Abstract
Thrombotic thrombocytopenic purpura (TTP) is the common name for adults with microangiopathic hemolytic anemia, thrombocytopenia, with or without neurologic or renal abnormalities, and without another etiology; children without renal failure are also described as TTP. The diagnosis of TTP is an indication for plasma exchange treatment, but beginning treatment requires sufficient confidence in the diagnosis to justify the risk of plasma exchange complications. Documentation of a severe deficiency of plasma ADAMTS13 activity, defined as less than 10% of normal, is not essential for the diagnosis of TTP. Some patients without severe ADAMTS13 deficiency may benefit from plasma exchange treatment; in addition, some patients with severe ADAMTS13 deficiency may subsequently be diagnosed with another cause for their clinical features. However, severe acquired ADAMTS13 deficiency does define a subgroup of patients who appear to benefit from treatment with corticosteroids and other immunosuppressive agents in addition to plasma exchange but who have a high risk for relapse. Approximately 80% of patients survive their acute episode, a survival rate that has not changed since the introduction of plasma exchange treatment. Although recovery may appear to be complete, many patients have persistent minor cognitive abnormalities. More effective as well as safer treatment for TTP is needed.
Introduction
Ten years ago, Blood began a new series entitled “How I treat …,”1 with a discussion of thrombotic thrombocytopenic purpura–hemolytic uremic syndrome (TTP-HUS).2 Although most of what I wrote then remains relevant, most of what I write now is new, beginning with how I name these syndromes and ending with how I anticipate long-term outcomes after recovery. I will focus on 4 topics: (1) definition of TTP, (2) diagnosis of TTP, (3) management of acute episodes, and (4) management of patients after recovery. Data from the Oklahoma TTP-HUS Registry provide the basis for how I treat patients with TTP. I have not performed a systematic literature review for this article; I have largely limited the references to Registry experience.
Definitions of TTP and HUS
Names matter. The name of a syndrome is clinically important because it can trigger treatment. For example, the name TTP implies a disorder that is fatal without effective treatment,3 whereas the name HUS implies that supportive care may be sufficient.4 Table 1 presents my current understanding of the common usage of the terms thrombotic microangiopathy (TMA), TTP, and HUS. Current definitions remain related more to the patient's age, presentation, and anticipated treatment rather than to a specific etiology.
Names of the syndromes
| Name . | Definition . | Comments . |
|---|---|---|
| Pathologic name | ||
| TMA | The characteristic histologic abnormalities (swelling of endothelial cells and the subendothelial space) of capillaries and arterioles that cause microvascular thrombosis and result in microangiopathic hemolytic anemia and thrombocytopenia | In addition to TTP and HUS, TMA may occur in other disorders, such as malignant hypertension, scleroderma, antiphospholipid antibody syndrome, systemic lupus erythematosus, preeclampsia, radiation nephropathy, renal allograft rejection, HIV infection, allogeneic HSCT, disseminated malignancy. |
| Clinical names | ||
| Typical HUS | A syndrome of microangiopathic hemolytic anemia, thrombocytopenia, and renal failure with a diarrhea prodrome caused by infection with Shiga toxin-producing bacteria | Occurs primarily in children younger than 5 years. Accounts for 90% to 95% of childhood HUS. E. coli O157:H7 is the most common etiology. |
| aHUS | A syndrome of microangiopathic hemolytic anemia, thrombocytopenia, and renal failure without a diarrhea prodrome | Occurs primarily in children younger than 5 years. Accounts for 5% to 10% of childhood HUS. Abnormalities of complement regulation may be the most common etiology. |
| TTP | Adults with microangiopathic hemolytic anemia and thrombocytopenia, with or without renal or neurologic abnormalities, without another etiology, such as systemic infection or another cause of TMA | Children without renal failure are also diagnosed as TTP. The diagnosis of TTP requires treatment with plasma exchange. |
| Congenital TTP (Upshaw-Schulman syndrome) | A rare syndrome caused by congenital ADAMTS13 deficiency | Symptoms may first occur at any age. Treatment with plasma infusion is sufficient. In some subjects, symptoms of TTP never occur. |
| Name . | Definition . | Comments . |
|---|---|---|
| Pathologic name | ||
| TMA | The characteristic histologic abnormalities (swelling of endothelial cells and the subendothelial space) of capillaries and arterioles that cause microvascular thrombosis and result in microangiopathic hemolytic anemia and thrombocytopenia | In addition to TTP and HUS, TMA may occur in other disorders, such as malignant hypertension, scleroderma, antiphospholipid antibody syndrome, systemic lupus erythematosus, preeclampsia, radiation nephropathy, renal allograft rejection, HIV infection, allogeneic HSCT, disseminated malignancy. |
| Clinical names | ||
| Typical HUS | A syndrome of microangiopathic hemolytic anemia, thrombocytopenia, and renal failure with a diarrhea prodrome caused by infection with Shiga toxin-producing bacteria | Occurs primarily in children younger than 5 years. Accounts for 90% to 95% of childhood HUS. E. coli O157:H7 is the most common etiology. |
| aHUS | A syndrome of microangiopathic hemolytic anemia, thrombocytopenia, and renal failure without a diarrhea prodrome | Occurs primarily in children younger than 5 years. Accounts for 5% to 10% of childhood HUS. Abnormalities of complement regulation may be the most common etiology. |
| TTP | Adults with microangiopathic hemolytic anemia and thrombocytopenia, with or without renal or neurologic abnormalities, without another etiology, such as systemic infection or another cause of TMA | Children without renal failure are also diagnosed as TTP. The diagnosis of TTP requires treatment with plasma exchange. |
| Congenital TTP (Upshaw-Schulman syndrome) | A rare syndrome caused by congenital ADAMTS13 deficiency | Symptoms may first occur at any age. Treatment with plasma infusion is sufficient. In some subjects, symptoms of TTP never occur. |
TMA is a descriptive name for the histologic abnormalities that are characteristic of all varieties of TTP and HUS.5 Some hematologists favor the use of the term TMA for all syndromes that may be described as TTP or HUS because it avoids the use of multiple terms to describe overlapping syndromes. However, TTP and HUS are the familiar clinical terms; therefore, I use them in this discussion. Histologic abnormalities that are similar to TTP and HUS, most often seen in renal biopsies, can also occur in multiple other disorders and cause microangiopathic hemolytic anemia and thrombocytopenia that can mimic the clinical presentation of TTP and HUS.5
