Abstract
Natural killer (NK) cells are innate immune lymphocytes that express a heterogeneous repertoire of germline-encoded receptors and undergo a distinct pattern of maturation. CD57 is a marker of terminal differentiation on human CD8+ T cells. Very few newborn or fetal NK cells express CD57; however, the frequency of CD57-bearing NK cells increases with age. We assessed the transcriptional, phenotypic, and functional differences between CD57+ and CD57− NK cells within the CD56dim mature NK subset. CD57+ NK cells express a repertoire of NK-cell receptors, suggestive of a more mature phenotype, and proliferate less when stimulated with target cells and/or cytokines. By contrast, a higher frequency of CD57+ NK cells produced interferon-γ and demonstrated more potent lytic activity when these cells were stimulated through the activating receptor CD16; however, they are less responsive to stimulation by interleukin-12 and interleukin-18. Finally, CD57 expression is induced on CD57−CD56dim NK cells after activation by interleukin-2. A combination of a mature phenotype, a higher cytotoxic capacity, a higher sensitivity to stimulation via CD16, with a decreased responsiveness to cytokines, and a decreased capacity to proliferate suggest that CD57+ NK cells are highly mature and might be terminally differentiated.
Introduction
Natural killer (NK) cells are a subset of lymphocytes composing 5% to 20% of peripheral blood mononuclear cells (PBMCs) in humans.1 NK cells participate in the innate immune response and play an important role in the defense against viral infections as well as in tumor surveillance and are also involved in shaping adaptive immune responses through their production of cytokines.2 Recently, studies have shown that NK cells share features with B and T cells of the adaptive immune system, as mouse NK cells demonstrate immune memory after viral infection.3
In humans, 2 NK-cell subsets have been characterized according to the cell-surface density of CD56 and expression of CD16 (FcγRIIIa). CD56dimCD16bright NK cells compose approximately 90% of circulating NK cells; CD56brightCD16−/dim NK cells constitute approximately 10%.4 CD56bright NK cells proliferate and produce interferon-γ (IFN-γ) in response to stimulation with interleukin-12 (IL-12), whereas CD56dim NK cells are more cytolytic and produce significant amounts of cytokine when their activating receptors are engaged.5-9 The CD56dim NK cells differentiate from the CD56bright NK cells.4,10-12 NK cells are a heterogeneous population with respect to the expression of killer cell immunoglobulin-like receptors (KIR), NKG2A, and natural cytotoxic receptors.
Similar to NK cells, CD8+ T cells are crucial to the recognition and clearance of virus-infected cells. Chronic stimulation of T cells, which occurs during rheumatoid arthritis, multiple myeloma, and cytomegalovirus and HIV infections, and after bone marrow transplantation, can result in the development of CD8+ T cells that are capable of cytokine secretion yet are incapable of cell division.13,14 Such failure to proliferate is generally attributed to replicative senescence or “clonal exhaustion” resulting from continual stimulation by antigens and/or cytokines. The expression of CD57 correlates with senescence in human CD8+ T cells.14-17 CD57 is a terminally sulfated glycan carbohydrate,13 which is commonly expressed on T cells in persons with chronic immune activation. CD57+ T cells increase in frequency with age.18 CD57+CD8+ and CD57+CD4+ T cells produce IFN-γ but are unable to proliferate in response to cognate peptide.19 Furthermore, CD57+ lymphocytes have undergone more rounds of cell division compared with CD57− memory T cells, and expression of CD57 correlates with T cells susceptible to activation-induced cell death (AICD). Although CD57 is generally regarded as a marker of senescence in CD8+ T cells,13,19,20 a recent study showed that CD57+CD8hi T cells are capable of proliferation in response to other stimuli.21 Thus, CD57+ T cells may not be “end-stage” effector T cells incapable of proliferation but may represent a highly differentiated subset of T cells capable of rapid cell division, cytotoxicity, and IFN-γ production, as well as secretion of IL-5.21
A total of 30% to 60% of CD56dimCD16bright NK cells in healthy adults express CD57, which is not expressed on immature CD56bright NK cells.7,22 Fetal and newborn NK cells do not express or express low amounts of CD57,23 and CD57 expression on NK cells increases with age.24,25 CD57 expression is linked to cellular maturity in CD8+ T cells and NK cells. Indeed, cells simultaneously expressing different cytolytic enzymes can be identified based on their expression of high levels of CD57.26 Although we originally reported the expression of CD57 on a subset of human NK cells many years ago,22 these studies were conducted before the appreciation of the receptor diversity that exists within the NK-cell population. Therefore, we have revisited the unique properties of NK cells expressing CD57 by defining their phenotype, transcriptional signature, and functional capacity.
Methods
NK-cell preparation and stimulation
PBMCs were obtained from leukocyte reduction Pall filters (Blood Centers of the Pacific) and from healthy adult volunteers after density gradient centrifugation over Ficoll-Paque (GE Healthcare). All volunteers gave informed consent to participate in this study. The University of California–San Francisco Committee on Human Research approved this study. PBMCs were analyzed by immunofluorescent staining (supplemental data, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
PBMCs were separated based on their buoyant density by suspension in 30% Percoll (GE Healthcare) in phosphate-buffered saline (PBS) layered over 40% Percoll, followed by centrifugation. The low buoyant density lymphocytes, enriched for NK cells, were cultured in complete RPMI 1640 media (10% fetal bovine serum, 1% penicillin and streptomycin, 1% glutamine) with or without 200 IU/mL human recombinant IL-2 (NCI BRB Preclinical Repository), with 50 ng/mL human IL-12 (PeproTech) and IL-18 (MBL) or with K562 or 721.221 cells at an effector/target ratio of 5:1 or 1:5. For antibody stimulation, plates were coated with N-[1-(2,3-dioleoyloxy)propyl]-N,N,N-trimethylammonium methylsulfate (Sigma-Aldrich) at 1 mg/mL and then with anti-CD56 (My31.13), anti-2B4 (C1.7), anti-NKG2D (140810), or anti-NKp46 (195314) at 10 μg/mL, or with anti-CD16 (Leu11a) at 10, 1, and 0.5 μg/mL. For degranulation assays, cells were cultured for 6 hours with fluorescein isothiocyanate (FITC) anti-CD107α (H4A3; eBioscience) and stained with phycoerythrin (PE) anti-CD56 (Leu19; BD Biosciences), PE-Cy5 anti-CD57, and allophycocyanin (APC) anti-CD3 (HIT3a; BioLegend), Pacific Blue anti-CD16, and near infrared live/dead fixable dead cell dye (Invitrogen). For intracellular cytokine staining, cells were cultured for 6 hours with brefeldin A (Golgi Plug; BD Biosciences) and stained with fluorescein isothiocyanate anti-CD57, PE anti-CD56, PerCPCy5.5 anti-CD3 (HIT3a; BioLegend), Pacific Blue anti-CD16, and near infrared live/dead dye. Cells were then fixed, permeabilized, and stained for intracellular IFN-γ with APC anti-IFN-γ (25723.11; BD Biosciences).
Cytotoxicity assays
For anti-CD16-induced cytotoxicity assays, 51Cr-labeled P815 target cells were incubated for 30 minutes at 37°C with anti-CD56 or anti-CD16 (1 μg/mL). Sorted CD57+ and CD57− NK cells (supplemental data) were resuspended in complete media with or without 100 IU/mL IL-2 and added with the targets at effector/target ratios from 20:1 to 0.625:1, each in triplicate. Cells were incubated for 5 hours at 37°C, and the released 51Cr was measured in a Microbeta counter (PerkinElmer Life and Analytical Sciences). Spontaneous lysis was determined by incubating target cells without effectors, and maximum lysis was determined by freezing and thawing target cells. The percentage of specific lysis = (lysis − average spontaneous lysis)/(average maximum lysis − average spontaneous lysis) × 100.
Proliferation assays
Sorted CD57+ and CD57− NK cells were labeled with 5μM carboxyfluorescein diacetate succinimidyl ester (CFSE; Invitrogen).27 CFSE-labeled cells were cultured in modified Eagle medium (Invitrogen) with 1% penicillin and streptomycin, 1% glutamine, 15% fetal calf serum (Hyclone), 0.05mM β-mercaptoethanol, and 100 IU/mL IL-2. NK cells were added to K562 stimulator cells expressing membrane-bound human IL-15 and human 4-1BB-ligand28 at a ratio 1:1.5. NK cells were harvested and stained with APC anti-CD3, PE-Cy5 anti-CD57, Alexa700 anti-CD56, biotin anti-CD16 (B73.1), Qdot605-streptavidin, and near infrared live/dead dye.
Apoptosis assay
Plates were coated with N-[1-(2,3-dioleoyloxy)propyl]-N,N,N-trimethylammonium methylsulfate, and then with 10 μg/mL anti-CD56 (DX32), anti-CD16 (L23a), or anti-CD95 (DX2). PBMCs were cultured for 6 hours with 200 IU/mL IL-2 and stained with PE-Cy5 anti-CD57, APC anti-CD3, Alexa700 anti-CD56, biotin anti-CD16, Qdot605-streptavidin, near infrared live/dead dye, and PE-annexin V (BD Biosciences). As a positive control, cells were stimulated with phorbol myristate acetate (50 ng/mL) and ionomycin (1 μg/mL).
Transcriptional analysis
CD57+CD3−CD56dimCD16+ and CD57−CD3−CD56dimCD16+ NK cells from 3 healthy adult donors were sorted into lysis solution (100 μL per 10 000 cells, RNAqueous-Micro kit; Ambion). RNA was analyzed by BioAnalyzer (Agilent) before amplification with the NuGEN WT-Ovation kit (Roche Diagnostics) and analyzing transcripts using Affymetrix Human Gene 1.0 ST arrays. Moderated paired t tests were used to identify differentially expressed genes (> 1.7-fold difference) between CD57+ and CD57− NK cells. The P values were set at less than .001 and less than .005. The microarray results are available at http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc = GSE23695.
Statistics
The 2-tailed paired t test was used in the phenotypic studies to compare CD57+ and CD57− NK cells. The nonparametric Wilcoxon matched pairs test was used in the functional analysis of CD57+ and CD57− NK cells. The statistical significance threshold was set at P less than .05.
Results
CD57 defines a subset of mature human NK cells
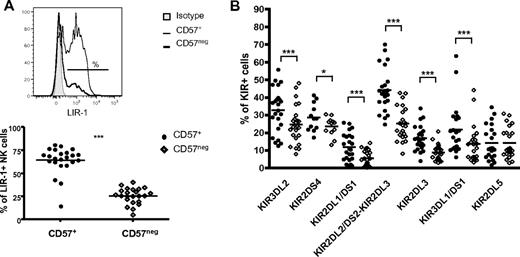
Our goal was to define the transcriptional signature, distinctive phenotype, and functional properties of CD57+ NK cells. CD57 is expressed on 30% to 60% of CD56dimCD16+ cells, which represent mature NK cells, whereas less than 1% of CD56bright NK cells, which are considered immature, express CD57 (Figure 1A; supplemental Figure 1), consistent with prior observations.4 Indeed, more than 98% of CD57+ NK cells were CD56dimCD16+ NK cells. CD56bright NK cells have higher surface density of NKp46, NKp30, NKG2A, and NKG2D compared with CD56dim NK cells.1,29 During maturation, the cell-surface density of CD56 and natural cytotoxic receptors decreases, whereas CD56dim NK cells acquire expression of CD16, KIRs, 2B4, and LIR-1. We hypothesized that within the CD56dimCD16+ NK-cell subset CD57 marks more mature NK cells, indicating a final stage of maturation. Thus, the phenotypic analysis was performed on CD56dimCD16+ NK cells (ie, with the exclusion of CD56bright cells as shown in supplemental Figure 1). CD45RA+CD62L+ and CD45RA+CD27+ T cells are considered naive, whereas CD45RO+ T cells are mature.30 NK cells are more than 95% CD45RA+ and rarely express CD45RO (data not shown). The percentage of CD57+ NK cells expressing CD27 and CD62L was significantly lower than CD57− NK cells (Figure 1B; CD27: 0.97% of CD57+ vs 6.67% of CD57−, CD62L: 4.17% vs 9.99%; P < .0005). Furthermore, NKp46, NKG2D, and NKp30 were expressed at a lower density (geometric mean fluorescent intensity [gMFI]) on the surface of CD57+ compared with CD57− NK cells (Figure 1C). Similarly, the percentage of NKG2A+ cells (44.7% of CD57+ vs 51.7% of CD57−, P < .0005) and the gMFI of NKG2A on these cells (2472 vs 2858.7, P < .05) were lower on CD57+ NK cells (data not shown). The difference of expression of these NK receptors between the 2 subsets was small in magnitude but consistently detected in all donors. On the other hand, the gMFI of CD16 correlated with NK-cell maturation and was significantly greater on CD57+ NK cells (Figure 1D; gMFI 10 682 vs 8636, P < .0005). KLRG1, an inhibitory receptor recognizing cadherins, was expressed on most CD56dimCD16+ NK cells, although at a lower frequency (Figure 1E; 47.7% of CD57+ vs 60% of CD57−, P < .0005) and density (gMFI 1041 vs1225, P < .0005, data not shown) on CD57+ NK cells. The frequency of NK cells expressing LIR-1 (Figure 2A) and most of the KIRs (Figure 2B) was higher on CD57+ NK cells. However, 2 KIRs, KIR2DL5 and KIR2DL4, deviated from this pattern. KIR2DL5 was the only KIR in which no significant difference was observed between CD57+ and CD57− NK cells (Figure 2B). KIR2DL4 was primarily expressed only on CD56bright NK cells (data not shown).31 The expression of other receptors (ie, 2B4, CD161, CD2, or DNAM-1 [CD226]) was not significantly different between the CD57+ and CD57− subsets (data not shown). Collectively, these data indicate different states of maturation within the CD56dimCD16+ NK-cell subset and suggest that CD57 is a marker of a more mature NK-cell subset.
CD57−CD56dimCD16+ NK cells are phenotypically less mature than CD57+ NK cells. (A) Flow cytometry was performed on total PBMCs, and CD3−CD56+ NK cells were gated (as shown in supplemental Figure 1) and analyzed for expression of CD57 and CD56. Two representative donors are shown. (B-E) Flow cytometry was performed on total PBMCs, and CD56dimCD16+ NK cells were gated on CD57+ and CD57− cells that were assessed for expression of different markers. Representative histograms for each marker are shown (shaded area represents isotype-matched control Ig; thin line, CD57+; and bold line, CD57−). The percentage of cells expressing CD62L and CD27 (B) and KLRG1 (D) was determined on each subset. The gMFI of NKp46, NKp30, NKG2D (C) and CD16 is shown (E). ● represents CD57+; and ◇, CD57−. ***P < .0005. (B-E) P values were determined comparing CD57+ and CD57− NK cells (n = 23, for KLRG1 n = 18 and for NKp30 n = 14).
CD57−CD56dimCD16+ NK cells are phenotypically less mature than CD57+ NK cells. (A) Flow cytometry was performed on total PBMCs, and CD3−CD56+ NK cells were gated (as shown in supplemental Figure 1) and analyzed for expression of CD57 and CD56. Two representative donors are shown. (B-E) Flow cytometry was performed on total PBMCs, and CD56dimCD16+ NK cells were gated on CD57+ and CD57− cells that were assessed for expression of different markers. Representative histograms for each marker are shown (shaded area represents isotype-matched control Ig; thin line, CD57+; and bold line, CD57−). The percentage of cells expressing CD62L and CD27 (B) and KLRG1 (D) was determined on each subset. The gMFI of NKp46, NKp30, NKG2D (C) and CD16 is shown (E). ● represents CD57+; and ◇, CD57−. ***P < .0005. (B-E) P values were determined comparing CD57+ and CD57− NK cells (n = 23, for KLRG1 n = 18 and for NKp30 n = 14).
More CD56dimCD16+CD57+ NK cells express KIR and LIR-1 than CD56dimCD16+CD57− NK cells. CD56dimCD16+ NK cells that are CD57+ or CD57− were assessed for the expression of LIR-1 and KIRs. (A) Representative histogram of LIR-1 expression (shaded area represents isotype-matched control Ig; thin line, CD57+; and bold line, CD57−). The percentage of cells expressing LIR-1 was determined in each subset. (B) The percentage of cells expressing each KIR was determined in each subset. ● represents CD57+; and ◇, CD57−. *P < .05. ***P < .0005. (A-B) P values were determined comparing CD57+ and CD57− NK cells (n = 23).
More CD56dimCD16+CD57+ NK cells express KIR and LIR-1 than CD56dimCD16+CD57− NK cells. CD56dimCD16+ NK cells that are CD57+ or CD57− were assessed for the expression of LIR-1 and KIRs. (A) Representative histogram of LIR-1 expression (shaded area represents isotype-matched control Ig; thin line, CD57+; and bold line, CD57−). The percentage of cells expressing LIR-1 was determined in each subset. (B) The percentage of cells expressing each KIR was determined in each subset. ● represents CD57+; and ◇, CD57−. *P < .05. ***P < .0005. (A-B) P values were determined comparing CD57+ and CD57− NK cells (n = 23).
CD57 is induced on CD56dim NK cells after activation
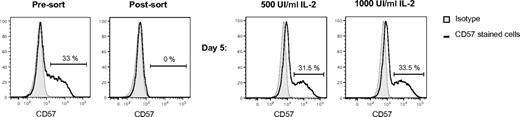
The differentiation of NK cells involves maturation from CD56bright to CD56dim NK cells,4,10-12 and we propose, from CD57−CD56dim to CD57+CD56dim cells. To test this hypothesis, we analyzed whether CD57− NK cells can acquire CD57 expression after stimulation. We sorted CD57−CD56dim NK cells and cultured them with IL-2 (500 or 1000 IU/mL) with or without stimulator cells for 5 days. After 5 days of activation with IL-2 (Figure 3) or with IL-2 and stimulator cells (data not shown), CD57 expression was induced on approximately 30% of the highly purified CD57−CD56dim NK cells. Under these conditions, once CD57 expression was acquired, it was stably expressed on the NK cells until the cultures were terminated at day 11 (data not shown). Thus, after appropriate stimuli, CD57 expression can be induced in CD57−CD56dim NK cells, suggesting that the acquisition of CD57 marks the transition to a late stage of NK-cell maturation.
CD57 is acquired after activation of CD57−CD56dim NK cells. Sorted CD57−CD56dim NK cells were cultured with 500 IU/mL or 1000 IU/mL IL-2 for 5 days. Representative histograms of CD57 expression by the NK cells before sort, after sort, and after stimulation are shown (shaded area represents isotype-matched control Ig; and bold line, CD57 stained cells).
CD57 is acquired after activation of CD57−CD56dim NK cells. Sorted CD57−CD56dim NK cells were cultured with 500 IU/mL or 1000 IU/mL IL-2 for 5 days. Representative histograms of CD57 expression by the NK cells before sort, after sort, and after stimulation are shown (shaded area represents isotype-matched control Ig; and bold line, CD57 stained cells).
Transcriptional signature of CD57+ and CD57− NK cells
To precisely define the differences between mature CD3−CD56dimCD16+ NK-cell subsets defined by CD57, we sorted CD57+ and CD57− NK cells, isolated RNA, and determined their transcriptional profile. We isolated the 2 subsets within the CD3−CD56dimCD16+ NK-cell population so that results obtained from the CD57− NK-cell population would be at a similar developmental stage (excluding less mature CD3−CD56brightCD16− NK cells). CD57+ and CD57− NK cells were isolated from 3 healthy adult blood donors, and the results were analyzed to identify genes that were differentially expressed in all donors.
Based on a cutoff of a 1.7-fold difference in transcript abundance and a P value of less than .001, 33 genes were different between the 2 subsets. Twenty genes were elevated in the CD57+ NK-cell subset compared with the CD57− NK cells, and 13 genes were expressed in lower amounts in the CD57+ NK cells (Table 1). Most of these genes are implicated in receptor signaling. Of particular note was the reduced transcription of IL-12 receptor-β2 on the CD57+ NK cells. CD57− NK cells express 4 times more IL-12Rβ2 mRNA than CD57+ NK cells (Table 1). This suggests a potential differential response to IL-12, a cytokine that is critical to IFN-γ production by NK cells. Remarkably, the expression of very few genes differed based on microarray analysis using a P value of less than .001. Using a less stringent threshold (P < .005), expression of several genes encoding receptors identified by our phenotypic analysis was determined to differ in these subsets. For example, as validated by our phenotypic studies, the KIR genes were more abundantly expressed in the CD57+ NK-cell population (see microarray results available at http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc = GSE23695).
Genes differentially expressed between CD57+ and CD57− NK cells
| Gene symbol . | Gene name . | Fold difference: CD57− vs CD57+ NK cells . |
|---|---|---|
| ENST00000365473 | Not determined | −4.434 |
| LRLE1 | Liver-related low express protein 1 | −3.548 |
| C5orf36 | Chromosome 5 open reading frame 36 | −3.998 |
| ENST00000410763 | Not determined | −3.868 |
| ENST00000386087 | Not determined | −3.802 |
| DOCK5 | Dedicator of cytokinesis 5 | −3.69 |
| ENST00000384268 | Not determined | −2.904 |
| CMKLR1 | Chemokine-like receptor 1 | −2.614 |
| ENST00000410744 | Not determined | −2.398 |
| ZNF570 | Zinc finger protein 570 | −2.376 |
| GPR141 | G protein-coupled receptor 141 | −2.312 |
| PALLD | Palladin, cytoskeletal associated protein | −2.096 |
| RPL7A; SNORD36B | Ribosomal protein L7a; small nucleolar RNA, C/D box 36B | −2.086 |
| RGS9 | Regulator of G protein signaling 9 | −1.972 |
| GOLM1 | Golgi membrane protein 1 | −1.962 |
| MTSS1 | Metastasis suppressor 1 | −1.896 |
| PATL2 | Protein associated with topoisomerase II homolog 2 (yeast) | −1.808 |
| ENST00000411036 | Not determined | −1.794 |
| SNORD56B | Small nucleolar RNA, C/D box 56B | −1.746 |
| MTERFD3 | MTERF domain containing 3 | −1.72 |
| FAM48B1; FAM48B2 | Family with sequence similarity 48, member B1; member B2 | 1.772 |
| FAM48B2; FAM48B1 | Family with sequence similarity 48, member B2; member B1 | 1.772 |
| LDB2 | LIM domain binding 2 | 1.928 |
| BC069702 | Not determined | 1.97 |
| UBASH3B | Ubiquitin associated and SH3 domain containing, B | 2.332 |
| ENST00000364309 | Not determined | 2.382 |
| ENST00000364948 | Not determined | 2.53 |
| ATP1B1 | ATPase, Na+/K+ transporting, β1 polypeptide | 2.548 |
| CLEC4A | C-type lectin domain family 4, member A | 2.864 |
| C3orf57 | Chromosome 3 open reading frame 57 | 3.092 |
| ENST00000364177 | Not determined | 3.272 |
| IL12RB2 | Interleukin 12 receptor, β2 | 4.252 |
| 7927706 | Not determined | 4.304 |
| Gene symbol . | Gene name . | Fold difference: CD57− vs CD57+ NK cells . |
|---|---|---|
| ENST00000365473 | Not determined | −4.434 |
| LRLE1 | Liver-related low express protein 1 | −3.548 |
| C5orf36 | Chromosome 5 open reading frame 36 | −3.998 |
| ENST00000410763 | Not determined | −3.868 |
| ENST00000386087 | Not determined | −3.802 |
| DOCK5 | Dedicator of cytokinesis 5 | −3.69 |
| ENST00000384268 | Not determined | −2.904 |
| CMKLR1 | Chemokine-like receptor 1 | −2.614 |
| ENST00000410744 | Not determined | −2.398 |
| ZNF570 | Zinc finger protein 570 | −2.376 |
| GPR141 | G protein-coupled receptor 141 | −2.312 |
| PALLD | Palladin, cytoskeletal associated protein | −2.096 |
| RPL7A; SNORD36B | Ribosomal protein L7a; small nucleolar RNA, C/D box 36B | −2.086 |
| RGS9 | Regulator of G protein signaling 9 | −1.972 |
| GOLM1 | Golgi membrane protein 1 | −1.962 |
| MTSS1 | Metastasis suppressor 1 | −1.896 |
| PATL2 | Protein associated with topoisomerase II homolog 2 (yeast) | −1.808 |
| ENST00000411036 | Not determined | −1.794 |
| SNORD56B | Small nucleolar RNA, C/D box 56B | −1.746 |
| MTERFD3 | MTERF domain containing 3 | −1.72 |
| FAM48B1; FAM48B2 | Family with sequence similarity 48, member B1; member B2 | 1.772 |
| FAM48B2; FAM48B1 | Family with sequence similarity 48, member B2; member B1 | 1.772 |
| LDB2 | LIM domain binding 2 | 1.928 |
| BC069702 | Not determined | 1.97 |
| UBASH3B | Ubiquitin associated and SH3 domain containing, B | 2.332 |
| ENST00000364309 | Not determined | 2.382 |
| ENST00000364948 | Not determined | 2.53 |
| ATP1B1 | ATPase, Na+/K+ transporting, β1 polypeptide | 2.548 |
| CLEC4A | C-type lectin domain family 4, member A | 2.864 |
| C3orf57 | Chromosome 3 open reading frame 57 | 3.092 |
| ENST00000364177 | Not determined | 3.272 |
| IL12RB2 | Interleukin 12 receptor, β2 | 4.252 |
| 7927706 | Not determined | 4.304 |
CD57+ NK cells are more responsive to stimulation by CD16 but less responsive to IL-12 and IL-18
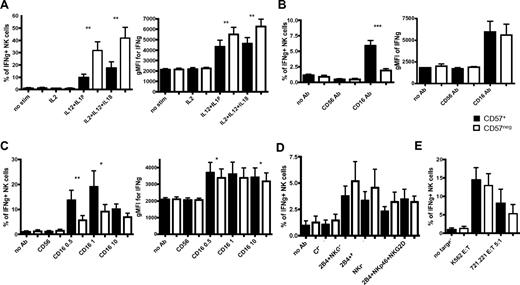
CD56bright NK cells are characterized by their ability to produce greater amounts of IFN-γ after stimulation with cytokines than CD56dim NK cells.6,8,32 We assessed whether CD56dimCD16+CD57+ and CD56dimCD16+CD57− NK cells differ in their capacity to produce IFN-γ. Based on the microarray analysis highlighting lower IL-12Rβ2 mRNA expression in CD57+ NK cells suggesting that these cells might be less responsive to IL-12, freshly isolated NK cells were cultured in IL-12 and IL-18 for 6 hours to induce IFN-γ. Approximately 3-fold less CD57+ NK cells produced IFN-γ in response to IL-12 plus IL-18 stimulation compared with CD57− NK cells (Figure 4A left). Moreover, the amount of IFN-γ produced by each CD57+ NK cells was lower than the amount produced by CD57− NK cells (Figure 4A right).
More CD57+CD56dimCD16+ NK cells produce IFN-γ in response to activation by CD16, but they are less responsive to IL-12 and IL-18. Enriched NK cells were activated for 6 hours with different cytokines (A), with anti-CD16 at 10 μg/mL (B), with anti-CD16 at different concentrations (0.5, 1, or 10 μg/mL) and 200 IU/mL IL-2 (C), with plate-bound antibodies at 10 μg/mL and 200 IU/mL IL-2 (D), or with K562 or 721.221 target cells and 200 IU/mL IL-2 (E). CD56dimCD16+ NK cells were gated on CD57+ and CD57− cells, and the percentages of cells expressing IFN-γ were determined in each subset (A-E). The gMFI of IFN-γ staining of each subset is shown (A-C). Only minimal amounts of IFN-γ were detected in NK cells cultured in either IL-12 or IL-18 alone (not shown), similar to the amount detected in NK cells cultured in IL-2 alone (A). Black bars represent CD57+; and white bars, CD57−. **P < .005. ***P < .0005. P values were determined between CD57+ and CD57− NK cells (n = 8).
More CD57+CD56dimCD16+ NK cells produce IFN-γ in response to activation by CD16, but they are less responsive to IL-12 and IL-18. Enriched NK cells were activated for 6 hours with different cytokines (A), with anti-CD16 at 10 μg/mL (B), with anti-CD16 at different concentrations (0.5, 1, or 10 μg/mL) and 200 IU/mL IL-2 (C), with plate-bound antibodies at 10 μg/mL and 200 IU/mL IL-2 (D), or with K562 or 721.221 target cells and 200 IU/mL IL-2 (E). CD56dimCD16+ NK cells were gated on CD57+ and CD57− cells, and the percentages of cells expressing IFN-γ were determined in each subset (A-E). The gMFI of IFN-γ staining of each subset is shown (A-C). Only minimal amounts of IFN-γ were detected in NK cells cultured in either IL-12 or IL-18 alone (not shown), similar to the amount detected in NK cells cultured in IL-2 alone (A). Black bars represent CD57+; and white bars, CD57−. **P < .005. ***P < .0005. P values were determined between CD57+ and CD57− NK cells (n = 8).
We explored whether this difference in IFN-γ production was an intrinsic property of the CD56dimCD16+CD57+ or CD56dimCD16+CD57− NK cells. After anti-CD16 stimulation of fresh NK cells, more CD57+ NK cells produce IFN-γ compared with CD57− NK cells (Figure 4B left), indicating that CD57+ NK cells are more responsive to signaling through the CD16 receptor. Under these conditions, the 2 subsets produced similar amounts of IFN-γ (Figure 4B right). To determine whether IL-2 would influence these differences, NK cells were stimulated with IL-2 and anti-CD16 at different concentrations. At the maximal concentration of anti-CD16, no significant difference was found in the percentage of CD57+ and CD57− NK cells producing IFN-γ; however, using lower concentrations, 2-fold more CD57+ NK cells produced IFN-γ compared with CD57− NK cells (Figure 4C left). Interestingly, CD57+ NK cells produced slightly more IFN-γ than CD57− NK cells in response to CD16 and IL-2 stimulation (Figure 4C right).
To address whether this observation was specific to CD16, freshly isolated NK cells were stimulated through other NK-activating receptors. Although CD16 stimulation alone induces resting NK cells to produce IFN-γ, other activating NK receptors need costimulation to induce these responses.33 Therefore, NK cells were stimulated simultaneously with anti-2B4, anti-NKp46, and anti-NKG2D. In most of the donors, this did not induce detectable IFN-γ production (data not shown). Thus, NK cells were activated with IL-2 plus stimulating antibodies to increase IFN-γ production. Under these conditions, similar frequencies of CD57+ and CD57− NK cells produced IFN-γ (Figure 4D) at similar amounts (data not shown), despite having greater per-cell expression of NKp46 and NKG2D on CD57− cells (Figure 1C).
To assess whether differences existed in these subsets after target cell stimulation, NK cells were cultured in the presence of target cells with or without IL-2. Without IL-2, less than 5% of NK cells produced IFN-γ and no significant difference was observed between the 2 subsets (data not shown). With IL-2, a higher percentage of NK cells produced IFN-γ, but no significant difference was detected (Figure 4E). During all these stimulations, CD57 expression was stable. These data indicate that both CD57+ and CD57− NK cells can produce IFN-γ in response to different stimulations; however, CD57+ NK cells are less responsive to IL-12 and IL-18 and more responsive to the activating Fc receptor CD16.
CD57+ NK cells have a reduced proliferative capacity
CD57+ CD8+ T cells proliferate poorly after stimulation with mitogens or cognate peptides.19 The proliferative capacity of NK cells decreases during maturation, as CD56bright NK cells exhibit a higher capacity to proliferate after stimulation with cytokines or dendritic cells than CD56dim NK cells.6,8,32 We assessed whether there is a difference in the proliferative capacity of CD57+ and CD57− NK cells. We sorted CD57+CD56dimCD16+ and CD57−CD56dimCD16+ NK cells. Cells were CFSE-labeled, and their proliferation was monitored in response to IL-2 plus target cells. CD57+ NK cells proliferated less than CD57− NK cells. CD57− NK cells divided more extensively (evidenced by greater dilution of CFSE) after 12 days of culture (Figure 5A). CD57+ NK cells proliferated less; and interestingly, fewer live cells were detected in the culture at each time point. Thus, CD57−CD56dimCD16+ NK cells have a greater proliferative capacity than CD57+ NK cells when stimulated with cytokines and target cells, suggesting that CD57+ NK cells, like CD57+ CD8+ T cells, have a proliferation defect in vitro.
CD57+CD56dimCD16+ NK cells proliferate less than CD57−CD56dimCD16+ NK cells in vitro but are not more sensitive to AICD. (A) CD57+ and CD57− sorted NK cells were CFSE-labeled and cultured in 100 IU/mL IL-2 with K562 cells expressing membrane-tethered IL-15 and 4-1BBL. Cells were analyzed by flow cytometry at different time points. Representative histograms of CFSE dilution over time (thin line represents CD57+; and bold line, CD57−; n = 7 for A, n = 3 for B). (B) Total PBMCs were activated with plate-bound antibodies (10 μg/mL) in 200 IU/mL IL-2 for 6 hours. Flow cytometry was performed, and CD56dimCD16+ NK cells were gated on CD57+ and CD57− cells. The percentages of cells expressing annexin V were determined for each subset (black bars represent CD57+; and white bars, CD57−). *P < .05. **P < .005. P values were determined between CD57+ NK cells and CD57− NK cells (n = 5).
CD57+CD56dimCD16+ NK cells proliferate less than CD57−CD56dimCD16+ NK cells in vitro but are not more sensitive to AICD. (A) CD57+ and CD57− sorted NK cells were CFSE-labeled and cultured in 100 IU/mL IL-2 with K562 cells expressing membrane-tethered IL-15 and 4-1BBL. Cells were analyzed by flow cytometry at different time points. Representative histograms of CFSE dilution over time (thin line represents CD57+; and bold line, CD57−; n = 7 for A, n = 3 for B). (B) Total PBMCs were activated with plate-bound antibodies (10 μg/mL) in 200 IU/mL IL-2 for 6 hours. Flow cytometry was performed, and CD56dimCD16+ NK cells were gated on CD57+ and CD57− cells. The percentages of cells expressing annexin V were determined for each subset (black bars represent CD57+; and white bars, CD57−). *P < .05. **P < .005. P values were determined between CD57+ NK cells and CD57− NK cells (n = 5).
CD57+ NK cells do not show increased susceptibility to AICD
CD57+ CD8+ T cells are more sensitive to AICD after stimulation with mitogens or antigenic peptides.19 We assessed the sensitivity of CD57+ and CD57− NK cells to AICD by staining for annexin V, an early marker of apoptosis. We detected no significant difference in annexin V staining between the CD57+ and CD57− NK cells ex vivo or after overnight culture in media without IL-2 (data not shown). CD16 is a potent activating receptor,33,34 and its stimulation induces AICD in NK cells.35,36 Therefore, cells were stimulated with IL-2 and antibodies against CD16 or CD95 (Fas), a death-inducing receptor expressed on NK and T cells. Similar amounts of CD95 were present on both CD57+ and CD57− NK cells (gMFI ∼ 180), as well as on CD57+ and CD57− T cells (gMFI ∼ 600) (supplemental Figure 2). After stimulation with anti-CD16 or anti-CD95, no significant difference in AICD was observed between CD57+ and CD57− NK cells. After stimulation with phorbol myristate acetate plus ionomycin, approximately 30% of the NK cells stained for annexin V, but no significant difference was detected comparing CD57+ and CD57− NK cells (Figure 5B). Under the same conditions, more CD57+ T cells underwent apoptosis than CD57− T cells (Figure 5B), consistent with prior observations.19 Therefore, unlike CD57+ T cells, CD57+ NK cells do not have an increased sensitivity to AICD after stimulation.
CD57+ NK cells mediate more potent CD16-induced cytotoxicity
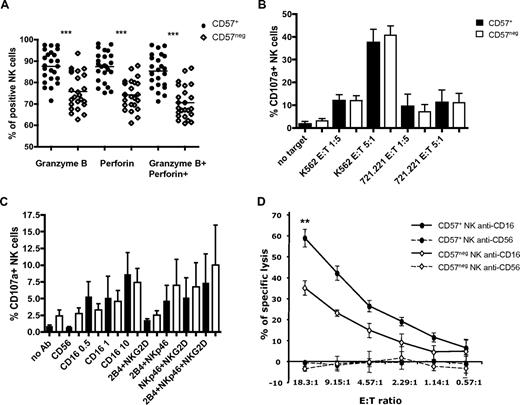
CD57 expression in T cells and NK cells correlates with expression of granzyme A, granzyme B, and perforin, suggesting that CD57 might be a marker of high cytolytic potential.26 In every donor tested, more CD57+CD56dimCD16+ NK cells expressed perforin and granzyme B than their CD57−CD56dimCD16+ counterpart (Figure 6A). CD57+ NK cells contained more of these cytotoxic proteins (gMFI for granzyme B was 929 in CD57+ vs 723 in CD57−, and gMFI for perforin was 646 vs 540, P < .0005; data not shown).
CD57+CD56dimCD16+ NK cells have a higher cytotoxic potential and mediate better CD16-induced cytotoxicity. (A) Percentages of NK cells that express perforin, granzyme B, or both cytotoxic molecules were assessed on CD57+CD56dimCD16+ NK cells and CD57−CD56dimCD16+ NK cells (● represents CD57+; and ◇, CD57−). ***P < .0005. P values were determined between CD57+ and CD57− NK cells (n = 23). (B-C) Low buoyant density lymphocytes were activated with K562 or 721.221 target cells (B) or with plate-bound antibodies (C) in media with 200 IU/mL IL-2. CD56dimCD16+ NK cells were gated on CD57+ and CD57− cells, and the percentages of CD107+ cells were determined on each subset (black bars represent CD57+; and white bars, CD57−). P values were determined between CD57+ and CD57− NK cells (n = 8). (D) Sorted CD57+ and CD57− CD56dimCD16+ NK cells were activated with P815 target cells coated with anti-CD56 or anti-CD16. *P < .05. P value was determined between the percentage of specific lysis of P815 plus anti-CD16 by CD57+ and CD57− NK cells (n = 6, in triplicate).
CD57+CD56dimCD16+ NK cells have a higher cytotoxic potential and mediate better CD16-induced cytotoxicity. (A) Percentages of NK cells that express perforin, granzyme B, or both cytotoxic molecules were assessed on CD57+CD56dimCD16+ NK cells and CD57−CD56dimCD16+ NK cells (● represents CD57+; and ◇, CD57−). ***P < .0005. P values were determined between CD57+ and CD57− NK cells (n = 23). (B-C) Low buoyant density lymphocytes were activated with K562 or 721.221 target cells (B) or with plate-bound antibodies (C) in media with 200 IU/mL IL-2. CD56dimCD16+ NK cells were gated on CD57+ and CD57− cells, and the percentages of CD107+ cells were determined on each subset (black bars represent CD57+; and white bars, CD57−). P values were determined between CD57+ and CD57− NK cells (n = 8). (D) Sorted CD57+ and CD57− CD56dimCD16+ NK cells were activated with P815 target cells coated with anti-CD56 or anti-CD16. *P < .05. P value was determined between the percentage of specific lysis of P815 plus anti-CD16 by CD57+ and CD57− NK cells (n = 6, in triplicate).
We tested whether CD57+ and CD57− NK cells differ in their capacity to degranulate (as measured by CD107α staining) under different stimulating conditions. As expected, activation with cytokines alone did not induce degranulation (data not shown). After stimulation with IL-2 and MHC class I–deficient target cells (K562 or 721.221), CD57+ and CD57− NK cells degranulated similarly (Figure 6B). We obtained similar results with fresh cells (data not shown). We also performed cytotoxicity assays using 51Cr-labeled K562 as targets and freshly isolated, sort-purified CD57+ and CD57− NK cells as effectors. Although a trend toward increased killing by CD57+ compared with CD57− NK cells was observed, it did not reach statistical significance (data not shown). This suggests that CD57+ NK cells were not better at killing these targets.37 Similarly, CD57+ and CD57− NK cells degranulated equivalently after stimulation using plate-bound antibodies against their activating receptors (Figure 6C).
Because CD57+ NK express higher amounts of CD16 on the cell surface and anti-CD16 antibody induces more IFN-γ production by CD57+ NK cells, we tested whether CD57+ NK cells can induce better killing of targets when activated via CD16. 51Cr-labeled Fc receptor-bearing P815 target cells were coated with anti-CD56 (negative control) or anti-CD16 and incubated with freshly isolated, sorted CD57+, and CD57− NK cells. In 4 of 6 donors assayed, CD57+ NK cells were more efficient (statistically significant) at killing P815 target cells coated with anti-CD16 than CD57− NK cells (Figure 6D). In the other 2 donors, there was a trend toward increased killing by CD57+ compared with CD57− NK cells, but it did not reach statistical significance. These data suggest that CD57+ NK cells are more sensitive to CD16 activation.
Discussion
Here, we defined the transcriptional signature, phenotypic properties, and functional capacity of human CD57+ NK cells. CD57 was expressed mainly by the CD56dimCD16+ mature subset of NK cells, as previously observed.7,22 The expression of differentiation markers and NK-cell receptors showed that in the mature CD56dimCD16+ NK population, CD57 defines a subset of highly mature cells. During maturation of the CD56bright into CD56dimCD16+ NK cells, the expression of NKp46, NKG2D, NKp30, and NKG2A decreases, and CD16 expression is acquired.1,7,29 Thus, the amount of CD16 expression on the cell surface correlates with the level of maturation of the NK cell. Similarly, the expression of LIR-1 and KIR are mainly observed on mature CD56dim NK cells. KIR expression can be acquired by the CD56bright, as well as by CD56dimKIR− NK cells in vitro and in vivo.11,12
Our analysis of expression of various receptors by CD57+ and CD57− CD56dimCD16+ NK cells indicates that CD57 clearly correlates with NK-cell maturation. Indeed, CD57− NK cells express higher amounts of NKp46, NKG2D, NKp30, and NKG2A; and interestingly, they also have a higher frequency of CD27+ and CD62L+ cells. CD27 expression on human NK cells correlates with an increased ability to proliferate and produce IFN-γ and with lower cytotoxic potential.38,39 Our studies revealed that CD57+ NK cells express higher amounts of CD16 and have a higher frequency of LIR-1+ and KIR+ cells. Interestingly, the frequency of CD57+ cells correlated with the number of inhibitory KIR expressed by the NK-cell population.50 Moreover, we demonstrated that CD57−CD56dim NK cells are induced to express CD57 after culture in IL-2 or when cocultured with target cells.37 Similarly, Malmberg et al50 have observed acquisition of CD57 on human NK cells developing in a scid mouse model system. Once acquired, CD57 is stably expressed on NK cells in the conditions analyzed. Altogether, these data indicate that NK cells mature from CD56bright to CD56dim acquiring the expression of CD16, KIR, LIR-1, and down-regulating natural cytotoxic receptor and NKG2D, and that the final stage of maturation is the acquisition of CD57 by the CD56dim NK subset.
CD56bright NK cells are characterized by their ability to produce greater amounts of IFN-γ compared with CD56dim NK cells after stimulation with IL-12; however, CD56dim NK cells have a greater capacity to degranulate in the presence of targets.5,6,8,29,32 Our transcriptional profiling showed that CD57+ NK cells express less of IL-12Rβ2 chain mRNA than CD57− cells, suggesting that they are less sensitive to IL-12. We found that, within the CD56dim NK-cell subset, CD57+ NK cells have a lower frequency of IFN-γ+ cells than CD57− and produce less IFN-γ per cell in response to IL-12 and IL-18 stimulation. These NK cells probably express less IL-12 receptor; however, we were unable to directly quantitate the amounts of IL-12Rβ2 on the NK-cell surface because of lack of a suitable monoclonal antibody.
Interestingly, more CD57+CD56dimCD16+ NK cells produced IFN-γ and in higher amounts than CD57−CD56dimCD16+ NK cells after ligation of CD16. This might be because CD57+ NK cells express higher amounts of CD16, although other mechanisms might also explain this difference. The expression of at least one self-MHC specific inhibitory receptor (eg, KIR, LIR-1 or NKG2A) by an NK cell correlates with a higher ability to kill and produce IFN-γ in response to stimulation via activating receptors.40-42 Among CD56dim cells, the CD57+ NK cells that express more KIR and LIR-1 might therefore be more responsible to stimulation through CD16, as indicated by our IFN-γ production and killing assays.
CD57 is considered a marker of senescent T cells that are unable to proliferate in response to antigenic peptides in vitro.19,20 This proliferative defect of CD57+CD8+ T cells could not be overcome by the addition of exogenous IL-2 or IL-15. Similarly, we found that CD57+ NK cells proliferate less than CD57− in response to IL-2- and IL-15-transduced target cells. More CD57+ NK cells express KIRs, and KIR expression correlates with a lower proliferative capacity.12,43
CD57+ NK cells not only proliferate poorly in vitro but also are less responsive to IL-2 and IL-15. It has been previously reported that CD56bright NK cells express IL-2Rα (CD25) and thus proliferate better in response to lower amounts of IL-2 than CD56dim NK cells.7,44 During NK-cell maturation, CD25 expression is diminished or lost, and it is possible that NK cells also decrease their surface expression of other cytokine receptors, such as CD122 (IL-2Rβ, a subunit of the shared receptor for IL-2 and IL-15), explaining why these cytokines cannot rescue the proliferation defect of CD57+ NK cells.
Interestingly, the majority of CD56dimCD16+ NK cells express KLRG1,45 and the frequency of KLRG1+ cells is slightly higher in the CD57− NK-cell subset. KLRG1 was shown previously to identify a subset of antigen-specific human T cells that are capable of secreting cytokines but fail to proliferate after stimulation.45 A study of antigen-specific human CD8+ T cells shows that these cells express KLRG1 during latent infections and that a subset also expresses CD57.46 CD57−KLRG1+ CD8+ T cells have a “memory” phenotype, but CD57+KLRG+ CD8+ T cells are senescent. These findings indicate that CD57, but not KLRG1, is a marker of replicative senescence. Similarly, a subset of γδ T cells expressing KLRG1 has an effector memory phenotype and can proliferate robustly.47 Thus, KLRG1 expression may be acquired during maturation but does not determine the proliferative capacity of the NK cells.
CD57+ T lymphocytes have undergone more rounds of cell division compared with other memory T-cell subsets, and expression of CD57 correlates with T-cell susceptibility to AICD.19 In contrast, CD57+ NK cells were not more sensitive to AICD than CD57− NK cells. Therefore, even if CD57 marks a lack of proliferative capacity for NK cells and T cells, CD57+ NK cells do not appear to share all the features of CD57+ CD8+ T cells. Nonetheless, CD57 is a marker of more mature cells that have a lower proliferative capacity and may have undergone more divisions, probably as a result of responding to pathogens or cytokines in vivo. The average telomere length in human NK cells decreases with age, and it is significantly shorter in CD56dimCD16+ NK cells compared with CD56brightCD16− NK cells.12,48 In the CD56dim subset, telomere length is very heterogeneous in NK cells expressing CD94, KIR, NKG2A, or CD161,48 suggesting that these receptors are not specific markers of maturation. Taken together, these data indicate that CD57 is a marker of highly mature NK cells within the CD56dimCD16bright subset. Our data showing that CD57+ NK cells are better at CD16-induced cytotoxicity and produce more IFN-γ in response to CD16 ligation argues that these cells are not “anergic” or “exhausted.”
It is tempting to speculate that CD57+ NK cells are long-lived cells that have encountered pathogens and represent human “memory” NK cells. In mice, we have shown that a subset of NK cells directly recognizing mouse cytomegalovirus are capable of extensive proliferation and give rise to long-lived progeny with characteristics of memory T cell.3 This finding is particularly intriguing given the interest in developing strategies to apply NK cells as therapeutic agents against a broad range of malignancies,29,49 and approaches to augment NK-cell function during chronic viral infections (eg, HIV-1 and hepatitis C virus). Thus, although speculative, we propose that CD57 might provide a marker of these “memory” NK cells in humans that will be of interest to monitor after infection, vaccination, or bone marrow transplantation to determine the outcome of the immune response.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Jessica Jarjoura and Natasja Nielsen for help with flow cytometry, Dr H. S. Warren for the CFSE protocol for human NK cells, Dr C. Barker and Dr A. Holloway (Gladstone Genomic and Bioinformatics core) for the microarray analysis, Dr J. P. Houchins for generously providing reagents, and the Lanier laboratory for helpful discussion.
This work was supported by the National Institutes of Health (grants AI64520, AI068129, and HL095470) and the Basic Scientist Award Program in HIV/AIDS (pilot grant). J.M.M. is supported in part by the National Institutes of Health (grant 5T32HL007185). L.L.L. is an American Cancer Society Professor. S.L.-V. is supported by a Cancer Research Institute/Irvington Institute postdoctoral fellowship.
National Institutes of Health
Authorship
Contribution: S.L.-V. designed and performed experiments, analyzed data, and wrote the paper; J.M.M. designed and performed experiments and assisted in the writing of the paper; S.P., V.A.Y., and J.A.-H. performed experiments; H.P. provided reagents; P.J.N. assisted in the writing of the paper; D.F.N. assisted in the writing of the paper and supervised research; and L.L.L. supervised research and edited the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Lewis L. Lanier, University of California, Department of Microbiology and Immunology, 513 Parnassus Ave, HSE 1001G, San Francisco, CA 94143; e-mail: lewis.lanier@ucsf.edu.