Abstract
Protein vaccines for T-cell immunity are not being prioritized because of poor immunogenicity. To overcome this hurdle, proteins are being targeted to maturing dendritic cells (DCs) within monoclonal antibodies (mAbs) to DC receptors. To extend the concept to humans, we immunized human immunoglobulin-expressing mice with human DEC205 (hDEC205) extracellular domain. 3D6 and 3G9 mAbs were selected for high-affinity binding to hDEC205. In addition, CD11c promoter hDEC205 transgenic mice were generated, and 3G9 was selectively targeted to DCs in these animals. When mAb heavy chain was engineered to express HIV Gag p24, the fusion mAb induced interferon-γ– and interleukin-2–producing CD4+ T cells in hDEC205 transgenic mice, if polynocinic polycytidylic acid was coadministered as an adjuvant. The T-cell response was broad, recognizing at least 3 Gag peptides, and high titers of anti-human immunoglobulin G antibody were made. Anti-hDEC205 also improved the cross-presentation of Gag to primed CD8+ T cells from HIV-infected individuals. In all tests, 3D6 and 3G9 targeting greatly enhanced immunization relative to nonbinding control mAb. These results provide preclinical evidence that in vivo hDEC205 targeting increases the efficiency with which proteins elicit specific immunity, setting the stage for proof-of-concept studies of these new protein vaccines in human subjects.
Introduction
Protein vaccines offer potential advantages with respect to ease and low costs of production, and ability to be administered repeatedly. Yet, protein vaccines characteristically suffer from poor immunogenicity for the Th1 type of T cell–mediated immunity that should help to resist global infectious diseases and cancers. The requirements for immunization are now better understood, particularly the importance of antigen delivery to dendritic cells (DCs) to achieve antigen presentation in vivo as well as DC maturation to differentiate the cells to overcome their normal role in inducing tolerance by different mechanisms.1-3 To meet these DC requirements, it is logical to explore several receptors on DCs to improve antigen uptake and presentation in vivo, as well as several adjuvants that guide DC function.4,5
DEC205/CD205 is a member of the multilectin family of C-type lectins expressed by mammalian cells.6 This family, which include the mannose receptor, CD206, and the phospholipase A2 receptor, have 8-10 external contiguous lectin domains and mediate adsorptive endocytosis.7 Among leukocytes, DEC205 is expressed at the highest levels by DCs within the T-cell areas of lymphoid tissues, which are the sites for the generation of immunity and tolerance. Although DCs are known to express many potential receptors to enhance antigen uptake and processing, DEC205 is currently the only receptor that has been visualized on most DCs in the T-cell areas of human lymphoid organs.8
Proteins can be targeted selectively to mouse DCs in vivo when incorporated into a monoclonal antibody (mAb) specific for DEC205.1,2 This targeting increases the efficiency of antigen presentation on major histocompatibility complex (MHC) class I and II molecules approximately 100-fold3,9-12 as well as strong and protective T-cell immunity.10 With synthetic double-stranded RNA as the only adjuvant, DEC205 targeting leads to Th1 immunity that is also durable, lasting for several months in mice.13 The improved cell-mediated immunity with polyriboinocinic polyribocytidylic acid (poly IC) as an adjuvant is due to the high levels of type I interferon (IFN) induced by this agonist through MDA5 and TLR3 pattern-recognition receptors. Specifically, the maturation of DCs to become immunostimulatory requires their expression of type I IFN receptors.14
To extend the concept of protein targeting to DCs to human therapeutics, a series of human immunoglobulin G (IgG) mAbs to human DEC205 (hDEC205) were produced from human Ig transgenic mice that lack mouse Ig genes, but carry the human Ig locus.15 While these mAbs also react with DEC205 in rhesus macaques, the latter are expensive to explore the many variables that are needed to move this vaccine strategy into proof-of-concept studies in humans. Therefore, we generated transgenic (Tg) mice expressing the hDEC205 receptor on DCs. Below, we describe the capacity of human anti–hDEC205-HIV Gag p24 fusion mAb to enhance humoral and cellular immunity in vivo when poly IC adjuvant is coadministered.
Methods
Cells
Chinese hamster ovary (CHO) cells were cultured in Dulbecco modified Eagle medium (DMEM; Invitrogen no. 11 995) 5%-10% fetal bovine serum (FBS; Sigma-Aldrich) or 5% Ultra-Low IgG FBS supplemented with 2-mercaptoethanol, antibiotic-antimycotic, and nonessential amino acids (all from GIBCO Invitrogen). Human monocyte-derived DCs (MoDCs) were generated from normal blood monocytes purchased as buffy coats from the New York Blood Center as previously described.16 Briefly, CD14+ cells were isolated using anti-CD14 beads (Miltenyi Biotec) and cultured 6 days in RPMI 1640 (Invitrogen) with 5% human serum (Gemini Bio-Products), interleukin-4 (IL-4; R&D Systems; 10 ng/mL), and granulocyte-macrophage colony-stimulating factor (GM-CSF; Immunex; 100 IU/mL). To mature MoDCs, lipopolysaccharide (LPS; 100 ng/mL) was added during the last 24 hours.
Expression of full-length human DEC205 and its external region
A synthetic open reading frame (ORF) for full-length hDEC205 with a V5 epitope tag and stable CHO cells expressing hDEC205 (CHO/hDEC205 cells) were previously described.17 In brief, a full-length hDEC205 ORF was amplified by polymerase chain reaction (PCR) from a human thymic cDNA library (Clontech), followed by cloning and sequencing. To detect the expression of full-length hDEC205 protein, the signal peptide was replaced with a mouse IgG signal peptide and a V5 epitope tag (V5-tagged hDEC205; GenBank accession no. AY682091). This synthetic hDEC205 ORF was inserted into a promoter of cytomegalovirus (pCMV) expression vector, transfected, and stably expressed as CHO/hDEC205 cells. Similarly, the full-length cDNA of mouse DEC205 (mDEC205; GenBank accession no. AF395445) was cloned from C57Bl/6 spleen cDNAs and also inserted into the pCMV for stable expression in CHO cells (CHO/mDEC205 cells).
A synthetic DNA for hDEC205 extracellular domain was generated similarly, modified with V5-tagged mouse IgG signal peptide, and fused in-frame with the human IgG1 (hIgG1) Fc domain amplified from human genomic DNA. The V5.hDEC205/hIgG1Fc construct (GenBank accession no. DQ407610) was inserted into the pCMV expression vector, transfected, stably expressed as CHO/V5.hDEC205/hIgG1Fc cells, and the culture supernatant was used to purify V5.hDEC205/hIgG1Fc fusion protein by affinity to Protein A Sepharose (GE Healthcare) as described.18
Antibodies
Murinized NLDC145 mAb (mNLDC145, the original V regions from rat anti-mDEC205 mAb modified to express mouse kappa and IgG1 constant domains) and hybrid mAbs fused with antigens were previously described.1 The cDNAs of mouse lambda and IgG2b heavy chains of mouse anti-hDEC205 mAb MG38.219 were cloned and expressed in 293T cells to produce genetically cloned MG38.2 as previously described.20 Peroxidase-conjugated anti-V5 antibody (Ab) was purchased from Invitrogen. Phycoerythrin (PE)–conjugated anti-human IgG was purchased from Jackson ImmunoResearch Laboratories. Fluorescein isothiocyanate (FITC)–anti-CD3, FITC-anti-B220, PerCP-anti-CD8, allophycocyanin (APC)–anti-CD4, and APC-anti-CD11c were from BD Biosciences Pharmingen.
Generation of CD11c promoter-human DEC205 Tg mice
CD11c promoter-hDEC205 (CD11c-hDEC205) Tg mice were generated in the C57BL/6 strain using standard microinjection techniques. Briefly, hDEC205 cDNA was cloned into a synthetic CD11c promoter (GenBank Accession no. DQ658851). The construct was linearized by digestion with restriction enzymes AatII and AscI, purified, and injected into fertilized pronuclei of C57BL/6 mice. Transgene-positive mice were mated with C57BL/6. Six founders were established and confirmed for germ-line transmission by PCR genotyping of tail DNA. The DEC205F 5′-TGGAAGAGACATGGAGAAACCT-3′ and DEC205R 5′-TCTCAGGCCAGTCCAGAAGTA-3′ primers recognize conserved sequences in human and mouse DEC205 genes, and distinguish amplicons of 165 and 293 bp from the hDEC205 cDNA transgene and mouse DEC205 gene. hDEC205 Tg mice also mated with B10.BR(H2K). C57BL/6 or B10.BR mice were purchased from Taconic Farms and mated with CD11c-hDEC205 Tg mice. All animals were maintained under specific pathogen-free conditions and used according to Rockefeller University institutional guidelines.
Immunization of human Ig Tg mice for human anti-hDEC205 mAbs
Human Ig Tg mice15 were immunized with 5-25 μg of purified V5.hDEC205/hIgG1Fc protein using either complete/incomplete Freund or RIBI monophosphoryl lipid A (MPL) plus trehalose dicorynomycolate (TDM) as adjuvants (Sigma-Aldrich). Mice with anti-hDEC205 titers were administered with fusion-protein intravenously 3-4 days before harvesting spleen cells for fusion with the murine myeloma cell line, P3 × 63Ag8.653. Hybridoma supernatant was harvested and initially screened for human IgGκ using a sandwich enzyme-linked immunosorbent assay (ELISA). Human IgGκ-positive supernatants were then assayed by fluorescence-activated cell sorting (FACS) for reactivity to CHO/hDEC205 cells and by ELISA to V5.hDEC205/hIgG1Fc-coated plates. Human IgGκ anti-hDEC205 hybridomas were subcloned and expanded before the purification of human anti-hDEC205 mAbs by protein A column chromatography. The 3D6-p24 and 3G9-p24 hybrid mAb proteins were made as an in-frame fusion of HIV Gag p24 cDNA at the C-terminus of the Ig heavy chains. DNA for simian immunodeficiency virus (SIV) Gag p27 or Gag p41, that is, amino acids 136-368 or 1-364 of SIV Gag p55 protein (from SIV mac 239) were also cloned in-frame into the 3G9 heavy chain. Fusion mAbs were expressed in 293T cells by transient transfection and purified by protein A chromatography. 3G9 or control human IgG1 antibodies were conjugated with Alexa 647 (Invitrogen), according to the manufacturer's instructions.
Flow cytometry
Control and transfectant CHO cells or human MoDCs were suspended in phosphate-buffered saline (PBS) containing 2% FBS (FACS buffer) and distributed into round-bottom 96-well plates at 2 × 105 cells/well. Fifty microliters of supernatant or purified anti-hDEC205 mAbs were added for 1 hour at 4°C. The cells were washed, incubated in 50 μL of PE–anti-human IgG (1:1000; Jackson ImmunoResearch Laboratories) in FACS buffer at 4°C for 1 hour, and assayed on a BD FACSCalibur flow cytometer at the Rockefeller University Flow Cytometry Resource Center.
Determination of affinity and rate constants of anti-hDEC205 mAbs by SPR
Binding affinity and kinetics of human anti-DEC205 mAbs were examined by surface plasmon resonance (SPR) using a Biacore 2000 SPR instrument (Biacore AB), according to the manufacturer's guidelines. Purified human DEC205 fusion (or control) protein was covalently linked to a Biacore CM5 sensor chip (carboxymethylated dextran covalently attached to a gold surface; Biacore) using standard amine-coupling chemistry with an Amine Coupling Kit provided by Biacore, according to its guidelines [Biacore, comprising coupling reagents N-hydroxysuccinimide and 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride]. Low levels of ligand were immobilized to limit any effects of mass transport of analyte on kinetic parameters, such that the RMAX observed was in the order of 200 RU. Binding was measured by flowing the mAbs over the sensor chip in HEPES buffer (HBS-N buffer, Biacore: HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid] 0.24%, NaCl 0.88%, q.s. water, filtered/degassed, and pre-equilibrated to room temperature with a 1:2000 dilution of Surfactant P20) at 1.25-200nM and a flow rate of 35 μL/min. Antigen-antibody association and dissociation kinetics were followed for approximately 300-600 seconds in each case. Corresponding controls were conducted using an unrelated protein for “background” subtraction. A single injection of 18mM NaOH for 17 seconds at 35 μL/min was used as the regeneration conditions throughout the study. Biacore's kinetics wizard was used to derive kinetic parameters from the concentration series of analyte diluted in HBS-NP running buffer. Association and dissociation curves were fitted to a 1:1 Langmuir binding model using Biacore kinetics wizard software, according to Biacore guidelines.
Western blot analysis and epitope mapping
Stably transfected CHO cells (above) and CHO cells expressing empty vector (CHO/Neo cells) were lysed with RIPA lysis buffer (150mM NaCl, 50mM Tris-HCl, pH 8.0, 1% NP-40, 0.5% desoxycholate, 0.1% sodium dodecyl sulfate [SDS]), including protease inhibitor cocktail (Roche Applied Science). Cell lysates were mixed with glycerol (5%-10% final) for loading and separated on 6% SDS-polyacrylamide gel electrophoresis (PAGE), blotted onto Hybond-P polyvinylidine difluoride membrane (GE Healthcare), incubated with 3G9 or NLDC145 mAb supernatants at 1:10, detected by anti-human (for 3G9) or -rat (for NLDC145) IgG conjugated with horseradish peroxidase (HRP) (Southern Biotech), and visualized with enhanced chemiluminescence Plus reagents (GE Healthcare). To detect V5-tagged protein by immunoblotting, HRP-conjugated anti-V5 Ab (1:1000; Invitrogen) was used.
To map the subdomain containing the epitope bound by mAb 3G9, a series of deletion constructs for hDEC205 extracellular domain were generated with a FLAG-tag sequence, that is, CR/FN (GenBank accession no. GU270871), CRD1/2 (accession no. GU270872), CRD3/4 (accession no. GU270873), CRD5/6 (accession no. GU270874), CRD7/8 (accession no. GU270875), and CRD9/10 (accession no. GU270876). Each construct was cloned into the pCMV vector and transiently expressed into 293T cells using Lipofectamine 2000 reagent (Invitrogen). Western blotting assays were done on transfectant cell lysates on 12% SDS-PAGE using mAbs 3G9 and L5 (anti-FLAG) as described above.
Immunohistochemical analysis of DEC205 expression
Spleen and lymph nodes from CD11c-hDEC205 Tg mice were harvested and frozen in Tissue-Tek OCT (optimal cutting temperature; Sakura Finetek USA). Then, 10-μm frozen sections were fixed 20 minutes in cold acetone, blocked with 5% mouse serum in FACS buffer, and stained in humidified chambers with purified human anti-hDEC205 mAbs, followed by mouse antihuman IgG-PE (Jackson ImmunoResearch Laboratories) and anti-B220–FITC (BD Biosciences). Sections were mounted in Aqua Poly/Mount (Polysciences) and stored at 4°C until examination by deconvolution microscopy (Olympus) and Zeiss LSM 510 laser scanning confocal microscopy (Carl Zeiss MicroImaging) at the Rockefeller University Bio-Imaging Resource Center.
In vivo targeting of 3G9 antibody
Briefly, 30 μg of Alexa 647–labeled 3G9 or control human IgG1 mAbs were injected intraperitoneally to wild-type or CD11c-hDEC205 Tg mice. Then, 12 hours later, spleen cells were prepared with collagenase and stained with FITC-conjugated anti-CD3, CD19, and DX-5 to exclude lymphocytes plus anti-CD8α, CD11c, and PDCA1 mAbs. Targeting of the Alexa 647–labeled mAbs to subsets of DCs was analyzed with a BD LSRII flow cytometer.
T-cell immunity after HIV and SIV Gag immunization of CD11c-hDEC205 Tg mice
Mice were injected intraperitoneally with 5 or 10 μg of 3G9-HIV Gag p24, 3D6-HIV Gag p24, 3G9-SIV Gag p41, 3G9-SIV Gag p27, or control Ig-HIV Gag p24 fusion mAbs plus adjuvants. To detect responses with a single immunization, the adjuvants were 50 μg of poly IC and 25 μg of anti-CD40 mAb 1C10, while 50 μg of poly IC alone was used 1 month apart for prime-boost immunizations.13 Spleens were harvested 2 weeks after the primary immunization or booster immunizations. SIV- or HIV-specific T cells were analyzed by intracellular cytokine staining as previously described.10,13 Spleen cells were stimulated with the entire HIV Gag p24, SIV Gag p17, or SIV Gag p27 peptide mix, or HIV Gag p24 peptide pools or, as a negative control, nonrelated peptide mix of Yersinia pestis LcrV21 at a concentration of 2 μg/mL, or medium alone, in the presence of a costimulatory anti-CD28 mAb (clone 37.51) for 6 hours. Brefeldin A (10 μg/mL) was added for the last 5 hours to accumulate intracellular cytokines. Cells were then washed with FACS buffer, incubated with Fcγ receptor-blocking mAb (2.4G2), and stained with Live/Dead Fixable Aqua viability dye (Invitrogen), FITC- or Alexa Fluor 700–anti-CD3, PE-, or PerCP-anti-CD8, and PerCP Cy 5.5- or APC-anti-CD4 for 20 minutes on ice. Following fixation and permeabilization using Cytofix/Cytoperm solution (BD Biosciences Pharmingen), cytokines were visualized with APC- or PE–anti-IFN-γ or anti-IL-2 antibodies for 15 minutes at room temperature for flow cytometry.
To assess the proliferation of primed T cells, bulk splenocytes were labeled with carboxyfluorescein succinimidyl ester (CFSE; 107 cells/mL, 1μM, 10 minutes, 37°C; Invitrogen) and cultured with anti-CD28 and either HIV Gag p24, nonreactive peptide mix, or anti-CD3 (0.1 μg/mL; positive control) in 96-well, round-bottomed plates. After 3 days, samples were restimulated 6 hours with p24 peptides and anti-CD28, adding Brefeldin A (10 μg/mL) for the last 5 hours. Cells were stained with PerCP-anti-CD3 and PE-anti-CD4 as described above, permeabilized, stained with APC-anti-IFN-γ mAb for 15 minutes, and 40 000 CD3+ events were acquired on BD FACSCalibur.
Humoral immunity after HIV Gag p24 immunization of CD11c-hDEC205 Tg mice
To assess B-cell immunity, groups of 3 CD11c-hDEC205 Tg mice were injected intraperitoneally with 10 μg of purified 3G9, 3D6, or isotype control human IgG1 mAbs in the presence of 50 μg of poly IC and 25 μg of anti-CD40 mAb 1C10. At day 25, mice were boosted intraperitoneally with 10 μg of the respective antibodies without adjuvants. Serum samples were collected at days −2, 5, 21, 31, 40, and 58. To measure specific antibody, 96-well ELISA plates (BD Falcon) were coated with 0.1 μg of control human IgG1 (the isotype of 3G9 and 3D6) in PBS per well overnight at 4°C. After washing 3× with PBS containing 0.1% Tween 20 (PBST), plates were incubated with blocking solution (5% normal goat serum in PBST) for 1 hour at 37°C. Then, serial dilutions of serum samples in blocking solution were added and incubated for 1 hour at 37°C. After washing, plates were incubated with HRP-conjugated goat antibodies (Southern Biotech) against mouse total IgG, IgG1, IgG2c, IgG2b, or IgG3 in blocking solution for 1 hour at 37°C. The levels of mouse antihuman IgG1 antibodies were visualized by colorimetric assay using OPD (o-phenylenediamine dihydrochloride; Sigma-Aldrich) in coded picture buffer (CPB) solution, that is, a 10-mg OPD tablet in 25 mL of citrate-phosphate buffer (25mM citric acid, 5 mM Na2HPO4) with 10 μL of 30% H2O2. The highest dilution showing an OD450 > 0.1 was recorded as the titer. Antibody titer data were plotted on log scales.
Cross-presentation of Gag to HIV-primed human CD8+ T cells
PBMCs from HIV-infected donors were labeled with 1μM CFSE and 106 cells plated in 96-deep well plates in 500 μL of RPMI with 5% human serum. PBMCs were cultured 7 days with no Gag antigen (negative control) or with HIV Gag peptides at 2 μg/mL or MG38.2-Gag p24, 3D6-p24, 3G9-p24, and control Ig-p24 at 1 μg/mL. To directly assess the function of DCs in presenting the Gag fusion mAbs, we used MoDCs in parallel to the PBMC assay described above. Immature DCs at day 5 of monocyte culture in GM-CSF and IL-4 were collected and pulsed overnight with medium, p24 peptides (2 μg/mL), or MG38.2-p24, 3D6-p24, 3G9-p24, and control Ig-p24 at 1 μg/mL. The antigen-pulsed MoDCs were matured by adding γ-irradiated CD40L-expressing cells (kindly provided by J. Banchereau, Dallas, TX) at a ratio of 1:5 (CD40L:DC) for 48 hours. CD40 ligation enhances cross-presentation by DCs.22 Syngeneic CD14− cells were thawed, rested for 2 hours at 37°C before CFSE labeling, plated in 48-well plates at 1.5-2 × 106/mL, and cultured 6 days with the antigen-pulsed mature MoDCs at a DC:T ratio of 1:30. After 6-7 days of culture, all samples were restimulated 8 hours ± p24 peptides (2 μg/mL) with 0.5 μg/mL anti-CD28 and CD49d (clones L293 and L25; BD Biosciences). Brefeldin A, at 10 μg/mL, was added for the last 6 hours. The cells were fixed, permeabilized, and stained with perCP-anti-CD3, APC-anti-CD8, and PE-anti-IFN-γ or its respective isotype control (all from BD Bioscience). Cells were analyzed with a BD FACSCalibur, collecting 50 000-100 000 high CD3+ events.
All human subject experiments were approved by The Rockefeller University Institutional Review Board.
Results
Production of hDEC205/hIgG1Fc fusion protein
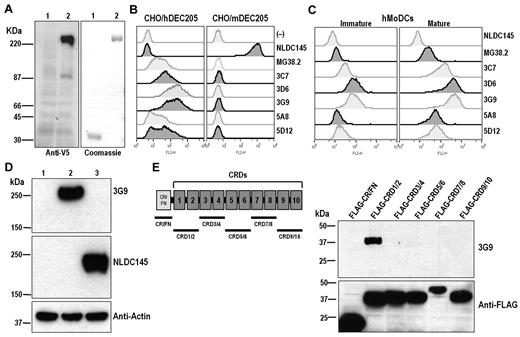
To express hDEC205, cDNAs encoding full-length DEC205 proteins from human and mouse were cloned by PCR based on published sequences.6,19 A V5 epitope tag was inserted into the N-terminus to detect the expression of recombinant hDEC205 protein or transgene. The cDNAs of V5-tagged hDEC205 (GenBank accession no. AY682091) and C57BL/6 mDEC205 (GenBank accession no. AF395445) were used to generate stable CHO cells expressing cell-surface DEC205. To prepare a secreted form of DEC205, the full-length extracellular domain of hDEC205 cDNA was fused to a human IgG1 Fc cassette (V5.hDEC205/hIgG1Fc; GenBank accession no. DQ407610), transfected, and stably expressed in CHO cells (Figure 1A left) to produce V5.hDEC205/hIgG1Fc protein of 90% or better purity after protein A (Figure 1A right). This protein was used to prepare mAbs.
Generation of human anti-hDEC205 monoclonal antibodies. (A) Expression of soluble human DEC205-human IgG1 Fc (V5.hDEC205/hIgG1Fc) fusion protein. Left: CHO cells, transfected with control plasmid (lane 1) and V5.hDEC205/hIgG1Fc (lane 2), were lysed and analyzed by Western blotting with anti-V5 antibody. Right: purified human IgG1 Fc alone (lane 1) or V5.hDEC205/hIgG1Fc fusion proteins (lane 2) produced by transfected CHO cells were analyzed on SDS-PAGE and stained by Coomassie blue. (B) FACS labeling of CHO/hDEC205 and CHO/mDEC205 cells and (C) human MoDCs stained at 1 μg/mL with human anti-hDEC205 mAbs, as well as the previously described NLDC145 rat anti-mDEC205 and MG38.2 mouse anti-hDEC205. The MoDCs were treated with/without LPS (100 ng/mL) to generate mature and immature DCs. (D) Lysates from CHO/Neo (lane 1), CHO/hDEC205 (lane 2), and CHO/mDEC205 (lane 3) cells were analyzed by Western blotting with mAbs 3G9 anti-hDEC205, NLDC145 anti-mDEC205, and anti-actin. (E) A series of deletion constructs in the extracellular domain of human DEC205 were generated (left, underlines), each with a FLAG tag, and each FLAG-tagged deletion construct was expressed in 293T cells. To map the 3G9 epitope on DEC205, each cell lysate was analyzed by Western blotting with mAbs 3G9 and L5 anti-FLAG.
Generation of human anti-hDEC205 monoclonal antibodies. (A) Expression of soluble human DEC205-human IgG1 Fc (V5.hDEC205/hIgG1Fc) fusion protein. Left: CHO cells, transfected with control plasmid (lane 1) and V5.hDEC205/hIgG1Fc (lane 2), were lysed and analyzed by Western blotting with anti-V5 antibody. Right: purified human IgG1 Fc alone (lane 1) or V5.hDEC205/hIgG1Fc fusion proteins (lane 2) produced by transfected CHO cells were analyzed on SDS-PAGE and stained by Coomassie blue. (B) FACS labeling of CHO/hDEC205 and CHO/mDEC205 cells and (C) human MoDCs stained at 1 μg/mL with human anti-hDEC205 mAbs, as well as the previously described NLDC145 rat anti-mDEC205 and MG38.2 mouse anti-hDEC205. The MoDCs were treated with/without LPS (100 ng/mL) to generate mature and immature DCs. (D) Lysates from CHO/Neo (lane 1), CHO/hDEC205 (lane 2), and CHO/mDEC205 (lane 3) cells were analyzed by Western blotting with mAbs 3G9 anti-hDEC205, NLDC145 anti-mDEC205, and anti-actin. (E) A series of deletion constructs in the extracellular domain of human DEC205 were generated (left, underlines), each with a FLAG tag, and each FLAG-tagged deletion construct was expressed in 293T cells. To map the 3G9 epitope on DEC205, each cell lysate was analyzed by Western blotting with mAbs 3G9 and L5 anti-FLAG.
Generation of human anti–human DEC205/CD205 mAbs
Human Ig Tg mice15 were used to generate human anti-hDEC205 mAbs by standard immunization and hybridoma technology. Both complete/incomplete Freund and RIBI MPL plus TDM adjuvants resulted in good antibody titers to hDEC205. Spleen cells harvested at peak titers were fused with murine P3 × 63Ag8.653 myeloma cells, cultured in selection media, and hybridoma supernatants initially screened for human IgGκ with an isotype-specific sandwich ELISA. Human IgGκ+ supernatants were assayed for reactivity to hDEC205 by FACS on CHO/hDEC205 cells and ELISA with V5.hDEC205/hIgG1Fc-coated plates.
Five selected mAbs, named 3C7, 3D6, 3G9, 5A8, and 5D12, were further characterized for binding to cell-surface hDEC205. All 5 mAbs and MG38.2, which is a mouse anti-hDEC205 mAb generated previously,19,20 bound to CHO/hDEC205 cells, but not to CHO/mDEC205 cells, while NLDC145 anti-mDEC205 mAb bound only to CHO/mDEC205 cells (Figure 1B). When new anti-hDEC205 mAbs were used to stain human MoDCs, binding was observed, and, as expected, mature hMoDCs expressed higher levels of hDEC205 (Figure 1C). The 5 new anti-hDEC205 mAbs exhibited stronger binding to hDEC205 than the earlier MG38.2 mAb. 3D6 and 3G9 mAbs showed the highest binding affinities to hDEC205 on both CHO/hDEC205 cells and hMoDCs (Figure 1B-C). In addition, the binding affinity and binding kinetics of human anti-hDEC205 mAbs were examined by Biacore SPR analysis, where 3D6, 3G9, and 5D12 mAbs exhibited the highest affinity to hDEC205 protein (Table 1). One of these new anti-hDEC205 mAbs, 3G9, also detected hDEC205 protein expressed in CHO/hDEC205 cells by Western blotting (Figure 1D), verifying that the mAb detected the appropriate antigen when expressed on the cell surface (Figure 1B-C) and by immunoblotting (Figure 1D). When blotting was used to determine the subdomain of hDEC205 protein reactive to 3G9, the epitope was in carbohydrate recognition domains (CRDs) 1 and 2 (CRD1/2; Figure 1E). As a control, we used either a nonbinding human IgG1 or 3G9 mAb with mutated complementarity determining region (CDR) sequences. The new human anti-hDEC205 mAbs were then tested for reactivity with hDEC205 in vivo.
Affinity and kinetic parameters (with background subtracted)
| mAb ID . | ka, 1/Ms . | kd, 1/s . | KA, 1/M . | KD, M . | RMax, RU . |
|---|---|---|---|---|---|
| 5A8 | 3.6 × 105 | 2.0 × 10−4 | 2.1 × 109 | 1.5 × 10−9 | 172 |
| 3C7 | 1.7 × 105 | 7.6 × 10−4 | 5.2 × 108 | 5.6 × 10−9 | 133 |
| 3D6 | 1.8 × 106 | 1.2 × 10−4 | 1.5 × 1010 | 8.0 × 10−11 | 294 |
| 5D12 | 5.4 × 105 | 3.2 × 10−4 | 2.0 × 109 | 7.0 × 10−10 | 272 |
| 3G9 | 9.0 × 105 | 1.9 × 10−4 | 4.7 × 109 | 2.4 × 10−10 | 268 |
| mAb ID . | ka, 1/Ms . | kd, 1/s . | KA, 1/M . | KD, M . | RMax, RU . |
|---|---|---|---|---|---|
| 5A8 | 3.6 × 105 | 2.0 × 10−4 | 2.1 × 109 | 1.5 × 10−9 | 172 |
| 3C7 | 1.7 × 105 | 7.6 × 10−4 | 5.2 × 108 | 5.6 × 10−9 | 133 |
| 3D6 | 1.8 × 106 | 1.2 × 10−4 | 1.5 × 1010 | 8.0 × 10−11 | 294 |
| 5D12 | 5.4 × 105 | 3.2 × 10−4 | 2.0 × 109 | 7.0 × 10−10 | 272 |
| 3G9 | 9.0 × 105 | 1.9 × 10−4 | 4.7 × 109 | 2.4 × 10−10 | 268 |
For each antibody, the figures shown are the mean of 2 separate series of experiments, using separately prepared sensor chips in each case, where: ka indicates kinetics of association; kd, kinetics of dissociation; KD, dissociation rate constant (measure of affinity); KA, association rate constant; and RMax, maximum SPR response signal.
Generation of hDEC205 Tg mice
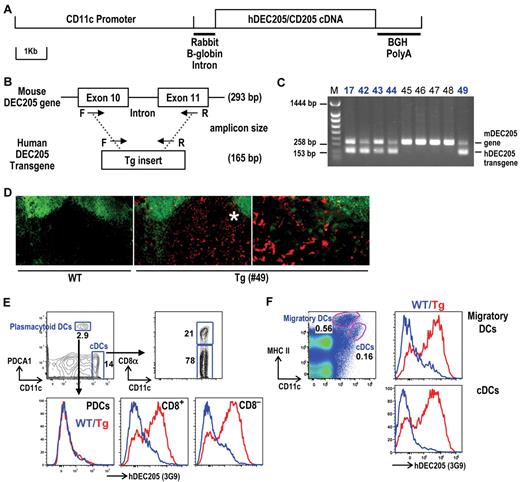
To establish the in vivo targeting activity of human anti-hDEC205 mAbs, we generated Tg mice expressing hDEC205 in mouse DCs. To do so, we used the promoter of the CD11c integrin α subunit, which is expressed at high levels on most DC subsets in mice. Full-length hDEC205 cDNA linked to a previously described 5.8-kb fragment of mouse CD11c promoter23 (CD11c-hDEC205; Figure 2A) was integrated into the genome of C57BL/6 mice. Because the sequences of human and mouse DEC205 cDNAs are highly homologous, a set of PCR primers was designed to discriminate hDEC205 cDNA transgene from the mDEC205 gene, which contains introns (Figure 2B). Using this primer pair, we identified several founders expressing the hDEC205 transgene through germ-line transmission to the next generation (Figure 2C). Because 3G9 mAb showed the strongest binding to hDEC205 in FACS and Western blot analyses (Figure 1B-E), we used 3G9 to detect hDEC205 in tissues of the founder lines (Figure 2C). In the initial screen by FACS, the no. 49 line exhibited the highest expression of hDEC205 in spleen and lymph nodes (data not shown). These results were confirmed by immunolabeling of tissue sections, such as in lymph nodes of hDEC205 Tg no. 49, but not wild-type, mice, hDEC205 was localized to dendritic profiles in the T-cell areas where DCs typically reside (Figure 2D).
Generation of hDEC205 transgenic mice. (A) Schematic of a linearized CD11c promoter-hDEC205 transgene. (B) PCR genotyping strategy for hDEC205 Tg mice, where a set of primers was selected for conserved sequences between mouse and human DEC205. The shorter (165 bp) PCR amplicon is generated from the hDEC205 intronless transgene and the longer (293 bp) from the mouse DEC205 gene. Arrows indicate the positions of F (forward) and R (reverse) primers. (C) A representative gel of 5 hDEC205 Tg founders after PCR of genomic DNA from newborn Tg mouse tails. M is for a lane loaded with molecular weight markers, and bold numbers (17, 42, 43, 44, and 49) indicate the lanes with samples from founder mice carrying the transgene. (D) Localization of hDEC205 in lymph nodes of hDEC205 Tg no. 49 and wild-type mice was visualized by immunohistochemistry. Acetone-fixed cryostat sections of subcutaneous lymph nodes were stained with 3G9 mAb, followed by secondary PE-conjugated antihuman IgG, and FITC-anti-B220 to discriminate B and T areas. Images were taken 200× magnification, but the region with the asterisk (*) is magnified 400× on the right using a Molecular Devices OlympusAX70 deconvolution microscope (Olympus) running METAMORPH Meta Imaging 3.0 (Universal Imaging Corporaration). (E) Expression of hDEC205 in the spleen of wild-type and hDEC205 Tg no. 49 mice. Lymphocyte lineage (CD3, CD19, and DX5) negative cells were surface stained with antibodies for hDEC205 (3G9-Alexa647), CD11c, CD8α, and PDCA1 to monitor hDEC205 expression in PDCs (PDCA1+), and cDCs (CD11c+ and CD8+ or CD8−). (F) Skin-draining lymph node cell suspension from wild-type and hDEC205 Tg no. 49 mice were stained with mAbs for hDEC205 (3G9-A647), CD11c, and MHC II to detect hDEC205 in migratory DCs and cDCs. In histograms, blue is for wild-type (WT) and red for hDEC205 Tg mice (Tg; E-F).
Generation of hDEC205 transgenic mice. (A) Schematic of a linearized CD11c promoter-hDEC205 transgene. (B) PCR genotyping strategy for hDEC205 Tg mice, where a set of primers was selected for conserved sequences between mouse and human DEC205. The shorter (165 bp) PCR amplicon is generated from the hDEC205 intronless transgene and the longer (293 bp) from the mouse DEC205 gene. Arrows indicate the positions of F (forward) and R (reverse) primers. (C) A representative gel of 5 hDEC205 Tg founders after PCR of genomic DNA from newborn Tg mouse tails. M is for a lane loaded with molecular weight markers, and bold numbers (17, 42, 43, 44, and 49) indicate the lanes with samples from founder mice carrying the transgene. (D) Localization of hDEC205 in lymph nodes of hDEC205 Tg no. 49 and wild-type mice was visualized by immunohistochemistry. Acetone-fixed cryostat sections of subcutaneous lymph nodes were stained with 3G9 mAb, followed by secondary PE-conjugated antihuman IgG, and FITC-anti-B220 to discriminate B and T areas. Images were taken 200× magnification, but the region with the asterisk (*) is magnified 400× on the right using a Molecular Devices OlympusAX70 deconvolution microscope (Olympus) running METAMORPH Meta Imaging 3.0 (Universal Imaging Corporaration). (E) Expression of hDEC205 in the spleen of wild-type and hDEC205 Tg no. 49 mice. Lymphocyte lineage (CD3, CD19, and DX5) negative cells were surface stained with antibodies for hDEC205 (3G9-Alexa647), CD11c, CD8α, and PDCA1 to monitor hDEC205 expression in PDCs (PDCA1+), and cDCs (CD11c+ and CD8+ or CD8−). (F) Skin-draining lymph node cell suspension from wild-type and hDEC205 Tg no. 49 mice were stained with mAbs for hDEC205 (3G9-A647), CD11c, and MHC II to detect hDEC205 in migratory DCs and cDCs. In histograms, blue is for wild-type (WT) and red for hDEC205 Tg mice (Tg; E-F).
To confirm hDEC205 expression on DCs from CD11c-hDEC205 Tg #49 mice, lymphoid cells were surface stained with 3G9 mAb. In the spleen, CD8+ and CD8− classical DCs (cDCs) expressed hDEC205, but plasmacytoid DCs (PDCs) did not (Figure 2E), while in lymph nodes, both cDCs and migratory DCs selectively expressed hDEC205 (Figure 2F). CD11c-hDEC205 Tg no. 49 mice were chosen to test anti-hDEC205 mAbs for DC targeting.
Targeting of 3G9 mAb to DCs in vivo
To assess the targeting of 3G9 mAb to DCs in vivo, 30 μg of Alexa 647–labeled 3G9 or control mAb was injected intraperitonally into wild-type and CD11c-hDEC205 Tg mice. Neither of the injected mAbs was detectable in wild-type mice, but in hDEC205 Tg mice, the injected 3G9 selectively labeled splenic cDCs, while the control human IgG1 did not label any targets (Figure 3). Thus, 3G9 mAb targets to hDEC205 on DCs in vivo.
Targeting of 3G9 to DCs in vivo. Wild-type and hDEC205 Tg mice were injected intraperitoneally with Alexa 647–labeled 3G9 or human IgG1 isotype (30 μg per mouse). Twelve hours later, lymphocyte lineage-negative (LIN−) splenic cell suspensions were stained with the indicated antibodies. In the histograms, blue is for wild-type (WT) and red for hDEC205 Tg mice (Tg).
Targeting of 3G9 to DCs in vivo. Wild-type and hDEC205 Tg mice were injected intraperitoneally with Alexa 647–labeled 3G9 or human IgG1 isotype (30 μg per mouse). Twelve hours later, lymphocyte lineage-negative (LIN−) splenic cell suspensions were stained with the indicated antibodies. In the histograms, blue is for wild-type (WT) and red for hDEC205 Tg mice (Tg).
Production of anti-hDEC205-p24 fusion mAbs
To evaluate the capacity of hDEC205 receptor on DCs to process and present antigens, 3D6 and 3G9 cDNAs were cloned and engineered to generate hybrid mAbs fused with HIV Gag p24 (Figure 4A). A control human IgG1 mAb Gag p24 fusion mAb was also generated by mutation of 3G9 parental CDR sequences. Following expression from mammalian cells and purification by protein A, the integrity and specificity of the Gag p24 fusion mAbs were confirmed by Coomassie stains, Western blots (Figure 4B), and FACS analyses with CHO/hDEC205 cells (Figure 4C). Next, we stained normal human spleen sections with 3G9-p24 fusion mAb. 3G9-p24 and MG38.2, a mouse anti-hDEC205, colabeled hDEC205+ DCs in T areas of the human spleen (Figure 4D). Therefore, the human anti-hDEC205-HIV Gag p24 fusion mAb vaccines were ready for testing in mice.
Expression and binding of hybrid anti-hDEC205-HIV Gag p24 fusion mAbs. (A) Schematic view of engineered mAb fused with HIV Gag p24. (B) Purified anti-hDEC205 mAbs 3D6 and 3G9 and their p24-fused hybrid mAbs were separated on 10% SDS-PAGE and stained with Coomassie blue (left) and blotted with anti-HIV Gag p24 antibodies (right). (C) Binding of hybrid mAbs to CHO/hDEC205, CHO/mDEC205, and control CHO/Neo cells using graded doses of purified recombinant p24-fused mAbs, followed by anti-hIgG-PE. (D) Detection of hDEC205 in normal human spleen sections after costaining with biotinylated 3G9-p24 (Alexa 555, red), MG38.2 mouse anti-hDEC205 (Alexa 488, green), and anti-hCD3 (APC, blue) to mark the T-cell areas. Images were taken 200× magnification using a Molecular Devices OlympusAX70 deconvolution microscope running METAMORPH Meta Imaging 3.0
Expression and binding of hybrid anti-hDEC205-HIV Gag p24 fusion mAbs. (A) Schematic view of engineered mAb fused with HIV Gag p24. (B) Purified anti-hDEC205 mAbs 3D6 and 3G9 and their p24-fused hybrid mAbs were separated on 10% SDS-PAGE and stained with Coomassie blue (left) and blotted with anti-HIV Gag p24 antibodies (right). (C) Binding of hybrid mAbs to CHO/hDEC205, CHO/mDEC205, and control CHO/Neo cells using graded doses of purified recombinant p24-fused mAbs, followed by anti-hIgG-PE. (D) Detection of hDEC205 in normal human spleen sections after costaining with biotinylated 3G9-p24 (Alexa 555, red), MG38.2 mouse anti-hDEC205 (Alexa 488, green), and anti-hCD3 (APC, blue) to mark the T-cell areas. Images were taken 200× magnification using a Molecular Devices OlympusAX70 deconvolution microscope running METAMORPH Meta Imaging 3.0
Antigens targeted to hDEC205 are efficiently presented to CD4+ T cells in vivo
The HIV Gag p24-conjugated anti-hDEC205 fusion mAbs with adjuvant induced high frequencies of IFN-γ (and IL-2; data not shown) producing CD4+ T cells in hDEC205 Tg mice, compared with control Ig-p24 immunization (Figure 5A). Pools 1 and 4 from the Gag peptide library were recognized by the CD4+ T cells (Figure 5A), which is in accordance with our previous immunizations with anti-mDEC205 Gag mAb.10 In 3 experiments of the primary response to fusion mAb with poly IC and anti-CD40 as adjuvants, 3G9-p24 induced significantly more IFN-γ (and IL-2; data not shown) producing CD4+ T cells than 3D6-p24 and many more T cells relative to nontargeted control Ig-p24 mAb (Figure 5A).
hDEC205 targeting with adjuvant induces Gag-specific CD4+ T-cell responses. (A) hDEC205 Tg mice were injected intraperitonally with 10 μg of purified 3G9-HIV Gag p24, 3D6-p24, or control Ig-p24 fusion mAbs along with 50 μg of poly IC and 25 μg of anti-CD40 mAb. After 2 weeks, splenic T-cell responses to pools of p24 peptides were analyzed. FACS plots from a representative experiment and histograms from 3 independent experiments are shown in percentiles of CD3+, IFN-γ–producing CD4+ T cells; similar results were obtained for IL-2–producing cells (not shown). Asterisks for statistically significant changes (*P ≤ .05, **P ≤ .01). (B) As in panel A, but prime-boost immunizations with 3G9-HIV Gag p24 (left, in blue) or 3G9-SIV Gag p27 (right, in red), plus only poly ICLC as an adjuvant. (C) SIV Gag p41 was the immunizing antigen to test responses to p17 and p27 regions of Gag. (D) As in panel A, showing IFN-γ–producing T cells after immunization with 3G9-HIV Gag p24, but not control anti-human DC-SIGN-p24 (left), and also specific proliferation (CFSE dilution) to HIV Gag p24, not HIV Gag p17 peptides (right). hDEC205 Tg in C57BL/6 background (A-C) and crossed with B10.BR (D).
hDEC205 targeting with adjuvant induces Gag-specific CD4+ T-cell responses. (A) hDEC205 Tg mice were injected intraperitonally with 10 μg of purified 3G9-HIV Gag p24, 3D6-p24, or control Ig-p24 fusion mAbs along with 50 μg of poly IC and 25 μg of anti-CD40 mAb. After 2 weeks, splenic T-cell responses to pools of p24 peptides were analyzed. FACS plots from a representative experiment and histograms from 3 independent experiments are shown in percentiles of CD3+, IFN-γ–producing CD4+ T cells; similar results were obtained for IL-2–producing cells (not shown). Asterisks for statistically significant changes (*P ≤ .05, **P ≤ .01). (B) As in panel A, but prime-boost immunizations with 3G9-HIV Gag p24 (left, in blue) or 3G9-SIV Gag p27 (right, in red), plus only poly ICLC as an adjuvant. (C) SIV Gag p41 was the immunizing antigen to test responses to p17 and p27 regions of Gag. (D) As in panel A, showing IFN-γ–producing T cells after immunization with 3G9-HIV Gag p24, but not control anti-human DC-SIGN-p24 (left), and also specific proliferation (CFSE dilution) to HIV Gag p24, not HIV Gag p17 peptides (right). hDEC205 Tg in C57BL/6 background (A-C) and crossed with B10.BR (D).
To extend the study to an adjuvant with a good safety profile in human subjects, we used poly ICLC (poly IC complexed with poly-L-lysine and carboxymethylcellulose) only to prime and boost mice 1 month apart with 3G9-HIV Gag p24 and 3G9-SIV Gag p27. The combination of targeted vaccine plus poly ICLC was immunogenic in hDEC205 Tg mice (Figure 5B). We also immunized with 3G9-SIV Gag p41 to test if epitopes from both SIV Gag p17 and SIV Gag p27 regions were immunogenic, and this was the case (Figure 5C). To evaluate the quality of the T-cell response, we showed that the T cells were capable of antigen-specific proliferative activity (Figure 5D). Thus, high frequencies of CD4+ T cells with potentially valuable properties, such as breadth and proliferative capacity, can be primed with anti-hDEC205 Gag fusion mAbs.
Induction of humoral responses to anti-hDEC205 antibodies in vivo
To examine humoral responses in hDEC205 Tg mice, 10 μg of 3D6, 3G9, or control human IgG1 mAbs were injected intraperitoneally with adjuvant. One immunization of hDEC205 Tg mice with anti-hDEC205 mAbs 3D6 and 3G9 was sufficient to induce high IgG antibodies against human IgG1 (Figure 6). These antibodies against human anti-hDEC205 mAbs included a spectrum of all IgG subclasses (ie, IgG1, IgG2b, IgG2c, and IgG3). There was no significant difference in total IgG titers between hDEC205 Tg mice immunized with 3D6 and 3G9 (Figure 6A); however, the mice immunized with 3D6 had slightly higher IgG2c and IgG3 titers than those immunized with 3G9 (Figures 6C,E). High antibody titers against anti-hDEC205 mAbs were induced with an additional round of immunization in the absence of adjuvant (Figure 6). In the same experiment, control human IgG1 mAb generated no detectable humoral IgG responses in hDEC205 Tg mice (Figure 6), indicating the greatly enhanced humoral responses to anti-3D6 and -3G9 mAb targeting.
Targeting to hDEC205 enhances humoral responses. At day 0, hDEC205 Tg mice were primed by intraperitonal injection with 10 μg of purified 3G9, 3D6, or control human IgG1 mAbs in the presence of 50 μg of poly IC and 25 μg of anti-CD40 mAb. At day 25, mice were boosted intraperitoneally with 10 μg of the same antibodies without adjuvants. Serum samples were collected at days −2, 5, 21, 31, 40, and 58. Serum antihuman IgG1 titers of total mouse IgG (A), IgG1 (B), IgG2c (C), IgG2b (D), and IgG3 (E) were measured by ELISA. Filled diamonds, squares, and triangles represent each group (3 mice per group). Asterisks denote statistical significances (*P ≤ .05; **P ≤ .01).
Targeting to hDEC205 enhances humoral responses. At day 0, hDEC205 Tg mice were primed by intraperitonal injection with 10 μg of purified 3G9, 3D6, or control human IgG1 mAbs in the presence of 50 μg of poly IC and 25 μg of anti-CD40 mAb. At day 25, mice were boosted intraperitoneally with 10 μg of the same antibodies without adjuvants. Serum samples were collected at days −2, 5, 21, 31, 40, and 58. Serum antihuman IgG1 titers of total mouse IgG (A), IgG1 (B), IgG2c (C), IgG2b (D), and IgG3 (E) were measured by ELISA. Filled diamonds, squares, and triangles represent each group (3 mice per group). Asterisks denote statistical significances (*P ≤ .05; **P ≤ .01).
Cross-presentation of human anti-hDEC205 HIV Gag to Gag primed T cells
To assess the capacity of the 3D6 and 3G9 mAbs to mediate cross-presentation, we studied HIV-primed CD8+ T cells from HIV-infected individuals as previously described,11 since we were unable to prime large numbers of CD8+ T cells to HIV Gag in hDEC205 Tg mice in the C57BL/6 background. We compared the human-Gag p24 fusion mAbs to mouse MG38.2 anti-hDEC205-p24 and nonbinding control Ig-p24. Either whole PBMCs (Figure 7A) or mixtures of MoDC and T cells (Figure 7B) were used as previously described.11 Anti-hDEC205 mAbs at just 1 μg/mL induced T-cell proliferation and IFN-γ production from primed CD8+ T cells, comparably to cells expanded with a pool of 50 peptides spanning the Gag p24 sequence (Figure 7). Thus, the human anti-hDEC205 mAbs mediate cross presentation.
Improved cross-presentation of Gag protein via DEC205 to human T cells. (A) PBMCs from a long-term nonprogressor patient (LB06) were CFSE labeled and cultured 7 days with the indicated sources of HIV Gag p24 antigen (top). At the end of the culture, the cells were restimulated 6 hours with a pool of 50 Gag 15-mer peptides (upper) or without peptides (lower) in the presence of anti-CD28 costimulatory mAb. Data show IFN-γ production by CD8+ T cells, most of which have extensively diluted their CFSE label as a result of cell division. (B) As in (A), but the cells are mixtures of MoDCs and autologous T cells from a chronically HIV-infected donor (LB11). The data are displayed as 2 color dot plots to measure CFSE dilution and IFN-γ production in CD3+CD8+ cells.
Improved cross-presentation of Gag protein via DEC205 to human T cells. (A) PBMCs from a long-term nonprogressor patient (LB06) were CFSE labeled and cultured 7 days with the indicated sources of HIV Gag p24 antigen (top). At the end of the culture, the cells were restimulated 6 hours with a pool of 50 Gag 15-mer peptides (upper) or without peptides (lower) in the presence of anti-CD28 costimulatory mAb. Data show IFN-γ production by CD8+ T cells, most of which have extensively diluted their CFSE label as a result of cell division. (B) As in (A), but the cells are mixtures of MoDCs and autologous T cells from a chronically HIV-infected donor (LB11). The data are displayed as 2 color dot plots to measure CFSE dilution and IFN-γ production in CD3+CD8+ cells.
Discussion
Protein vaccines offer considerable potential relative to vector-delivered vaccines in terms of reduced expense of production, ease of transport and storage, and capacity to repeatedly immunize without the obstacle of vector immunity. To render protein vaccines immunogenic, we have previously shown the value of targeting to receptors on DCs along with coadministration of adjuvants that render DCs immunostimulatory. In our studies, we have emphasized the DEC205 receptor for targeting and poly IC as an adjuvant.
To bring this potentially protective strategy into the clinic, it would help to have fully human anti-hDEC205 fusion mAbs. Human mAbs to hDEC205 proved feasible to produce using human DEC205 extracellular domain to immunize mice carrying human, not mouse, Ig genes. We selected 2 mAbs, 3G9 and 3D6, for study because they both bound to human and rhesus DEC205 at high affinity. To carry out in vivo immunization experiments, we also developed a Tg mouse expressing full-length hDEC205 in DCs under control of the CD11c promoter and assessed the immunological responses of hDEC205 targeting in vivo. These mice express hDEC205 exclusively in CD11chigh DCs, although the frequency of DEC205 expressing DCs is not 100% and varies among individual Tg mice. Targeting of anti-hDEC205 mAb selectively labeled DCs, and these DCs were found in the T-cell areas of the lymph nodes.
We then found that these Tg mice developed strong CD4+ T-cell responses to hDEC205-targeted HIV and SIV Gag proteins, but not control Ig-targeted Gag. The T-cell responses showed 2 valuable features: breadth or capacity to recognize several different Gag peptides and proliferative capacity. In prior research,13 we found that the T-cell responses to DEC205-targeted antigen in mice are of the Th1 and not Th2 or Th17 types. We have been verifying this in ongoing studies in rhesus macaques, where we used the 3G9-HIV gag p24 fusion mAb along with poly IC to immunize nonhuman primates. However, Gag-specific CD8+ T cells were not detected in our immunized C57BL/6 mice, which are not known to present HIV Gag on MHC class I.10 To verify that the new anti-hDEC205 mAbs could enhance cross-presentation, we carried out experiments with CD8+ T cells from HIV-infected individuals. We had shown previously that mouse antihuman DEC-targeted gag p24 was reliably cross-presented to human CD8+ T cells. Here, we verified that human 3G9 and 3D6 anti-DEC205 greatly enhanced cross-presentation relative to control fusion mAbs, and were as active as pools of Gag peptides to load antigen into DCs. We did not assess the behavior of regulatory Foxp3+ T cells in the current paper, because, in a prior study, we showed that the use of poly IC as an adjuvant blocks the induction of Treg by DEC205-targeted antigen.24
To assess B-cell responses, we followed antibody formation to the human IgG following 3G9 and 3D6 immunization. The new targeting mAbs, but not nontargeting control, elicited high titers of anti-human IgG. These B- and T-cell findings propend feasibility to use protein vaccines to induce immunity to clinically relevant antigens as long as these are delivered efficiently to DCs together with appropriate adjuvants. Since DEC205 targeting enhances the immunogenicity of protein vaccines, which offer potential advantages with respect to ease and low costs of production, and ability to be administered repeatedly, we propose to test human DEC-targeted protein antigens as stand-alone vaccines and in combination with other approaches, such as recombinant viral vectors.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank members of the Steinman Laboratory for their valuable discussion. We also thank Judy Adams for her help with graphics, and So-Young Joe, Anna Charalambous, Joseph D. Schauer, James Storey, and George Ward for their technical help.
This work was supported by National Institutes of Health grants AI 40 045 and AI 40 874 (to R.M.S.) and AI 057158 (to C.G.P.), R21AI082331-01 (to Y.D.), the Bill and Melinda Gates Foundation Center for AIDS Vaccine Discovery (to R.M.S.), grants from NRF of Korea funded by Korean Ministry, MEST (2010-000856 and 2010K000830 to H.-W.L., 2010-0002810 to Y.D.), and the New York Community Trust (the Francis Florio Fund for Blood Diseases to C.C.).
National Institutes of Health
Authorship
Contribution: C.C., J.-H.C., L.V., L.-Z.H., C.T., L.B., Y.D., G.N., S.H.P., D.B.D., E.S., M.P., and C.G.P. performed research; H.-W.L. contributed vital new reagents; and C.C., J.-H.C., C.T., T.K., R.M.S., and C.G.P. designed research, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: L.V., L-Z.H., and T.K. are employees of Celldex Therapeutics, which is developing human DEC205-based vaccines. R.M.S. is a paid member of the scientific advisory board and holds stock options in Celldex Therapeutics. The remaining authors declare no competing financial interests.
Correspondence: Ralph M. Steinman or Choe Gyu Park, Laboratory of Cellular Physiology and Immunology and Chris Browne Center for Immunology and Immune Diseases, The Rockefeller University, 1230 York Ave, New York, NY 10065; e-mail: steinma@mail.rockefeller.edu or parkc@mail.rockefeller.edu.
References
Author notes
C.C. and J.-H.C. contributed equally to this work.