“Typical HUS,” often described as “diarrhea-associated HUS” or simply as HUS, is the name used for children with abdominal pain, diarrhea, microangiopathic hemolytic anemia, thrombocytopenia, and renal failure.4 The abdominal pain and diarrhea, which is often overtly bloody, are manifestations of hemorrhagic enterocolitis caused by Shiga toxin-producing bacteria, most commonly Escherichia coli O157:H7.4 Much less often, children present with microangiopathic hemolytic anemia, thrombocytopenia, and renal failure without a prodrome of diarrhea; therefore, they are named “atypical HUS,” often described as atypical HUS (aHUS) or “diarrhea-negative HUS.”6 The most common causes of aHUS appear to be abnormalities of complement regulation resulting in uncontrolled complement activation.7 These children (and adults) often have recurrent HUS episodes, which also distinguishes them from typical diarrhea-associated HUS. Many different factors that can control complement activation have been reported to be altered in aHUS.7,8 Recently, aHUS has been used as a name to specifically imply an abnormality of complement regulation in children or adults8 ; however, in practice, aHUS continues to describe children with microangiopathic hemolytic anemia, thrombocytopenia, and renal failure but without a diarrhea prodrome.6 Rarely, children have microangiopathic hemolytic anemia and thrombocytopenia without renal failure; these children are described as having TTP. The standard management for children with typical, diarrhea-associated HUS is supportive care without plasma exchange. However, plasma exchange has been used uncommonly and empirically for children with typical HUS when severe neurologic abnormalities occur. For children with aHUS in whom an abnormality of complement regulation is suspected, current opinion suggests that plasma exchange should be used.8,9
TTP has been the name used for many years for adults with microangiopathic hemolytic anemia and thrombocytopenia, with or without renal failure or neurologic abnormalities, and without another cause for TMA.3,10-14 These were the inclusion criteria for the randomized clinical trial that documented the benefit of plasma exchange treatment,11 and they have become the definition and diagnostic criteria for TTP. Before the plasma exchange era, survival of patients with TTP was only 10%.3 When plasma exchange was reported to increase survival to 78%, compared with 51% survival for patients treated with plasma infusion,11 the diagnosis of TTP became an indication for plasma exchange treatment. A companion study reported outcomes of 24 patients with oliguric renal failure who were excluded from the randomized clinical trial because they could not be assigned to the plasma infusion group because of the risk for volume overload.12 The survival of these 24 patients, all of whom were treated with plasma exchange, was 83%,12 the same as the survival of patients treated with plasma exchange in the clinical trial.11 These observations document that plasma exchange is beneficial for patients who meet the diagnostic criteria for TTP, with or without renal failure.
After the discovery that TTP is associated with a severe deficiency of ADAMTS13 activity,15,16 it was suggested that ADAMTS13 deficiency may become the definition and diagnostic criterion for TTP. But many patients who fulfill the diagnostic criteria for TTP do not have severe ADAMTS13 deficiency.14,17 Therefore, I continue to define all patients who meet the traditional diagnostic criteria11,12 as TTP. Although TTP is the name commonly used for adults in clinical practice, the hybrid name TTP-HUS is sometimes used for patients who have severe renal failure and for whom plasma exchange is appropriate.
TTP is almost always acquired. Patients with congenital TTP (Upshaw-Schulman syndrome) caused by a mutation of the ADAMTS13 gene18 are rare; we have not documented congenital absence of ADAMTS13 activity in any of the patients in the Oklahoma Registry.
Oklahoma TTP-HUS Registry
The Registry is a population-based cohort of consecutive patients since January 1, 1989 with TTP or HUS identified at the time of a request for plasma exchange treatment.14,17 All patients in 58 of Oklahoma's 77 counties are included in the Registry because the Oklahoma Blood Institute is the sole provider of plasma exchange for all hospitals in these counties and because plasma exchange is the standard treatment in this region for all adults diagnosed with TTP or HUS, children who are diagnosed with TTP, and some children diagnosed with HUS. Most children with typical HUS are not included in the Registry because they are not treated with plasma exchange. The Registry enrolled 398 patients with their first episode of clinically diagnosed TTP or HUS through December 31, 2009. ADAMTS13 activity has been measured by Drs Johanna Kremer Hovinga and Bernhard Lämmle (Berne, Switzerland) in serum collected immediately before the first plasma exchange since November 13, 199514 ; through December 31, 2009, ADAMTS13 activity was measured in 283 (93%) of 304 patients. ADAMTS13 activity is the same when measured in either serum or plasma.14 Testing for inhibitors of ADAMTS13 activity was performed on all samples with activities less than 20%.14 Because we send samples to Berne about twice per year, results are typically available in several months. Severe deficiency of ADAMTS13 activity is defined as less than 10% of normal.14 The Registry is approved by the Institutional Review Boards of the University of Oklahoma Health Sciences Center and all participating hospitals.
Because all patients for whom plasma exchange is requested for treatment of TTP or HUS are identified, there is no selection or referral bias. No patients referred for plasma exchange with a diagnosis of TTP or HUS have been excluded from our analyses. This has allowed an accurate determination of the incidence of TTP.19 But because plasma exchange is requested by many physicians who may have quite different sensitivities for the diagnosis of TTP, there is greater heterogeneity among Registry patients than in case series of selected patients. To provide consistency, I have seen and participated in the evaluation and management of 298 (90%) of the 332 patients enrolled since January 1, 1995.
Clinical categories of TTP
To provide additional consistency, we developed categories based on patients' clinical features at the time of their first episode (Table 2).17 This has allowed identification of distinct clinical features as well as distinct disparities of gender and race20 among the different clinical categories of these syndromes. Results of ADAMTS13 measurements are not known when clinical categories are assigned.
Clinical categories TTP and frequency of severe ADAMTS13 deficiency: data from all patients who had ADAMTS13 measurements (1995-2009)
| Category . | All patients (n = 283) . | Patients with ADAMTS13 < 10% (n = 65) . |
|---|---|---|
| Allogeneic HSCT | 10 | 1 (10%) |
| Pregnant/postpartum | 15 | 3 (21%) |
| Drug-associated (n = 38) | ||
| Quinine | 20 | 0 |
| Chemotherapeutic agents, calcineurin inhibitor | 12 | 0 |
| Other drugs | 6 | 0 |
| Bloody diarrhea | 25 | 2 (8%) |
| Additional/alternative disorders (n = 88) | ||
| SLE | 21 | 2 (10%) |
| Other autoimmune disorders | 16 | 1 (7%) |
| Infections | 23 | 4 (17%) |
| Malignancies | 10 | 1 (10%) |
| Malignant hypertension | 6 | 0 |
| Other disorders | 12 | 0 |
| Idiopathic | 107 | 51 (48%) |
| Category . | All patients (n = 283) . | Patients with ADAMTS13 < 10% (n = 65) . |
|---|---|---|
| Allogeneic HSCT | 10 | 1 (10%) |
| Pregnant/postpartum | 15 | 3 (21%) |
| Drug-associated (n = 38) | ||
| Quinine | 20 | 0 |
| Chemotherapeutic agents, calcineurin inhibitor | 12 | 0 |
| Other drugs | 6 | 0 |
| Bloody diarrhea | 25 | 2 (8%) |
| Additional/alternative disorders (n = 88) | ||
| SLE | 21 | 2 (10%) |
| Other autoimmune disorders | 16 | 1 (7%) |
| Infections | 23 | 4 (17%) |
| Malignancies | 10 | 1 (10%) |
| Malignant hypertension | 6 | 0 |
| Other disorders | 12 | 0 |
| Idiopathic | 107 | 51 (48%) |
Data are presented for 283 consecutive patients in whom ADAMTS13 was measured immediately before their first plasma exchange treatment (November 13, 1995 through December 31, 2009). Patients were assigned to clinical categories based on their clinical features during their initial episode and without knowledge of the results of the ADAMTS13 measurement, as previously described.17 Chemotherapeutic agents were mitomycin C (4), gemcitabine (4), carmustine (1), and pentostatin (1). The calcineurin inhibitor was cyclosporine (2). Other drugs were alendronate, clopidogrel, cocaine, ticlopidine, trimethoprim-sulfamethoxazole, and vancomycin (1 each). Other autoimmune disorders included immune thrombocytopenic purpura/Evan syndrome (5), antiphospholipid antibody syndrome (2), scleroderma (2), Wegener granulomatosis (2), and once each for bronchiolitis obliterans with organizing pneumonia, Hamman-Rich syndrome, periarteritis nodosa, Sjögren syndrome, and unspecified vasculitis. Other disorders included multiorgan failure (11) and congenital hemolytic anemia (1).
Patients who developed clinical features of TMA after allogeneic hematopoietic stem cell transplantation (HSCT) were previously described as TTP and treated with plasma exchange. However, plasma exchange was apparently not effective. Twenty-two of our 23 patients died; autopsies documented that systemic infections were the most common cause of death and demonstrated no evidence of systemic microvascular thrombosis, the characteristic pathologic feature of TTP.21-23 These patients had renal TMA,22 probably of multiple etiologies, that contributed to their microangiopathic hemolytic anemia and thrombocytopenia. To avoid the risks of unnecessary plasma exchange, the name of this syndrome has been changed to HSCT-associated TMA.24-26 We have not treated a patient with HSCT-associated TMA with plasma exchange since 2003.
Pregnancy/postpartum is a distinct category for 2 reasons. First, the diagnosis of TTP is uniquely difficult in pregnant and postpartum women because the clinical features of preeclampsia and the HELLP syndrome (hemolysis, elevated liver enzymes, low platelets) may mimic TTP.27 Second, pregnancy can be a trigger for an acute episode of TTP.28 Most pregnant/postpartum women who develop TTP, including the 3 Registry patients with severe ADAMTS13 deficiency, present near term or postpartum.28
TTP is categorized as drug-associated when a drug reported to be associated with TTP or HUS was taken before diagnosis or if there was a compelling temporal association of a previously unreported drug with the onset of symptoms. Drugs may cause TTP by 2 mechanisms: an acute immune-mediated reaction or dose-dependent toxicity. Quinine is the most common drug associated with TTP (Table 2); an immune-mediated mechanism has been documented by the demonstration of quinine-dependent antibodies that react with platelets as well as other cells.29-33 Patients with quinine-associated TTP have a characteristic presentation: a sudden onset of chills, fever, nausea, vomiting, diarrhea, and anuric acute renal failure beginning within several hours of ingestion of a quinine tablet, usually taken for leg cramps, or a quinine-containing beverage, such as tonic water. In patients with this presentation, a detailed search for exposure to quinine, or another drug that may cause immune-mediated reactions, is critical. Chemotherapeutic agents and calcineurin inhibitors cause dose-dependent renal TMA manifested by a gradual onset of renal failure. For other drugs, both the mechanism and association with TTP are less clear. Although an association of TTP with clopidogrel has been reported, TTP has been attributed to clopidogrel in only one Registry patient, and in that patient the association with clopidogrel was uncertain.
The category of patients who present with overtly bloody diarrhea,34 similar to children with typical HUS,4 is based on the suspected etiology of infection with E coli O157:H7 or a related Shiga toxin–producing bacteria. Bloody diarrhea in the 2 Registry patients who were subsequently documented to have severe ADAMTS13 deficiency was attributed to microvascular thrombi causing ischemic colitis that may be pathologically similar to Shiga toxin-induced hemorrhagic enterocolitis.35
Patients diagnosed with TTP may have additional disorders or they may be discovered to have an alternative disorder after plasma exchange has begun that could have caused their clinical features. Among the additional disorders, systemic lupus erythematosus (SLE) is most common. The distinction of TTP from SLE may not be possible because both disorders have similar clinical features.36 Among alternative disorders, systemic infections37 and malignancies38,39 are most common; when they are diagnosed, plasma exchange is stopped. Patients described as multiorgan failure were critically ill without a clear etiology; plasma exchange has sometimes been attempted as an extreme measure, even though TTP was only a remote possibility.
The remaining patients are categorized as idiopathic. This category is also heterogeneous. Some patients have severe neurologic abnormalities and acute renal failure, whereas others have no neurologic or renal abnormalities and are not critically ill. Some patients appear to have preceding events, such as acute pancreatitis,40 whereas others have no preceding illness. Some patients without severe ADAMTS13 deficiency may have abnormal ADAMTS13 function.14 Comparison of idiopathic patients with and without severe ADAMTS13 deficiency demonstrates similarities but also remarkable differences (Table 3). Patients without severe ADAMTS13 deficiency were older and had higher platelet counts, a greater frequency of renal failure, and less risk for relapse than patients with severe ADAMTS13 deficiency. Both groups had the same frequency of neurologic abnormalities and the same survival. Only 28 (50%) of the 56 patients with ADAMTS13 activity more than or equal to 10% had normal activity (> 50%), 18 (32%) had mildly deficient activity (25%-50%), and 10 (18%) had moderately deficient activity (10%-24%).
Comparison of patients with idiopathic TTP with and without severe ADAMTS13 deficiency
| Demographic/clinical feature . | ADAMTS13 < 10% (n = 51) . | ADAMTS13 ≥ 10% (n = 56) . | P . |
|---|---|---|---|
| Median age, y (range) | 41 (9-71) | 57 (2-85) | < .001 |
| Sex (% women) | 80 | 66 | .096 |
| Race (% black) | 37 | 20 | .043 |
| Neurologic abnormalities, % | |||
| Major | 35 | 39 | .760 |
| Minor | 31 | 34 | |
| None | 34 | 27 | |
| Laboratory data (median) | |||
| Platelet count, μL | 17 000 | 43 500 | < .001 |
| Hematocrit, percentage | 25 | 27 | .043 |
| LDH, U/L | 1257 | 989 | .242 |
| Creatinine, mg/dL | 1.1 | 3.3 | < .001 |
| Renal failure, μmol/L | 97.2 | 291.7 | |
| Acute renal failure | 6 | 50 | < .001 |
| Mild renal insufficiency | 41 | 32 | |
| Normal renal function | 53 | 18 | |
| Clinical course | |||
| Dialysis, % | 4 | 43 | < .001 |
| Death, % | 18 | 21 | .623 |
| Plasma exchange,* median (range) | 19 (3-79) | 9 (3-71) | < .001 |
| Relapse,* % | 40 | 7 | < .001 |
| Demographic/clinical feature . | ADAMTS13 < 10% (n = 51) . | ADAMTS13 ≥ 10% (n = 56) . | P . |
|---|---|---|---|
| Median age, y (range) | 41 (9-71) | 57 (2-85) | < .001 |
| Sex (% women) | 80 | 66 | .096 |
| Race (% black) | 37 | 20 | .043 |
| Neurologic abnormalities, % | |||
| Major | 35 | 39 | .760 |
| Minor | 31 | 34 | |
| None | 34 | 27 | |
| Laboratory data (median) | |||
| Platelet count, μL | 17 000 | 43 500 | < .001 |
| Hematocrit, percentage | 25 | 27 | .043 |
| LDH, U/L | 1257 | 989 | .242 |
| Creatinine, mg/dL | 1.1 | 3.3 | < .001 |
| Renal failure, μmol/L | 97.2 | 291.7 | |
| Acute renal failure | 6 | 50 | < .001 |
| Mild renal insufficiency | 41 | 32 | |
| Normal renal function | 53 | 18 | |
| Clinical course | |||
| Dialysis, % | 4 | 43 | < .001 |
| Death, % | 18 | 21 | .623 |
| Plasma exchange,* median (range) | 19 (3-79) | 9 (3-71) | < .001 |
| Relapse,* % | 40 | 7 | < .001 |
Data are presented for the 107 patients with idiopathic TTP, comparing patients with ADAMTS13 activity less than 10% with patients with ADAMTS13 activity more than or equal to 10%. Among the 56 patients with ADAMTS13 activity more than or equal to 10%, 28 patients had normal ADAMTS13 activity (> 50%), 18 patients had mildly deficient activity (25%-50%), and 10 patients had moderately deficient activity (10%–24%). Neurologic abnormalities and renal failure categories have been previously defined.17 Neurologic abnormalities and laboratory data are from the day of diagnosis, defined as the day of the first plasma exchange treatment. Renal failure categories included data at diagnosis plus or minus 7 days. Laboratory data and the number of plasma exchange treatments are presented as median values. Deaths occurred within 30 days of the last plasma exchange treatment. The 3 patients who initially had ADAMTS13 activity more than or equal to 10% and who subsequently relapsed included 2 previously described patients with abnormal but not initially deficient ADAMTS13 activity14 (Table 5, patients 4 and 5) and one child with atypical HUS.
Number of plasma exchange treatments and frequency of relapse are presented for the 42 surviving patients with ADAMTS13 less than 10% and the 44 surviving patients with ADAMTS13 activity more than or equal to 10%.
Although HIV infection has been previously proposed as a cause of TTP, among Registry patients the frequency of HIV infection is similar to the prevalence of HIV infection in the population.41 In some patients, the occurrence of HIV infection may be merely coincidental; in others, HIV-related conditions may mimic TTP.41 Two of the Registry's 6 patients with HIV infection were categorized as idiopathic; the other 4 were discovered to have alternative disorders: HIV-associated nephropathy with malignant hypertension (3 patients) and systemic Kaposi sarcoma discovered at autopsy (one patient).
Diagnosis of TTP
Prompt diagnosis of TTP is critical to begin plasma exchange, but diagnosis may be difficult because of the absence of explicit criteria. The combination of urgent yet difficult diagnosis has resulted in an 8-fold increase in the frequency of patients diagnosed with TTP.42 Part of the increased frequency may be better recognition of TTP, but part may also be overdiagnosis of TTP.
The diagnosis of TTP is based on the presenting clinical features. Measurements of ADAMTS13 activity are not required and may not be appropriate for the critical initial management decision to begin or not begin plasma exchange. Severe ADAMTS13 deficiency does not identify all patients who may respond to plasma exchange treatment, some patients with severe ADAMTS13 deficiency may have other disorders, and different assay methods may vary in their detection of patients with severe deficiency.14 Documentation of severe ADAMTS13 deficiency does provide supporting evidence for the diagnosis of TTP; therefore, patients with severe ADAMTS13 deficiency can serve as a model for the presenting features and clinical outcomes of TTP.
The clinical features of patients with severe ADAMTS13 deficiency on the day of their diagnosis (defined for our analyses as the day of the first plasma exchange treatment17 ) are presented in Table 4. Neurologic abnormalities were present in 43 (66%) patients; 23 (35%) had major abnormalities, defined as coma, stroke, seizures, or focal abnormalities17 ; some patients had focal abnormalities in addition to having a seizure or stroke. More important is the observation that 20 (31%) patients had only minor symptoms, such as confusion or headache, which may or may not have represented neurologic abnormalities, and 22 (34%) had no neurologic abnormalities. New neurologic abnormalities may occur during the course of treatment. Three patients who had no neurologic abnormalities and 7 patients who had only minor abnormalities at the time of their diagnosis subsequently had major neurologic abnormalities, but 19 (29%) patients never had neurologic abnormalities. The other common presenting features were gastrointestinal symptoms, possibly related to intestinal ischemia, and symptoms of weakness and fatigue, possibly related to anemia. Half of patients had bleeding symptoms; dyspnea, chest pain, fever, and cough were uncommon. The range of laboratory data was great. Anemia usually became more severe during the week after diagnosis. Most, but not all, patients had very high levels of lactate dehydrogenase. Acute renal failure17 occurred in 6 (9%) patients; 29 (45%) patients had increased serum creatinine; in 30 (46%) patients, all creatinine levels preceding diagnosis and throughout their clinical course were less than 1.5 mg/dL. Cardiac involvement may be common in TTP,43 some patients had elevations of serum troponin I, but evidence for acute myocardial infarction was very rare. In the era before effective plasma exchange treatment, microvascular thrombi in the cardiac conduction system associated with sudden death have been described in TTP.44
Presenting features and clinical course of 65 consecutive patients with their first episode of TTP associated with severe ADAMTS13 deficiency
| Presenting features and clinical course . | No. (%) of patients . |
|---|---|
| History and physical examination | |
| Gastrointestinal symptoms (pain, nausea, vomiting, diarrhea) | 45 (69) |
| Neurologic abnormalities | 43 (66) |
| Major abnormalities | 23 (35) |
| Focal abnormalities | 17 (26) |
| Seizure | 6 (9) |
| Stroke | 6 (9) |
| Coma | 2 (3) |
| Minor abnormalities (eg, confusion, headache) | 20 (31) |
| Weakness | 41 (63) |
| Bleeding, purpura, hematuria | 35 (54) |
| Dyspnea | 19 (29) |
| Fever | 15 (23) |
| Chest pain | 14 (22) |
| Cough | 6 (9) |
| Laboratory data | |
| Hematocrit, percentage | 25 (15-43) |
| Platelet count, ×103/μL | 16 (2-124) |
| LDH, U/L | (256-3783) |
| Creatinine, μmol/L [mg/dL] | 97.2 (61.9-406.6)[1.1 (0.7-4.6)] |
| Patients with the “classic pentad” (thrombocytopenia, microangiopathic hemolytic anemia, neurologic and renal abnormalities, fever) | 3 (5) |
| Death | 13 (20) |
| Plasma exchange (in 52 survivors), number (range) | 18 (2-79) |
| Relapse in 52 survivors (median follow-up, 6.3 y; range, 0.3-13.8 y) | 35 |
| Presenting features and clinical course . | No. (%) of patients . |
|---|---|
| History and physical examination | |
| Gastrointestinal symptoms (pain, nausea, vomiting, diarrhea) | 45 (69) |
| Neurologic abnormalities | 43 (66) |
| Major abnormalities | 23 (35) |
| Focal abnormalities | 17 (26) |
| Seizure | 6 (9) |
| Stroke | 6 (9) |
| Coma | 2 (3) |
| Minor abnormalities (eg, confusion, headache) | 20 (31) |
| Weakness | 41 (63) |
| Bleeding, purpura, hematuria | 35 (54) |
| Dyspnea | 19 (29) |
| Fever | 15 (23) |
| Chest pain | 14 (22) |
| Cough | 6 (9) |
| Laboratory data | |
| Hematocrit, percentage | 25 (15-43) |
| Platelet count, ×103/μL | 16 (2-124) |
| LDH, U/L | (256-3783) |
| Creatinine, μmol/L [mg/dL] | 97.2 (61.9-406.6)[1.1 (0.7-4.6)] |
| Patients with the “classic pentad” (thrombocytopenia, microangiopathic hemolytic anemia, neurologic and renal abnormalities, fever) | 3 (5) |
| Death | 13 (20) |
| Plasma exchange (in 52 survivors), number (range) | 18 (2-79) |
| Relapse in 52 survivors (median follow-up, 6.3 y; range, 0.3-13.8 y) | 35 |
Data from all 65 patients presenting between November 13, 1995 and December 31, 2009 with ADAMTS13 activity less than 10%, by immunoblotting and/or FRETS assays, are presented. Neurologic abnormalities and renal failure categories have been previously defined.17 Neurologic abnormalities and laboratory data are from the day of diagnosis, defined as the day of the first plasma exchange treatment. Renal failure categories included data at diagnosis plus or minus 7 days. Laboratory data and the number of plasma exchange treatments are presented as median values and ranges. LDH values are normalized to an upper limit of normal of 200 U/L. The woman with a hematocrit of 43% had not been transfused; she had red cell fragments on her peripheral blood smear, a platelet count of 22 000/μL, LDH of 1613 U/L, and neurologic abnormalities; 2 days later, her hematocrit was 22%. No other untransfused patient had a hematocrit greater than 33% on the day of diagnosis. The woman with a platelet count of 124 000/μL had not received a platelet transfusion; she was comatose, had a hematocrit of 25% with red cell fragments on the peripheral blood smear, LDH of 418 U/L, and acute renal failure. The next highest (untransfused) platelet count on the day of diagnosis was 69 000/μL. Both the woman with the hematocrit of 43% and the woman with a platelet count of 124 000/μL had idiopathic TTP. Data are from the day of diagnosis for determining the “pentad” of clinical features; both major and minor neurologic abnormalities were included; serum creatinine greater than 1.5 mg/dL was considered a renal abnormality. Deaths occurred within 30 days of the last plasma exchange treatment.
The most important observation from these patients is that the so-called “classic pentad” of clinical features, described 44 years ago before effective treatment of TTP was available,3 occurred in only 3 (5%) patients. Two of these patients were subsequently discovered to have systemic infections as the cause of their clinical features; one had a previously established diagnosis of SLE. Therefore, the “classic pentad” of clinical features is not relevant to current practice.
Multiple disorders may cause TMA with microangiopathic hemolytic anemia and thrombocytopenia, mimicking the clinical features of TTP (Table 5).27,36-39,45 If a systemic infection, malignancy, or malignant hypertension was determined to be the probable cause of the presenting clinical features, plasma exchange was stopped.
Common disorders that may mimic the clinical features of TTP
| Disorder . | Comments . |
|---|---|
| Preeclampsia, HELLP syndrome | Can cause microangiopathic hemolytic anemia, thrombocytopenia, renal failure, and minor neurologic abnormalities. May first present after delivery. TTP diagnosed if major neurologic abnormalities occur or if abnormalities fail to resolve within 3 days after delivery.27 |
| Autoimmune disorders | May be indistinguishable from TTP. Some patients may have both TTP and an additional autoimmune disorder, such as SLE or APLA.36 |
| Systemic infection | Multiple etiologies of sepsis (bacteria, fungi, rickettsiae, and viruses) can cause thrombocytopenia and microangiopathic hemolytic anemia without signs of DIC.37 Sepsis suggested by high fever with chills and pulmonary infiltrates, which rarely if ever occur in TTP. |
| Systemic malignancy | Multiple malignancies can cause thrombocytopenia and microangiopathic hemolytic anemia without signs of DIC. Malignancy suggested by hepatic and pulmonary involvement, which rarely if ever occur in TTP.10 Nucleated red cells and immature white cells on the peripheral blood smear suggest marrow involvement that may be diagnosed by marrow biopsy.38,39 |
| Malignant hypertension | Can cause thrombocytopenia, microangiopathic hemolytic anemia, renal failure, and severe neurologic abnormalities.45 |
| Disorder . | Comments . |
|---|---|
| Preeclampsia, HELLP syndrome | Can cause microangiopathic hemolytic anemia, thrombocytopenia, renal failure, and minor neurologic abnormalities. May first present after delivery. TTP diagnosed if major neurologic abnormalities occur or if abnormalities fail to resolve within 3 days after delivery.27 |
| Autoimmune disorders | May be indistinguishable from TTP. Some patients may have both TTP and an additional autoimmune disorder, such as SLE or APLA.36 |
| Systemic infection | Multiple etiologies of sepsis (bacteria, fungi, rickettsiae, and viruses) can cause thrombocytopenia and microangiopathic hemolytic anemia without signs of DIC.37 Sepsis suggested by high fever with chills and pulmonary infiltrates, which rarely if ever occur in TTP. |
| Systemic malignancy | Multiple malignancies can cause thrombocytopenia and microangiopathic hemolytic anemia without signs of DIC. Malignancy suggested by hepatic and pulmonary involvement, which rarely if ever occur in TTP.10 Nucleated red cells and immature white cells on the peripheral blood smear suggest marrow involvement that may be diagnosed by marrow biopsy.38,39 |
| Malignant hypertension | Can cause thrombocytopenia, microangiopathic hemolytic anemia, renal failure, and severe neurologic abnormalities.45 |
APLA indicates antiphospholipid antibody syndrome; and DIC, disseminated intravascular coagulation.
Documentation of severe ADAMTS13 deficiency may not conclusively confirm the diagnosis of TTP. For example, among our 65 patients with severe ADAMTS13 deficiency, 6 were discovered to have alternative disorders as the probable cause of their presenting clinical features after plasma exchange had begun: 5 had systemic infections (including the HSCT patient) and one had systemic Kaposi sarcoma discovered at autopsy (Table 2).14
Management of acute episodes
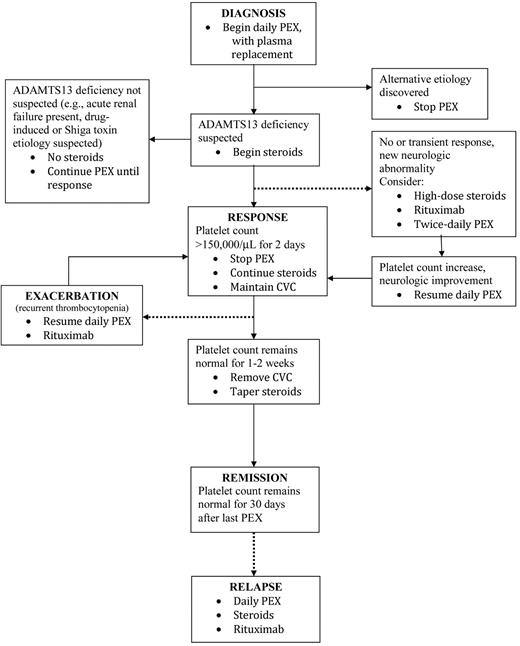
I treat all adults who fulfill the diagnostic criteria for TTP (microangiopathic hemolytic anemia and thrombocytopenia, with or without renal failure or neurologic abnormalities, and without another cause for TMA) with plasma exchange (Figure 1). Plasma replacement is essential; replacement with one plasma volume is appropriate; all plasma products (fresh-frozen plasma, 24-hour plasma, cryoprecipitate-poor plasma) appear to have equivalent efficacy. Because the inclusion criteria for the studies that documented the benefit of plasma exchange were only microangiopathic hemolytic anemia and thrombocytopenia, the patients enrolled in these 2 studies may be assumed to have had multiple etiologies for their TTP.11,12 Plasma infusion can provide temporary benefit until plasma exchange can be begun.11 The assumption that plasma exchange may work by replacing ADAMTS13 and removing autoantibodies that inhibit its activity46 may not apply to all patients because response to plasma exchange may be the same in patients without a severe deficiency of ADAMTS13.17 Table 3 documents that survival rates of patients with severe ADAMTS13 deficiency (82%) are the same in patients without severe ADAMTS13 deficiency (79%). Adults with a history suggesting E coli O157:H7 infection or a suspected drug association are treated with plasma exchange because the etiology cannot be certain when the decision for plasma exchange must be made, and these patients can be critically ill with a high frequency of severe neurologic abnormalities, renal failure, and death.33,34
The clinical course of patients with TTP may be complex and cannot be easily represented by a single diagram. Continued search for alternative etiologies for the patient's clinical features is critical, even after beginning plasma exchange. The clinical basis for suspecting severe ADAMTS13 deficiency is described in the text. Exacerbations of TTP, either while continuing daily plasma exchange or after plasma exchange is stopped, and relapses rarely occur in patients without ADAMTS13 deficiency. Although rituximab may be appropriate for 3 different situations illustrated in this algorithm, we have never used more than a single course of rituximab for any patient. Definitions for response, exacerbation, remission, and relapse have been previously described.17 Broken lines represent complications that occur in a minority of patients. PEX indicates plasma exchange. CVC indicates central venous catheter.
The clinical course of patients with TTP may be complex and cannot be easily represented by a single diagram. Continued search for alternative etiologies for the patient's clinical features is critical, even after beginning plasma exchange. The clinical basis for suspecting severe ADAMTS13 deficiency is described in the text. Exacerbations of TTP, either while continuing daily plasma exchange or after plasma exchange is stopped, and relapses rarely occur in patients without ADAMTS13 deficiency. Although rituximab may be appropriate for 3 different situations illustrated in this algorithm, we have never used more than a single course of rituximab for any patient. Definitions for response, exacerbation, remission, and relapse have been previously described.17 Broken lines represent complications that occur in a minority of patients. PEX indicates plasma exchange. CVC indicates central venous catheter.
Complications of plasma exchange treatment
The decision to begin plasma exchange is a balance between confidence in the diagnosis and risks of the procedure. For patients in whom there is doubt about the diagnosis of TTP, the risks of plasma exchange are sufficiently great that hesitation may be prudent. We have prospectively documented the presence or absence of complications of plasma exchange on all 249 patients treated for their first episode of TTP, 1996 to 2008; 64 (26%) patients had 83 major complications, including 7 (2.8%) deaths (4 caused by central venous catheter-related sepsis, 3 by hemorrhage caused by catheter insertion) and 2 cardiac arrests with pulseless electrical activity (Table 6).47-50
Major complications of plasma exchange treatment for TTP or HUS: experience of the Oklahoma TTP-HUS Registry with 249 consecutive patients treated for a diagnosis of TTP or HUS (1996-2008)
| Complication . | No. (%) . | Comments (no. of patients) . |
|---|---|---|
| Death | 7 (3) | Bacterial sepsis (n = 4); hemorrhage from catheter insertion (n = 3) |
| Nonfatal cardiac arrest | 2 (1) | Right ventricle perforation by catheter insertion guide wire with cardiac tamponade (n = 1); plasma allergic reaction (n = 1) |
| Catheter insertion complications | 5 (2) | Pulmonary (n = 2) and retroperitoneal (n = 1) hemorrhage requiring transfusion; pneumothorax requiring chest tube (n = 2) |
| Systemic infection | 29 (12) | Documented bacteremia (n = 24) or fungemia (n = 2); suspected bacteremia treated with systemic antibiotics (n = 3) |
| Catheter obstruction | 17 (7) | Requiring catheter removal and insertion of a new catheter |
| Hypotension | 7 (3) | Requiring vasopressor treatment |
| Venous thrombosis | 5 (2) | Requiring anticoagulant treatment |
| Complication . | No. (%) . | Comments (no. of patients) . |
|---|---|---|
| Death | 7 (3) | Bacterial sepsis (n = 4); hemorrhage from catheter insertion (n = 3) |
| Nonfatal cardiac arrest | 2 (1) | Right ventricle perforation by catheter insertion guide wire with cardiac tamponade (n = 1); plasma allergic reaction (n = 1) |
| Catheter insertion complications | 5 (2) | Pulmonary (n = 2) and retroperitoneal (n = 1) hemorrhage requiring transfusion; pneumothorax requiring chest tube (n = 2) |
| Systemic infection | 29 (12) | Documented bacteremia (n = 24) or fungemia (n = 2); suspected bacteremia treated with systemic antibiotics (n = 3) |
| Catheter obstruction | 17 (7) | Requiring catheter removal and insertion of a new catheter |
| Hypotension | 7 (3) | Requiring vasopressor treatment |
| Venous thrombosis | 5 (2) | Requiring anticoagulant treatment |
Platelet transfusions
Although decades of dogma describe the danger of platelet transfusions in patients with TTP, the only supporting observations are dramatic case reports. A systematic review of published case reports and case series together with an analysis of our experience did not document a risk from platelet transfusions.51 Most of our patients received a platelet transfusion. In most of these patients, the platelet transfusions were given as part of the common initial care for patients with severe thrombocytopenia and anemia, before the diagnosis of TTP was considered. No adverse events were identified.51 Although it is prudent to be cautious, the appropriate use of platelet transfusions for prevention or management of hemorrhage is also appropriate for patients with TTP. In patients with TTP, severe thrombocytopenia itself is not an appropriate indication for platelet transfusion.
Adjuvant corticosteroid treatment
After beginning plasma exchange, the next decision is whether to begin corticosteroids. Their value may be the suppression of autoantibodies inhibiting ADAMTS13 activity, and their potential benefit may be limited to patients with severe ADAMTS13 deficiency. Patients who are unlikely to have severe ADAMTS13 deficiency, such as patients with severe renal failure or a history and clinical features suggesting drug-associated TTP or an E coli O157:H7 infection, are not treated with corticosteroids. Patients in whom the etiology is unclear are treated with corticosteroids. Clinical criteria are sufficient for this decision.
Response to plasma exchange treatment
The response to plasma exchange, with or without corticosteroids, is judged by the platelet count. An increased platelet count is anticipated after the second or third daily treatment, and often the platelet count reaches normal in 1 week. Neurologic recovery may be the first sign of response, and complete recovery from critical neurologic abnormalities, such as coma and hemiparesis, may occur. Lactate dehydrogenase levels decrease after the initial plasma exchange but return to normal is less predictable. Anemia develops and also recovers more slowly than thrombocytopenia. Renal failure is the last abnormality to recover; recovery of normal renal function is often uncertain.
The clinical course of patients who do not have severe ADAMTS13 deficiency is variable. Patients with drug or Shiga toxin-associated TTP usually recover promptly and exacerbations rarely occur. Other patients with unknown etiologies may not respond to plasma exchange. Treatment intensification may provide no additional benefit.
The clinical course of patients with severe ADAMTS13 deficiency is also variable. Some patients respond quickly and completely, with neither exacerbation nor relapse; others may have a prolonged course with multiple exacerbations and critical complications. Some data suggest that patients with higher titers of anti-ADAMTS13 autoantibodies are at greater risk for complications and death.14,52 I adjust the initial corticosteroid dose according to the severity of the patient's illness. For patients who are alert and comfortable, without neurologic abnormalities, I use prednisone, 1 mg/kg per day. For patients who are acutely ill, I initially use higher doses, such as methylprednisolone, 125 mg 2 to 4 times daily.
In some patients, the platelet count may not increase even after 4 to 7 days of plasma exchange treatment, or the platelet count may initially increase and then fall again, despite continued daily plasma exchange, sometimes in association with new neurologic abnormalities or signs of cardiac ischemia. These patients require escalation of treatment intensity based on the severity of their illness; clinical criteria are sufficient for these decisions. High-dose corticosteroids, such as methylprednisolone, 1000 mg/day for 3 days, can be given immediately. Rituximab may also be given, using the conventional regimen of 375 mg/m2 weekly for 4 weeks.14,53,54 This dose may be excessive for autoimmune disorders, such as TTP, perhaps explaining why rituximab is effective, even though much may be removed by subsequent plasma exchanges.55 For refractory and critically ill patients, twice-daily plasma exchange, each procedure replacing one plasma volume, appears to provide additional benefit56 ; once-daily plasma exchange can be resumed as soon as clinical signs improve and the platelet count begins to increase. If all of these treatments seem insufficient, additional immunosuppression with cyclophos-phamide, vincristine, or cyclosporine57 may be used. Although splenectomy is still performed as treatment for patients with refractory or relapsing TTP, we have not done a splenectomy for TTP since 2000.
I stop plasma exchange after the platelet count has been normal for 2 days, rather than tapering treatment frequency. The response will be durable in most patients. Corticosteroid treatment is maintained (prednisone, 1 mg/kg per day), and the patient is usually evaluated twice weekly for 2 weeks. In some patients, TTP remains active and thrombocytopenia recurs when plasma exchange is stopped; these exacerbations may occur after missing only one day of plasma exchange, usually occur within one week, and almost always occur within 2 weeks of stopping plasma exchange.37 When recurrent thrombocytopenia occurs, suggesting an exacerbation of TTP, the first and most critical step is to exclude sepsis related to the plasma exchange catheter. The catheter is left in for one to 2 weeks (unless a femoral catheter was used) to avoid the risks of inserting a new catheter; it is not left in longer because of the increasing risk for infection. When the platelet count has remained normal for one to 2 weeks, the catheter is withdrawn and prednisone is quickly tapered and discontinued.
Rituximab may also be appropriate for patients who have an exacerbation after a response or a relapse after a remission (Figure 1). Fourteen (22%) of the 65 patients in Table 4 have been treated with rituximab: 8 during their initial episode and 6 for a relapse; none has subsequently relapsed (median follow-up after rituximab, 30 months; range, 3-78 months).
Managing patients after recovery
After a remission occurs, patients need gradually fewer routine blood counts over several months; after this, they need only routine care from their primary physician. However, both the patient and her physician need to maintain contact with the hematologist in case a relapse is suspected. Although routine blood counts are not necessary after several months, a platelet count is absolutely necessary when symptoms of any illness occur, to immediately diagnose a possible recurrence of TTP.
Measurement of ADAMTS13 activity during remission
Although TTP appears to be a disorder with acute episodes followed by complete recovery, many patients have persistent or intermittent ADAMTS13 deficiency after recovery. Among 41 patients who initially had severe ADAMTS13 deficiency and who have had one to 4 measurements of ADAMTS13 activity during remission, 7 (17%) have had ADAMTS13 activity less than 10% and 19 (46%) have had ADAMTS13 less than 50% at some time during their remissions; 9 (22%) have had an ADAMTS13 inhibitor.14 Severe ADAMTS13 deficiency during remission was not associated with clinical signs of TTP, and its clinical importance related to risk for relapse is uncertain.14 Therefore, I do not routinely measure ADAMTS13 activity after recovery; we only do annual measurements as part of our research program.
Long-term outcomes
Table 7 summarizes the principal potential problems that may occur after recovery from TTP.
Long-term outcomes after recovery from TTP
| Outcome . | Comments . |
|---|---|
| Relapse | In patients with ADAMTS13 deficiency, the risk is estimated to be 41% at 7.5 years; the greatest risk is in the first year after recovery. Patients without ADAMTS13 deficiency rarely relapse.14 |
| Pregnancy complications | Women who have had TTP may have a higher risk for pregnancy-related complications. Recurrent TTP is uncommon with a subsequent pregnancy.58 |
| Other autoimmune disorders | Patients with acquired (autoimmune) ADAMTS13 deficiency may be at risk for developing other autoimmune disorders.36 |
| Minor cognitive abnormalities | Common; manifested by problems with memory, concentration, and fatigue.62 |
| Outcome . | Comments . |
|---|---|
| Relapse | In patients with ADAMTS13 deficiency, the risk is estimated to be 41% at 7.5 years; the greatest risk is in the first year after recovery. Patients without ADAMTS13 deficiency rarely relapse.14 |
| Pregnancy complications | Women who have had TTP may have a higher risk for pregnancy-related complications. Recurrent TTP is uncommon with a subsequent pregnancy.58 |
| Other autoimmune disorders | Patients with acquired (autoimmune) ADAMTS13 deficiency may be at risk for developing other autoimmune disorders.36 |
| Minor cognitive abnormalities | Common; manifested by problems with memory, concentration, and fatigue.62 |
Risk for relapse
Eighteen (35%) of the 52 surviving patients with severe ADAMTS13 deficiency have relapsed (median follow-up, 6.3 years; range, 0.3-13.8 years). The estimated risk for relapse is 41% at 7.5 years; relapses are most common during the first year after recovery.14 Relapse rarely occurs in patients without severe ADAMTS13 deficiency. I do not use maintenance treatment to prevent relapses, but the increasing use of rituximab for management of acute episodes may decrease the occurrence of relapses.14,53,54 Survival with a relapse may be better than with an initial episode because of prompt recognition and treatment.14
Risk of subsequent pregnancies
Because pregnancy is a recognized risk factor for triggering acute episodes of TTP,28 pregnancy is often assumed to be associated with a high risk for a relapse. This assumption seemed to be supported by case reports of subsequent pregnancies associated with recurrent TTP,58 but case reports may preferentially report dramatic events rather than uncomplicated pregnancies. In the Oklahoma Registry, 27 women have had 51 subsequent pregnancies, including 6 women with severe ADAMTS13 deficiency who have had 12 subsequent pregnancies58 (data updated through June 30, 2010). Six women (22%) have been diagnosed with TTP during one subsequent pregnancy; these 6 women have also had 9 additional subsequent pregnancies without TTP recurrence. Five of these 6 women were initially diagnosed with TTP before 1995 (therefore, ADAMTS13 was not measured); in 3 the probable cause for thrombocytopenia was preeclampsia. One woman with severe ADAMTS13 deficiency who has had 2 pregnancies after her episode of TTP had a relapse 30 days after her second pregnancy. Therefore, I do not discourage women who have recovered from TTP and who wish to become pregnant, although I discuss the potential risk for a recurrent episode of TTP. I do advise careful management by a specialist in high-risk obstetrics, with platelet counts at each prenatal visit.
Risk for developing additional autoimmune disorders
Three (7%) of the 42 surviving patients with idiopathic TTP and severe ADAMTS13 deficiency have subsequently developed SLE. Our preliminary data suggest that the frequency of rheumatic disease-associated autoantibodies is significantly greater in patients with severe ADAMTS13 deficiency than in the normal population. Therefore, we specifically assess criteria for SLE during initial episodes and follow-up evaluations.
Cognitive abnormalities
Finally, more real than these potential risks are the persistent, subtle sequelae of TTP that occur in most patients after recovery. For the past 14 years, we have had regular meetings for our former patients.59,60 At these meetings, the consistent concern has been the patients' impression that their recovery has been incomplete. They have trouble concentrating, seem more forgetful, and have less endurance. The affected patients are not disabled; they have returned to their normal lives and occupations, but daily activities are more difficult. Our meetings have functioned as a focus group, suggesting themes for research. As a result of our meetings, we have documented significant abnormalities of health-related quality of life61 and cognitive ability62 that did not improve with time since an acute episode. When we presented these data to our patient group, I was surprised that the response was a sense of relief; patients felt comfort in the knowledge that their symptoms were common among TTP survivors and that they had been objectively documented.
Patient support
Our patient group meetings have served to address the sense of isolation that our patients experience from having had a very serious but nearly unknown illness. In addition to our group meetings, we have also used our website (www.ouhsc.edu/platelets) to provide information for patients and their families.
Future directions
The diagnosis of TTP needs to become more precise. Measurement of ADAMTS13 activity has been an important advance in understanding the pathogenesis of TTP, but it does not identify all patients who can benefit from plasma exchange treatment. The treatment of TTP needs to become more effective, more accessible, and safer. Survival rates for TTP have not changed since the introduction of plasma exchange. Plasma exchange can be logistically difficult, and it has a high risk for serious complications. Much about TTP has changed during the past 10 years, but much more must be achieved.
Acknowledgments
The author thanks his constant colleagues during the past 10 years, Drs Sara Vesely and Deirdra Terrell (University of Oklahoma) and Johanna Kremer Hovinga and Bernhard Lämmle (University of Berne, Switzerland), who have been responsible for what we have learned from the Oklahoma TTP-HUS Registry and who helped me interpret our observations to prepare this article.
The Oklahoma TTP-HUS Registry is supported by the Hematology Research Fund of the University of Oklahoma.
Authorship
Contribution: J.N.G. wrote the manuscript.
Conflict-of-interest disclosure: J.N.G. is a consultant for Baxter, Inc for rADAMTS13 development. He has no conflicts of interest with the topic or data of this manuscript.
Correspondence: James N. George, Department of Biostatistics & Epidemiology, CHB 237, University of Oklahoma Health Sciences Center, PO Box 73190, Oklahoma City, OK 73126-0901; e-mail: james-george@ouhsc.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal