TNF-α), and IL-2 (BD PharMmingen). The cells were resuspended in 1% paraformaldehyde (Electron Microscopy Systems) in phosphate-buffered saline (PBS) and analyzed by flow cytometry with the use of FACSCalibur. List mode data files were analyzed with FlowJo software (TreeStar). In all cases, ≥ 100 000 live events were collected for analysis.
HTLV-1 serum antibody and provirus detection
Reactivity to specific viral antigens in the sera of infected animals was detected with the use of a commercial HTLV-1 Western immunoblot assay (GeneLabs Diagnostics), adapted for rabbit serum by the use of alkaline phosphatase–conjugated goat anti–rabbit immunoglobulin G (1:1000 dilution; Chemicon). Real-time PCR was performed on genomic DNA extracted from the rabbits' or macaques' tissue with the DNeasy tissue kit, according to the manufacturer's protocol. The QIAGEN method was used for the DNA elution stepin with 10mM Tris (tris(hydroxymethyl)aminomethane; pH8.0). The quantity and quality of the DNA were assessed by Nanodrop. Five hundred nanograms of genomic DNA was subjected to real-time PCR. The TaqMan probe and PCR primers for the real-time PCR were designed within the integrase gene of HTLV-1 × 1MT. The sequence of the TaqMan probe was 5′-TGT CCA CCT GCC ATT AAG CCC GA-3′, the DNA primers sequences used had the following sequence: forward primer, 5′-GCA GAG GAG GAA ATT ACC CAG TAC-3′; reverse primer, 5′-CAA TTT TAC CCA GGC ATT TAA TGT-3′. Reaction conditions were as follows: the 25 μL of PCR mixture for HTLV-1 and rabbit or macaque albumin DNA consisted of 500 ng of DNA extracted from PBMCs; 200nM primers; 100nM probe; 2× TaqMan Universal PCR Mastermix (Applied Biosystems) which consists of 10mM Tris-HCl (pH 8.3); 50mM KCl; 5mM MgCl2; 300μM each of deoxyadenosine triphosphate, deoxycytidine triphosphate, and deoxyguanosine triphosphate; 600μM deoxyuridine triphosphate; 0.625 U of AmpliTaq Gold DNA polymerase; and 0.25 U of uracil N-glycosylase. Used for HTLV-1 and rabbit or macaque albumin DNA amplification, 1 cycle at 50°C for 2 minutes and 1 cycle at 95°C for 10 minutes were followed by a 2-step PCR procedure consisting of 15 seconds at 95°C and 1 minute at 60°C for 50 cycles. The amplification was performed with the ABI Prism 7500 Sequence Detector system (Applied Biosystems). The normalized value of the HTLV-1 proviral DNA load was calculated as HTLV-1 DNA copy number/rabbit or macaque albumin gene copy number and expressed as the number of HTLV-1 proviral DNA copies per 106 PBMCs.
DNA sequencing of the p12, p30, and HBZ genes were performed from ex vivo samples from rabbit and macaques cellular DNA, as well as from the cell lines' DNA before inoculation to ascertain the identity of the viral mutants. In all cases, we amplified a DNA fragment of 667 nucleotides spanning nucleotide position 6431 to 7098 of the HTLV-1 genome with the use of the oligonucleotides primers, p12-Fwd, 5′-CACCTCGCCTTCCAACTG-3′; p12-p30-Rev, 5′-GGAGTATTTGCGCATGGCC-3′, used for amplification of p12-p30 region.
The PCR conditions used were 94°C for 2 minutes, 94°C for 30 seconds, 55°C for 30 seconds, 68°C for 50 seconds, 40 cycles, 68°C for 7 minutes, 4°C; PCR product was purified with the QIAquick Gel Extraction Kit (QIAGEN) and was subsequently cloned into pCR4 TOPO vector (Invitrogen) according to the manufacturer's protocol. At least 5 independent clones were purified and sequenced from each animal. Internet-available tools http://www.expasy.ch/tools/dna.html and http://www.ebi.ac.uk/Tools/clustalw2/index.html were used for sequences analysis.
In the case of the stably infected 729 B-cell lines, we obtained the DNA sequence of 8 clones for the p30KO, of 9 clones for the p12KO and the 29S, and of 10 clones for the HBZKO and the 29G.
Southern blot analysis was performed to confirm the presence of HTLV-1 DNA in the genome of the 29S, 29G, 30KO, 12KO, and HBZKO stably transfected 729 B-cell lines. The cleaved DNA was separated on a 0.8% agarose/Tris borate ethylenediaminetetraacetic acid gel and transferred to nitrocellulose, and the blot was annealed to a 32P-deoxycytidine triphosphate–labeled genomic HTLV-1 probe (Lofstrand Lab). Blots were developed on a Kodak Biomax Film at −80°C overnight.
In vitro infection of human DCs
Blood from healthy donors was collected according to institutional review board protocols approved by the US National Institutes of Health, the PBMCs were isolated by Ficoll-gradient centrifugation, and the lymphocytes and monocytes were separated by countercurrent elutriation. The monocyte fraction was plated in 6-well plates at 50 000/well, then converted to monocyte-derived DCs (MDDCs) by culturing for 7-10 days in serum-free RPMI 1640 media supplemented with 20% bovine serum albumin, insulin, and transferrin (StemCell Technologies), containing transforming growth factor-β (10 ng/mL; R&D Systems), granulocyte-macrophage colony-stimulating factor (50 ng/mL), and IL-4 (50 ng/mL) (PeproTech). Twenty hours before infection, MDDCs were harvested with Cell Dissociation Solution (Sigma) and then incubated with viral-containing supernatant from the appropriate stably producing 729 cells line for 3-16 hours as indicated. After the incubation, the cells were incubated in trypsin for 10 minutes at 37°C to remove virus bound to the cell surface and washed twice with media containing 20% fetal calf serum. The MDDCs were then either harvested at 16 hours (for studies of viral entry) or replated in 6-well plates at 500 000/well (for studies of viral infection). The level of entry was determined by the level of intracellular HTLV p19 Gag determined by flow cytometry at 16 hours from infection. To assess early infection, cells were harvested 48 hours after infection, and the amount of intracellular Tax was determined by flow cytometry. Cells were fixed and analyzed as previously described.42 HTLV-1 infection was determined by p19 Gag detection by enzyme-linked immunoabsorbent assay in the culture supernatants.
Results
p12 and p30 but not HBZ proteins are critical for HTLV-1 infection of human DCs
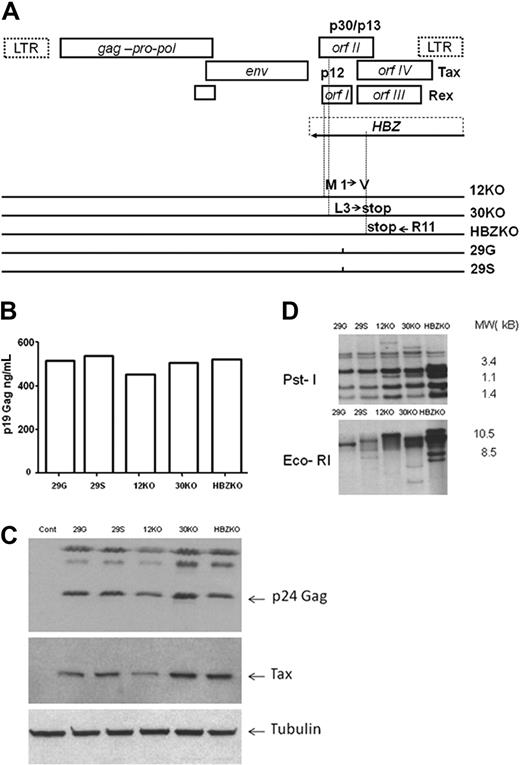
The mutations introduced in the HTLV-1 molecular clones to ablate p12, p30, and HBZ (Figure 1A) changed amino acids in other reading frames as indicated in Table 1. As wild-type control viruses, we used 2 provirus clones that carried either a serine 29S, or a glycine 29G, at amino acid position 29 of orf-I because these amino acids are found in type A and B HTLV-1 strains, respectively.19 The expression of intracellular p24Gag measured by Western blot and p19Gag measured by enzyme-linked immunoabsorbent assay in the supernatant of 293 cells transfected with the HTLV-1 mutants and wild-type viruses was equivalent (data not shown). We generated stable 729 B-cell lines expressing each mutant, and the cell lines expressed equivalent levels of p19 Gag in the supernatant (Figure 1B). Analysis of cell lysates by Western blot showed the expression of p24 Gag and Tax proteins (Figure 1C). The presence of provirus DNA by Southern blot analysis of genomic DNA was shown with the use of the restriction enzymes EcoRI that does not cleave in the provirus and PstI that cleaves within the HTLV-1 genome several times (Figure 1D).
Strategy for the ablation of p12, p30, and HBZ and characterization of the mutant viruses and cell lines. (A) Genetic organization of the HTLV-1 provirus genome and schematic representation of the overlapping orf I-IV (top). Amino acid changes in the mutant molecular clones (bottom). LTR indicates long terminal repeat. (B) Level of p19 Gag produced in the supernatants of the 729 B cell–infected cell lines measured by enzyme-linked immunoabsorbent assay. (C) Western blot analysis of the cell lysates from the 729 B-cell lines infected with the HTLV-1 mutant viruses with the use of antibodies to p24 Gag and to Tax. An antibody to tubulin was used as a control for equal loading of proteins. (D) Southern blot analysis of genomic DNA from the infected 729 B-cell lines. The numbers on the right represent the migration of the molecular weight (MW) marker.
Strategy for the ablation of p12, p30, and HBZ and characterization of the mutant viruses and cell lines. (A) Genetic organization of the HTLV-1 provirus genome and schematic representation of the overlapping orf I-IV (top). Amino acid changes in the mutant molecular clones (bottom). LTR indicates long terminal repeat. (B) Level of p19 Gag produced in the supernatants of the 729 B cell–infected cell lines measured by enzyme-linked immunoabsorbent assay. (C) Western blot analysis of the cell lysates from the 729 B-cell lines infected with the HTLV-1 mutant viruses with the use of antibodies to p24 Gag and to Tax. An antibody to tubulin was used as a control for equal loading of proteins. (D) Southern blot analysis of genomic DNA from the infected 729 B-cell lines. The numbers on the right represent the migration of the molecular weight (MW) marker.
Effect on orf-I, -II, -III, -IV and HBZ of the genetic mutations introduced in the12KO, 30KO, and pHBZKO viruses
| . | orf-I . | orf-II . | orf-III . | orf-IV . | HBZ . |
|---|---|---|---|---|---|
| p12ko | M1→L | Isogenic | Isogenic | Isogenic | H151→Q |
| p30ko | Isogenic | L3→stop | Isogenic | Isogenic | S153→Y |
| pHBZko | Isogenic | Isogenic | Isogenic | Isogenic | R11→stop |
| . | orf-I . | orf-II . | orf-III . | orf-IV . | HBZ . |
|---|---|---|---|---|---|
| p12ko | M1→L | Isogenic | Isogenic | Isogenic | H151→Q |
| p30ko | Isogenic | L3→stop | Isogenic | Isogenic | S153→Y |
| pHBZko | Isogenic | Isogenic | Isogenic | Isogenic | R11→stop |
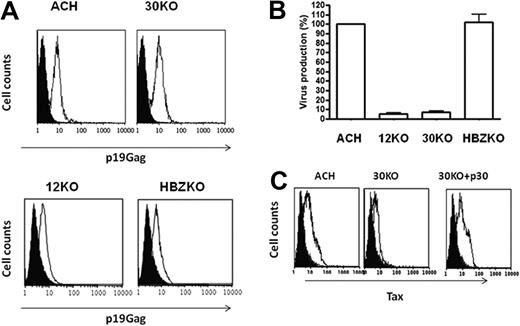
We next investigated whether the ablation of p12, p30, or HBZ altered the ability of HTLV-1 to productively infect DCs, because primary DCs are productively and stably infected in vitro by cell-free HTLV-142 and DCs isolated from HTLV-1–infected persons contain provirus.43 Exposure of MDDCs to the ACH wt, 30KO,12KO, and HBZKO mutant viruses showed that all viruses entered the cells as demonstrated by intracellular staining for the p19Gag (Figure 2A). However, in the case of both the 12KO and 30KO viruses, productive infection of DCs was not sustained over time, whereas the absence of HBZ did not affect virus replication in this cell type (Figure 2B). To further confirm the requirement of p30 and p12 in MDDC infection, we tested whether expression of p30 or p12 in MDDCs would rescue viral production by transfection with p30 or p12 expression plasmids of DCs. The coexpression of p30 convincingly but transiently rescued the expression of the 30KO virus (Figure 2C), whereas coexpression of p12 with the 12KO virus did not rescue significantly tax expression (data not shown), suggesting that p12 may be needed earlier than p30 in infection of this cell type. The HBZKO virus replicated to a similar extent than the wild-type HTLV-1 in human DCs (Figure 2B).
12KO, 30KO, and HBZKO infectivity in primary human DCs. (A) The level of entry of HTLV ACH (WT), 30KO, 12KO, and HBZKO virions was determined by intracellular staining for the p19 Gag protein (white histogram) by fluorescence-activated cell sorting at 16 hours after virus exposure. The black histogram represents the staining of cells with the isotype control antibody. (B) Level of infection of DCs at 14 days after virus exposure, determined by the concentration of viral particles in the culture supernatant by the p19 Gag enzyme-linked immunoabsorbent assay. The results are presented as the percentage of HTLV-1 WT expression of the viral mutants. (C) Infection of DCs by 30KO in the presence (right) and absence (middle) of coexpression of the p30 c-DNA relative to WT HTLV (left), determined by the level of intracellular Tax at 1 week after infection. The black histogram refers to the antibody isotype control and the white histogram to the staining with the anti-Tax antibody.
12KO, 30KO, and HBZKO infectivity in primary human DCs. (A) The level of entry of HTLV ACH (WT), 30KO, 12KO, and HBZKO virions was determined by intracellular staining for the p19 Gag protein (white histogram) by fluorescence-activated cell sorting at 16 hours after virus exposure. The black histogram represents the staining of cells with the isotype control antibody. (B) Level of infection of DCs at 14 days after virus exposure, determined by the concentration of viral particles in the culture supernatant by the p19 Gag enzyme-linked immunoabsorbent assay. The results are presented as the percentage of HTLV-1 WT expression of the viral mutants. (C) Infection of DCs by 30KO in the presence (right) and absence (middle) of coexpression of the p30 c-DNA relative to WT HTLV (left), determined by the level of intracellular Tax at 1 week after infection. The black histogram refers to the antibody isotype control and the white histogram to the staining with the anti-Tax antibody.
Point mutations in HBZ, p12, or p30 are stable in rabbits and only the absence of HBZ affects provirus level
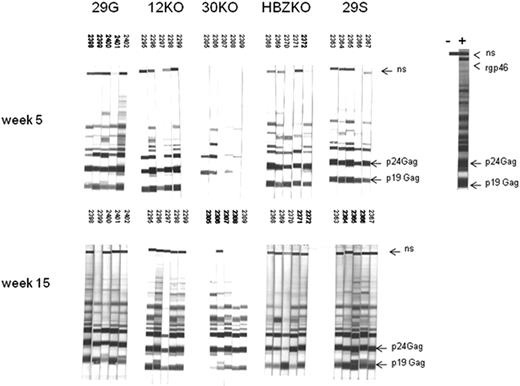
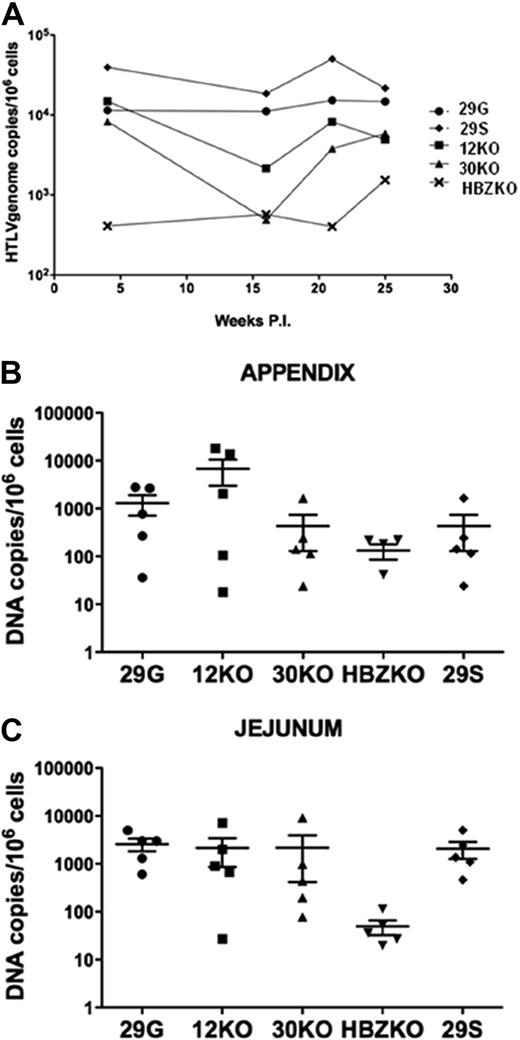
Groups of 5 rabbits each were inoculated with either the lethally γ-irradiated un-infected 729 B cells as a control or with cells expressing the different viral mutants. The sera from all animals were negative at the time of challenge (data not shown) and became reactive to HTLV-1 antigens in an HTLV-1 Western blot assay by week 5 or 15 (Figure 3). The sera from the control rabbits were negative at all time points in the same assay (data not shown). HTLV-1 infection was confirmed in the DNA from PBMCs and tissues by quantitative real-time PCR with the use of primers specific for the HTLV-1 integrase. Statistical analysis of the data in PBMCs with the use of the repeated measures of variance of the log-transformed copy numbers, and the P values for pairwise differences at each week, corrected by the Hochberg method, showed only a significant (P < .05), but transient (week 16), difference in virus levels among the animals infected with the 12KO or 30KO viruses and the wild-type 29S and 29G (Figure 4A). In contrast, animals infected with the HBZKO virus consistently had significantly (P < .05) lower levels of viral DNA in blood throughout the study, compared with the wild-type viruses (Figure 4A).
Detection of HTLV-1 antigens by the sera of the infected rabbits. Sera from each rabbit infected with the HTLV-1 molecular clones were collected at weeks 0, 5, and 15 and reacted with Western blot strips carrying HTLV-1 antigens. All sera were negative in Western blot at week 0 (data not shown) but reacted with the p24 and p19 Gag proteins by weeks 5 and 15 from infection. The rgp46 refers to the recombinant peptide from the HTLV envelope protein; ns stands for a nonspecific HTLV-1 protein.
Detection of HTLV-1 antigens by the sera of the infected rabbits. Sera from each rabbit infected with the HTLV-1 molecular clones were collected at weeks 0, 5, and 15 and reacted with Western blot strips carrying HTLV-1 antigens. All sera were negative in Western blot at week 0 (data not shown) but reacted with the p24 and p19 Gag proteins by weeks 5 and 15 from infection. The rgp46 refers to the recombinant peptide from the HTLV envelope protein; ns stands for a nonspecific HTLV-1 protein.
Virus level in the blood and tissues of the infected rabbits. Copies of HTLV-1 viral DNA per million of mononuclear cells in the blood of all infected animals overtime expresses as average (A) or presented for each animal at the time of killing in the appendix (B) and jejunum (C). The horizontal bars represent the average values. The statistical analysis of the data in blood was performed with the repeated measures of variance of the log-transformed copy numbers, and the P values for pairwise differences at each week were corrected by the Hochberg method.
Virus level in the blood and tissues of the infected rabbits. Copies of HTLV-1 viral DNA per million of mononuclear cells in the blood of all infected animals overtime expresses as average (A) or presented for each animal at the time of killing in the appendix (B) and jejunum (C). The horizontal bars represent the average values. The statistical analysis of the data in blood was performed with the repeated measures of variance of the log-transformed copy numbers, and the P values for pairwise differences at each week were corrected by the Hochberg method.
Results from the statistical analysis of viral DNA in tissues concurred with the results in blood. In the appendix, the null hypothesis that all 5 groups had the same mean value of viral DNA was close to being rejected (P = .058 by the exact Kruskal-Wallis test). The ranges of the 29G, 12KO, 30KO, and 29S groups mostly overlapped, and the group that was most different was the HBZKO. Although the median of the 29S group was not the highest, its range was the smallest, and the difference between the 29S and HBZKO groups reaches P = .0079 by the exact Wilcoxon rank sum (or Mann-Whitney) test.
The null hypothesis of equality across the 5 groups in the jejunum samples was rejected (P = .011 by the exact Kruskal-Wallis test). The significance was due entirely to the HBZKO group, because the other 4 were not different from each other (P = .49 by the exact Kruskal-Wallis test), but HBZKO was significantly (P < .015) different in any other group of 4. Pairwise tests have the pHBZKO group lower than the p29G and p29S groups with P = .0079 and lower than the p12KO group with P = .032 (Wilcoxon rank sum test; Figure 4C).
We investigated the possible occurrence of in vivo reversion of the HTLV-1 mutants by sequencing viral DNA obtained from the rabbits' PBMCs at the time the rabbits were killed. Analysis of the DNA sequences obtained from a minimum of 5 to a maximum of 10 independent clones from each animal showed no in vivo reversion of the genetic mutations introduced in the HTLV-1 mutants (Table 2). Because we had verified by DNA sequence the nature of the viruses present in each cell inoculums before infection of the rabbits and because of the concordance between the virus inocula and the viruses recovered from these animals at the time they were killed, we conclude that these data are consistent with the interpretation that none of these genes are necessary for infectivity in rabbits. Only HBZ appeared to be important to maintain higher virus load in this animal species, as also demonstrated by others.39
HTLV-1 DNA sequence from ex vivo PBMCs of the infected rabbits at time of killing
| Animal no. . | DNA sequence . | Clones with mutated viral sequence/no. of clones sequenced . | ||
|---|---|---|---|---|
| WT . | . | p12KO . | ||
| 2295 | catgctgttt | → | ctctgctgttt | 9/9 |
| 2296 | catgctgttt | → | ctctgctgttt | 9/9 |
| 2297 | catgctgttt | → | ctctgctgttt | 5/5 |
| 2298 | catgctgttt | → | ctctgctgttt | 7/7 |
| 2299 | catgctgttt | → | ctctgctgttt | 5/5 |
| WT | p30KO | |||
| 2305 | gcactatgctgt | → | gcataatgctgt | 8/8 |
| 2306 | gcactatgctgt | → | gcataatgctgt | 7/7 |
| 2308 | gcactatgctgt | → | gcataatgctgt | 6/6 |
| 2309 | gcactatgctgt | → | gcataatgctgt | 5/5 |
| WT | HBZKO | |||
| 2368 | tcgaaacagcc | tcgtaacagcc | 5/5 | |
| 2369 | tcgaaacagcc | tcgtaacagcc | 8/8 | |
| 2370 | tcgaaacagcc | tcgtaacagcc | 6/6 | |
| 2371 | tcgaaacagcc | tcgtaacagcc | 7/7 | |
| 2372 | tcgaaacagcc | tcgtaacagcc | 5/5 | |
| Animal no. . | DNA sequence . | Clones with mutated viral sequence/no. of clones sequenced . | ||
|---|---|---|---|---|
| WT . | . | p12KO . | ||
| 2295 | catgctgttt | → | ctctgctgttt | 9/9 |
| 2296 | catgctgttt | → | ctctgctgttt | 9/9 |
| 2297 | catgctgttt | → | ctctgctgttt | 5/5 |
| 2298 | catgctgttt | → | ctctgctgttt | 7/7 |
| 2299 | catgctgttt | → | ctctgctgttt | 5/5 |
| WT | p30KO | |||
| 2305 | gcactatgctgt | → | gcataatgctgt | 8/8 |
| 2306 | gcactatgctgt | → | gcataatgctgt | 7/7 |
| 2308 | gcactatgctgt | → | gcataatgctgt | 6/6 |
| 2309 | gcactatgctgt | → | gcataatgctgt | 5/5 |
| WT | HBZKO | |||
| 2368 | tcgaaacagcc | tcgtaacagcc | 5/5 | |
| 2369 | tcgaaacagcc | tcgtaacagcc | 8/8 | |
| 2370 | tcgaaacagcc | tcgtaacagcc | 6/6 | |
| 2371 | tcgaaacagcc | tcgtaacagcc | 7/7 | |
| 2372 | tcgaaacagcc | tcgtaacagcc | 5/5 | |
Boldface lowercase letters indicate the wild-type sequenced (left) and the mutated one (right).
WT indicates wild-type HTLV-1 sequence.
Critical requirement of p12, HBZ, and p30 for HTLV-1 infectivity in macaques
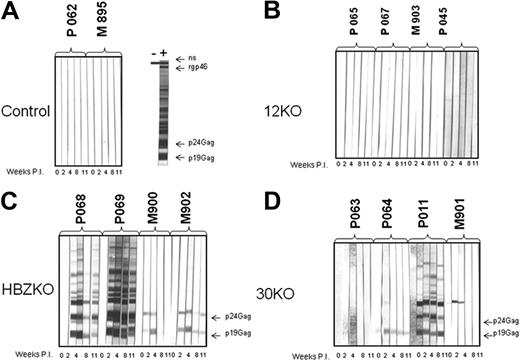
Nonhuman primates, including Rhesus macaques, are susceptible to HTLV-1 infection and to develop HTLV-1–associated diseases, including leukemia,44-46 whereas rabbits do not.47 We therefore assessed the effect of mutations in p12, p30, and HBZ in viral persistence in an animal model that may be more relevant for HTLV-1 infectivity and pathogenesis in humans.46 Four naive Rhesus macaques each were inoculated with the irradiated 729 B-cell lines, expressing the 12KO, 30KO, and HBZKO viruses, which we also used in rabbits. Two macaques were inoculated with uninfected irradiated 729 B cells as control. We elected not to use the 29S and 29G wild-type viruses in this study because the data in rabbits did not show differences in the infectivity of these 2 wild-type viruses. In addition, the wild-type virus ACH (that carries a serine at position 29), has been previously shown to be infectious in macaques.48 The serologic response to HTLV-1, measured by Western blot, was negative in the control animals exposed to the irradiated 729 B cells (Figure 5A). Unexpectedly however, the macaques exposed to the 12KO virus did not seroconvert (Figure 5B). Of the animal exposed to p30KO, only animal P011 fully seroconverted, whereas animals P064 and M901 recognized a single viral protein, and animal P063 failed to recognize any viral protein (Figure 5D). In contrast, the 4 animals exposed to HBZKO fully (P068 and P069) or partially (M900 and M902) seroconverted (Figure 5C). Quantitative real-time PCR for viral DNA in tissues of the 2 control animals P062 and M895 was consistently negative (Table 3). Of the 4 animals exposed to the 12KO virus, the presence of viral DNA was detected in 2 of the seronegative animals (P067 and M903) at a single time point (Table 3; Figure 5B). Of the animals exposed to the 30KO virus, P063 was the sole animal consistently negative for viral sequences and failed to seroconvert (Table 3; Figure 5D). The 3 animals infected with 30KO, which either fully seroconverted (P011) or partly sero-converted (P064 and M901), were positive by DNA PCR (Figure 5D; Table 3). In the HBZKO group, all animals were positive for viral DNA in tissues. Animal P069, the strongest positive in the serologic assay (Figure 5C), had the highest virus load and repeatedly scored positive for provirus, in contrast to the other macaque in the study (Table 3). Thus, most animals that were completely or partially positive in the Western blot assay were also positive for viral DNA sequences in blood or tissues or both. The exceptions were the 2 macaques P067 and M903 exposed to p12KO that remained seronegative despite the detection of viral DNA in tissues at a single time point. Two animals that were killed at week 58 after inoculation (P068 and P069) had detectable levels of HTLV-1 DNA in mesenteric lymph nodes (P068) or jejunum (P069; data not shown).
Detection of HTLV-1 antigens by the sera of the infected macaques. Sera from each macaque exposed to the HTLV-1 molecular clones were collected at weeks 0, 2, 4, 8, and 11 and reacted with Western blot strips carrying HTLV-1 antigens. Macaque P062 and M985, which were exposed only to uninfected 729 B cells as control, did not seroconvert (A). None of the 12KO-inoculated macaques seroconverted to HTLV-1 antigens (B). All animals inoculated with the HBZKO either fully seroconverted (animals P068 and P069) or partially seroconverted (PM902 and M900) to HTLV-1 antigens (C); full seroconversion was observed only in animal P0011 inoculated with the 30KO virus (D).
Detection of HTLV-1 antigens by the sera of the infected macaques. Sera from each macaque exposed to the HTLV-1 molecular clones were collected at weeks 0, 2, 4, 8, and 11 and reacted with Western blot strips carrying HTLV-1 antigens. Macaque P062 and M985, which were exposed only to uninfected 729 B cells as control, did not seroconvert (A). None of the 12KO-inoculated macaques seroconverted to HTLV-1 antigens (B). All animals inoculated with the HBZKO either fully seroconverted (animals P068 and P069) or partially seroconverted (PM902 and M900) to HTLV-1 antigens (C); full seroconversion was observed only in animal P0011 inoculated with the 30KO virus (D).
Copies of HTLV-1 integrase DNA in blood and tissues/106 cells in the infected macaques
| . | PBMC . | LN . | BM . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Weeks after inoculation . | Weeks after inoculation . | Weeks after inoculation . | ||||||||
| −4 . | 2 . | 4 . | 8 . | 10 . | 12 . | 14 . | 12 . | 46 . | 46 . | |
| p12KO | ||||||||||
| P065 | — | — | — | — | — | — | — | — | — | — |
| P067 | — | — | — | 45 | — | — | — | — | — | — |
| M903 | — | — | — | — | — | — | 14 | — | — | — |
| M045 | — | — | — | — | — | — | — | — | — | — |
| p30KO | ||||||||||
| P063 | — | — | — | — | — | — | — | — | — | — |
| P064 | — | — | — | — | — | — | 13 | — | — | 44 |
| P011 | — | — | — | — | 25 | 87 | — | — | — | — |
| M901 | — | — | — | — | — | 22 | — | — | — | — |
| pHBZKO | ||||||||||
| P068 | — | — | — | — | — | 25 | — | — | — | 42 |
| P069 | — | 16 | 49 | 93 | 91 | 116 | 38 | 27.35 | — | — |
| M900 | — | — | — | — | — | 11 | — | — | — | — |
| M902 | — | — | — | — | — | 40 | — | — | — | — |
| Controls | ||||||||||
| P062 | — | — | — | — | — | — | — | — | — | — |
| M895 | — | — | — | — | — | — | — | — | — | — |
| . | PBMC . | LN . | BM . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Weeks after inoculation . | Weeks after inoculation . | Weeks after inoculation . | ||||||||
| −4 . | 2 . | 4 . | 8 . | 10 . | 12 . | 14 . | 12 . | 46 . | 46 . | |
| p12KO | ||||||||||
| P065 | — | — | — | — | — | — | — | — | — | — |
| P067 | — | — | — | 45 | — | — | — | — | — | — |
| M903 | — | — | — | — | — | — | 14 | — | — | — |
| M045 | — | — | — | — | — | — | — | — | — | — |
| p30KO | ||||||||||
| P063 | — | — | — | — | — | — | — | — | — | — |
| P064 | — | — | — | — | — | — | 13 | — | — | 44 |
| P011 | — | — | — | — | 25 | 87 | — | — | — | — |
| M901 | — | — | — | — | — | 22 | — | — | — | — |
| pHBZKO | ||||||||||
| P068 | — | — | — | — | — | 25 | — | — | — | 42 |
| P069 | — | 16 | 49 | 93 | 91 | 116 | 38 | 27.35 | — | — |
| M900 | — | — | — | — | — | 11 | — | — | — | — |
| M902 | — | — | — | — | — | 40 | — | — | — | — |
| Controls | ||||||||||
| P062 | — | — | — | — | — | — | — | — | — | — |
| M895 | — | — | — | — | — | — | — | — | — | — |
LN indicates lymph node; BM, bone marrow; and —, viral DNA not detectable.
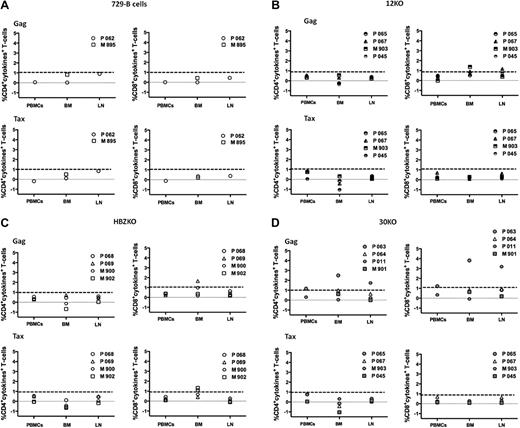
We next analyzed HTLV-1–specific functional T-cell responses on cryopreserved mononuclear cells from blood, bone marrow, and peripheral lymph nodes collected at week 12 after infection. To do this, overlapping peptide pools (15-mers with 11 amino acid overlap) encompassing the entire HTLV-1 Tax and Gag proteins were used. The ability of CD4+ and CD8+ T cells to produce cytokines (interferon-γ, tumor necrosis factor-α, and IL-2) after peptide stimulation, was assessed with multiparametric flow cytometry. After stimulation with Gag and Tax peptides, we observed background cytokine production in ≤ 1% of CD4+ and CD8+ T cells in the control animals inoculated with the uninfected 729 B-cell lines (Figure 6A). CD8+ T-cell responses to Gag above the background threshold were observed in the seronegative animals M903 and P067 from the p12KO group (Figure 6B), the seropositive animals P069 from the HBZKO group, and the P063 (seronegative) and p011 (seropositive) animals from the 30KO group (Figure 6C-D). CD4+ T-cell responses to Gag were found only in animals P063 and P011 from the 30KO group (Figure 6 D). The CD4+ T-cell responses to Tax were negative in all animals, and only animals P068 and M902 had CD8+ T-cell responses to Tax (Figure 6B-D).
HTLV-1 Tax and Gag–specific T-cell responses in macaques. Percentage of CD4+ T-cells (left) or CD8+T-cells (right) producing IL-2, tumor necrosis factor-α, or interferon-γ after stimulation with Gag (top) or Tax (bottom) overlapping peptides in control macaques (A), macaques exposed to 12KO (B) or HBZKO (C), and 30KO (D). All the data presented have been subtracted from the unstimulated cells background. The bolded dotted lines represent the background threshold of cytokine production observed in the control macaques. BM indicates bone marrow; LN, lymph node.
HTLV-1 Tax and Gag–specific T-cell responses in macaques. Percentage of CD4+ T-cells (left) or CD8+T-cells (right) producing IL-2, tumor necrosis factor-α, or interferon-γ after stimulation with Gag (top) or Tax (bottom) overlapping peptides in control macaques (A), macaques exposed to 12KO (B) or HBZKO (C), and 30KO (D). All the data presented have been subtracted from the unstimulated cells background. The bolded dotted lines represent the background threshold of cytokine production observed in the control macaques. BM indicates bone marrow; LN, lymph node.
Rapid in vivo reversion of the genetic mutations in the infected macaques
We studied the HTLV-1 genotype in the macaques that became infected to assess the in vivo stability of the genetic mutations introduced in the HTLV-1 molecular clones. We were able to amplify viral DNA fragments from the bone marrow or blood from 2 of the macaques inoculated with the 30KO virus (P064 and P011) and 3 of the macaques (P068, P902, and P069) inoculated with the HBZKO virus. We found that the 2 nucleotide mutations TA introduced in the 30KO virus was restored to CT in 10 of 10 clones in the bone marrow and in the blood of animals P064 and P011 at week 46 and week 12, respectively (Table 4). In the group of animals inoculated with HBZKO, we also observed reversion of the single point mutation T to C in 10 of 10 clones obtained from the bone marrow of animal P068 at week 46 and in 1 of 5 clones in the blood of animal M902 at week 12 (Table 4). The repeated detection of viral DNA in the blood of macaque P069 provided the opportunity to study the dynamic of variants selection over time. Indeed, we observed that at week 4, all the viral genomes present in the PBMCs corresponded to the inoculated HBZKO virus, whereas by week 8 from infection, 1 of 10 clones had reverted to the wild-type genotype, and by week 12 the wild-type virus had almost completely replaced the HBZKO virus in blood (Table 4).
In vivo reversion of the genetic mutation introduced in the 30KO and HBZKO viruses in the tissues of the infected macaques
| Animal no. . | Weeks after infection . | Clones with mutated viral sequence/no. of clones sequenced . |
|---|---|---|
| p30 KO | ||
| P064 (BM) | 46 | 0/10 |
| P011 (PBMC) | 12 | 0/10 |
| pHBZ KO | ||
| P068 (BM) | 46 | 0/10 |
| M902 (PBMC) | 12 | 1/6 |
| P069 (PBMC) | 4 | 10/10 |
| 8 | 9/10 | |
| 10 | 3/10 | |
| 12 | 1/10 |
| Animal no. . | Weeks after infection . | Clones with mutated viral sequence/no. of clones sequenced . |
|---|---|---|
| p30 KO | ||
| P064 (BM) | 46 | 0/10 |
| P011 (PBMC) | 12 | 0/10 |
| pHBZ KO | ||
| P068 (BM) | 46 | 0/10 |
| M902 (PBMC) | 12 | 1/6 |
| P069 (PBMC) | 4 | 10/10 |
| 8 | 9/10 | |
| 10 | 3/10 | |
| 12 | 1/10 |
BM indicates bone marrow.
Discussion
HTLV-1 expression is regulated by viral proteins that target the viral replication cycle5,6 and by viral proteins that regulate the proliferation and survival of T cells5 that together with DCs are the primary targets for HTLV-1 infection.42 HBZ antagonizes Tax activity by competing with cyclic adenosine monophosphate response element binding/ATF6,7 ; the p30 protein targets viral RNA and specifically decreases the transport of the tax/rex mRNA to the cytoplasm and viral production.20 The p12/p8 complex, on one hand, favors cell division and virus transmission and, on the other hand, spares infected cells from the host natural killer and cytotoxic T cells by down-regulating cell-surface ICAM-1 and ICAM-212 and the MHC-I molecules.8 In activated T cells, however, none of these genes are required for viral replication,36 suggesting that in vitro experiments in T cells give limited information needed to understand the role of these genes in vivo. Although the effect of the ablation of positive regulators of viral replication, such as the Tax or Rex proteins, can be easily assessed in vitro and in vivo,49 the role of the other nonstructural genes p12, p30, and HBZ in viral infectivity has been more difficult to uncover. Indeed, because the expression of these viral genes in vitro is detectable only with reverse transcription PCR,4 there is still a considerable degree of skepticism about their importance in viral replication. In the current work, we have explored the infectivity of HTLV-1 mutated in these genes at first, in primary human DCs in vitro, and in 2 animal species susceptible to HTLV-1 infection, rabbits and Rhesus macaques. Previous studies have evaluated the effect of mutations of the p12 and p30 genes either by deletion or insertion of several nucleotides within molecular clones of HTLV-1. However, in all the mutants described in those studies, the HBZ gene was also truncated,36-38 precluding an assessment of a specific role for p12 and p30 in HTLV-1 infection, particularly because HBZ ablation alone decreases virus level in rabbits.39 We therefore mutated p12, p30, and HBZ, changing 1 or 2 nucleotides and analyzed the phenotype of the resulting viruses in transient expression assays, in long-term infected B-cell lines, in primary human DCs in vitro, and in the rabbit and macaque models in vivo. The rabbit model was chosen at first because we wanted to compare our data with published data of those from other laboratories and because we wanted to test the stability of single-point mutations in this small nonpathogenic animal model. Rhesus macaques, however, were chosen because they are susceptible to HTLV-1 infection46 and because nonhuman primates develop HTLV-associated diseases, including leukemia,44,45 and may be a more relevant model for HTLV-1 infection of humans. We found important differences in the ability of the HTLV-1 mutants to replicate in these 2 animal species. In rabbits, HBZ, p12, and p30 are not necessary for viral infectivity because all animals seroconverted to HTLV-1 antigens and had sustained provirus DNA levels in blood and tissues. The rabbits infected with HBZKO, however, experienced a significantly reduced virus load, as also previously reported with a similar mutant virus.39 Importantly, we did not observe reversion of single genetic point mutations even in the rabbits infected with HBZKO, suggesting an overall low degree of selective pressure to maintain expression of these nonstructural genes in this animal species. Of note, a prior HTLV-1 mutant, whereby the p30 gene was truncated by the insertion of 24 nucleotides that affected also HBZ, was reported to revert in rabbits precisely to the HTLV-1 wild type, raising the possibility that the simultaneous mutations in both HBZ and p30 may have undergone a higher selective pressure in vivo.37 An alternative and more plausible hypothesis is that the inserted fragment may have been eliminated to maintain the optimal size of the viral genome, because reversion was not observed in other mutants carrying a deletion that affected both genes.36,37
Rhesus macaques are susceptible to HTLV-1 infection,46 and orf-I is essential for the establishment of infection in this species, because none of the 4 inoculated animals seroconverted to HTLV-1 antigens and only 2 were found positive for viral DNA sequence in tissues at a single time point. The ablation of p30 also severely affected virus infectivity, but to a lesser degree, because in 2 of the inoculated macaques, sufficient level of viral replication occurred to allow the selection of viruses that reverted to the wild type. In the case of HBZKO, all 4 animals partly or fully seroconverted and had viral DNA in tissues, and genetic reversion to the HTLV-1 wild type was documented in 3 of the 4 macaques.
In the generation of the p12 and p30 KO viruses, the introduction of point mutations did not truncate HBZ but, nevertheless, introduced amino acid change in HBZ of histidine at position 151 to a glutamine and of serine at position 153 to a tyrosine, respectively (Table 1). Thus, there is the possibility that the phenotype observed for the p12 and the p30 mutants may have been influenced by the amino acid change introduced in HBZ. Interestingly, the in vivo phenotype of the 12KO, 30KO, and HBZ viruses paralleled their ability to infect human DCs in vitro. The HBZKO virus infected DCs similar to the HTLV-1 wild type, whereas the ablation of p12 and p30 resulted in impairment of viral infectivity in this cell type. Indeed, p12 appeared to be necessary very early on because p12 overexpression could not rescue viral infectivity. Because p12 interacts with the 16-kKa subunit of the vacuolar adenosine triphosphatase,50 it is possible that p12 is needed very early to modulate the endosomal pH and either protect virus from degradation or favor membrane fusion and virus entry or both. The p30 protein requirement in DCs probably relates to the observation that p30 affects the release of cytokines important for maturation of human macrophage.51 Further work is necessary to address both of these hypotheses.
An unexpected finding was that the genetic reversion to wild-type HTLV-1 did not occur in rabbits but readily occurred in macaques, although the viral DNA copies per 106cells in PBMCs was 2-3 logs more in rabbits than in macaques. These data suggest the possibility that de novo virus transmission that requires reverse transcription may be more frequent in macaques than in rabbits. It is possible that in rabbits virus levels may be maintained more by mitotic replication of infected cells than de novo infection. It is of great interest to better understand the basis for the differences that we have observed in these 2 animal species. The current data, however, show that the macaque model may be more relevant and better suited to test therapeutic approaches to target the p12, p30, and HBZ gene products. These gene products may be a good target because they are all essential for the efficient establishment and maintenance of HTLV-1 infection in this animal species.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Stephen Hughes for helpful suggestions, Jake R. Fullen for study assistance, and Teresa Habina and Monica Vaccari for editorial help. We also thank Dr L. Ratner for providing the pACH molecular clone, Dr P. Green for providing the pH6Neo plasmids, and Dr D. Venzon for help with the statistical evaluation of the data.
This work was supported by the Intramural Program at the National Cancer Institute, National Institutes of Health, Bethesda, MD.
National Institutes of Health
Authorship
Contribution: V.W.V. generated the HTLV-1 mutants and coordinated the rabbit studies; A.H. coordinated the macaque studies and measured the cellular immune responses in macaques; V.A., I.B., C.F., and V.C. amplified viral DNA from tissues of the infected animals and obtained their DNA; K.J. and F.R. performed the experiments in DCs; H.K.C. performed real-time DNA-PCR on the tissues; R.F. provided molecular clone p12KO; R.W.P. performed the HTLV-1 p19 Gag enzyme-linked immunoabsorbent assay; M.G.F. expanded and irradiated the 729 B-cell lines and performed Western blot on the animals' sera; C.N. provided recombinant viruses y expressing p12 and p30; G.F. contributed to the experimental design and wrote the paper with the help of all coauthors.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Genoveffa Franchini, NCI, 9000 Rockville Pike, 41/D804, Bethesda, MD 20892-5065; e-mail: franchig@mail.nih.gov.
References
Author notes
V.W.V. and A.H. contributed equally to this study

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal