Abstract
Cyclic adenosine monophosphate (cAMP)-dependent signaling modulates platelet function at sites of vascular injury. Here we show that thrombospondin-1 (TSP-1) prevents cAMP/protein kinase A (PKA) signaling through a CD36-dependent mechanism. Prostaglandin E1 (PGE1) induced a robust inhibition of both platelet aggregation and platelet arrest under physiologic conditions of flow. Exogenous TSP-1 reduced significantly PGE1-mediated inhibition of both platelet aggregation and platelet arrest. TSP-1 prevented PGE1-stimulated cAMP accrual and phosphorylation of PKA substrates, through a mechanism requiring phosphodiesterase3A. TSP-1 also inhibited VASP phosphorylation stimulated by the nonhydrolyzable cAMP analog, 8-bromo-cAMP, indicating that it may regulate cAMP-mediated activation of PKA. The inhibitory effect of TSP-1 on cAMP signaling could be reproduced with a peptide possessing a CD36 binding sequence of TSP-1, while the effects of TSP-1 were prevented by a CD36 blocking antibody. TSP-1 and the CD36 binding peptide induced phosphorylation of Src kinases, p38 and JNK. Moreover, inhibition of Src kinases blocked TSP-1–mediated regulation of cAMP concentrations and the phosphorylation of VASP, indicating that TSP-1 modulated the cAMP/PKA signaling events through a tyrosine kinase-dependent pathway downstream of CD36. These data reveal a new role for TSP-1 in promoting platelet aggregation through modulation of the cAMP-PKA signaling pathway.
Introduction
Vascular injury leads to exposure of prothrombotic extracellular matrix proteins like von Willebrand factor and collagen, which trap and activate platelets. Activation of platelets leads to the release of soluble platelet agonists, adenosine diphosphate (ADP) and thromboxane A2 (TXA2), which act in a paracrine fashion to further enhance platelet activity and ensure rapid hemostasis.1 However, platelets also play an important role in atherothrombosis, the pathology that underpins myocardial infarction and stroke. Platelet activation is counterbalanced by negative signaling cascades, which modulate excessive activation and return platelets to their quiescent state after transient activation. This is achieved primarily through endothelial-derived factors such as nitric oxide and prostacyclin (PGI2).2 In platelets, PGI2 and prostaglandin E1 (PGE1) stimulate transmembrane adenylyl cyclase (AC) through receptors coupled to Gαs leading to the formation cyclic adenosine 3′,5′monophosphate (cAMP). In turn, elevated cAMP concentrations activate protein kinase A (PKA), which is thought to blunt platelet activation through phosphorylation of various target proteins.2 The loss of cAMP-mediated signaling leads to increased sensitivity to platelet agonists and accumulation of platelets at sites of vascular injury.3 However, the factors that modulate platelet regulation by PGI2-induced signaling pathways remain unclear.
Thrombospondin-1 (TSP-1) is a homotrimeric, multidomain glycoprotein that is present in the extracellular matrix, circulates in plasma in low concentrations, and is stored in platelet α granules, where it is released on activation increasing TSP-1-plasma concentrations by 100-fold.4 TSP-1 potentially interacts with several platelet receptors including integrins αvβ3 and αIIbβ3, CD36, and integrin-associated protein (IAP or CD47).5,6 Synthetic peptides based on the C-terminal of TSP-1 stimulate platelet spreading on immobilized fibrinogen, activate αIIbβ3 forming signaling complexes containing c-Src, FAK, and Syk, leading to outside-in signaling,7 potentiate α2β1-induced platelet adhesion,8 and induce phosphorylation of Syk, SLP-76, and PLCγ2 in a Src kinase–dependent fashion.9,10 However, some of these TSP-1 peptides have nonspecific effects.9 Importantly, there is no evidence that the full TSP-1 protein mediates platelet activation. Hence, a definitive role for TSP-1 in platelet activation has yet to be established. In contrast, TSP-1 does play a role in platelet-endothelium interactions. Under flow, both free and endothelium bound TSP-1 can mediate platelet-endothelial cell association by a mechanism that involves both CD47 and CD36.11 TSP-1 also protects endothelium-bound von Willebrand factor from ADAMTS13-mediated degradation,12 enhancing the dynamic recruitment of platelets into developing thrombi. As TSP-1 signaling does not activate platelets directly, we hypothesized that it may promote platelet activity indirectly by reducing sensitivity to platelet inhibitors. Indeed, a study by Isenberg and colleagues suggested that this was the case for nitric oxide-mediated regulation of platelets.13 To further examine this possibility, we studied the effects of TSP-1 on platelet sensitivity to PGE1-stimulated cAMP/PKA signaling. We report here that TSP-1 triggers CD36-dependent signals that reduce platelet sensitivity to PGE1, diminishing its ability to inhibit platelet aggregation and arrest under conditions of flow. Our data suggest that TSP-1 modulates platelet inhibitory pathways stimulated by endothelial-derived mediators rather than causing direct activation of platelets.
Methods
Materials
The CD36 stimulatory peptide derived from the second type 1 repeat (amino acids 488-500 of TSP-1) of the parent TSP-1 molecule (488VTAGGGVQKRSRL500) and CD47 (7N3; amino acids 1101-1112 of TSP1) activatory peptide (1101FIRVVMYEGKK1112), based on the receptor binding sequences of TSP-1,5,14 were purchased from Peptides International. Trimeric platelet-derived TSP-1, JNK inhibitor 1, PP1, PP3, and SB202190 were from Calbiochem. Anti-CD36 antibody FA6-152 was from Abcam. PhosphoVASP-ser,157 phospho-PKA substrate antibody (recognizing RRXS*/T*), phospho-JNK, phospho-MAPK, and phosphoSrc-tyr418 antibodies were from Cell Signaling. Anti–β-tubulin and immunoglobulin G (IgG) control were from Upstate. cAMP enzyme immunoassay kits were from GE Healthcare. The PDE3 inhibitor milrinone was purchased from Tocris. PGE1 and all other chemicals were from Sigma-Aldrich.
Platelet preparation
Human blood was taken from drug-free volunteers by clean venepuncture using acid citrate dextrose (29.9mM sodium citrate, 113.8mM glucose, 72.6mM sodium chloride and 2.9mM citric acid, pH 6.4) as anticoagulant. All studies were approved by the Postgraduate Medical Institute Faculty Ethics Committee of the University of Hull. Platelet-rich plasma was obtained by centrifugation of whole blood at 200 × g at 20°C for 20 minutes. Platelet-rich plasma was treated with citric acid (0.3mM) and centrifuged at 1900 rpm for 12 minutes. The platelet pellet was then suspended in wash buffer (36mM citric acid, 10mM EDTA [ethylenediaminetetraacetic acid], 5mM glucose, 5mM KCl, 9mM NaCl) and spun once more. Platelets were finally resuspended at a concentration of 3 × 108 platelets/mL in modified Tyrode buffer (150mM NaCl, 5mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid], 0.55mM NaH2PO4, 7mM NaHCO3, 2.7mM KCl, 0.5mM MgCl2, 5.6mM glucose) unless otherwise stated.
Platelet aggregation
Washed platelets (3 × 108 platelets/mL) were preincubated with TSP-1 (0.1-10 μg/mL for 1-15 minutes) at 37°C before treatment with PGE1 (0.1μM). After 1 minute incubation with PGE1, platelets were stimulated with collagen (5 μg/mL). Aggregation was recorded under constant stirring conditions (900 rpm) for 4 minutes using a Chronolog aggregation module-dual channel light aggregometer.
Platelet aggregation under flow
Platelets (4 × 108 platelets/mL) were incubated with the fluorescent dye, DIOC6 (1μM), at 37°C for 10 minutes and then incubated at 37°C for 2 minutes with PGE1 (10μM), before being reconstituted with autologous washed red blood cells at a ratio of 1:1 to give a final count of 2 × 108 platelets/mL. In some cases platelets were preincubated with TSP-1 (10 μg/mL for 15 minutes) before addition of PGE1. Flow studies were performed using glass capillary tubes (Cambridge Biosciences), coated with immobilized fibrinogen (1 mg/mL) for 12 hours. Platelets were perfused through fibrinogen-coated capillary tubes at 800/s−1 for 2 minutes, followed by washing for 4 minutes at the same shear rate. Adherent platelets/thrombi were visualized using fluorescence microscopy (IX71; Olympus). Data were presented as percentage area covered by adhering platelets (percent surface coverage), since this methodology cannot fully discriminate between individual platelets and platelet aggregates. For each experiment, 8 random fields of view were used to calculate surface coverage (percent) using ImageJ software
Immunoblotting
For signaling studies suspended platelets (3 × 108 platelets/mL) were incubated with apyrase (2 U/mL), indomethacin (10μM), RGDS (Arg-Gly-Asp-Ser; 500μM), and EGTA (ethyleneglycoltetraacetic acid; 1mM) to prevent secondary signaling events. Platelets were then treated with TSP-1 (0-10 μg/mL; for 0.5-60 minutes), CD36 stimulatory peptide (0.001-10μM; for 15 minutes), or CD47 stimulatory peptide (0.001-10μM; for 15 minutes) before addition of PGE1 (0.1μM) for 1 minute or 8 Br-cAMP (500μM) for 15 minutes before termination with Laemmli buffer. In some cases, platelets were incubated with the CD36 blocking antibody FA6-152 (1 μg/mL) or an IgG control for 15 minutes before addition of TSP-1. Proteins were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene fluoride membranes. Membranes were blocked for 60 minutes with 10% bovine serum albumin dissolved in Tris-buffered saline-Tween (0.1%), then incubated with anti-VASP-phospho-ser157 (1:1000), anti–phospho-PKA substrate (1:1000), anti–phosphoSrc-tyr418 (1:1000), anti–phospho-p38 (1:1000), anti-phosphoJNK (1:1000), anti-phosphoErk antibodies (1:1000), or an anti–β-tubulin antibody (1:1000). Immunoblots were processed as described previously.15
cAMP measurement
Washed platelets (2 × 108 platelets/mL) were preincubated with TSP-1 (10 μg/mL), CD36 binding peptide (10μM), or fibrinogen (10 μg/mL) for 10 minutes followed by treatment with PGE1 (0.1μM) for 30 seconds. In some cases, platelets were preincubated with kinase inhibitors for 15 minutes before addition of TSP-1 or CD36 binding peptide. For experiments examining the role of phosphodiesterase 3A, platelets were incubated with milrinone (10μM) for 15 minutes before addition of TSP-1 or CD36 peptide. Reactions were terminated by addition of lysis buffer containing dodecyltrimethylammonium bromide, and cAMP levels were assayed with a commercially available enzyme immunoassay system and expressed as fmol cAMP/1 × 107 platelets. All assays were carried out in triplicate and with independent platelet donors.
Statistical analysis
Results are expressed as means ± SEM and were analyzed using the Student t test or analysis of variance. The results were considered significant when P values were less than .05.
Results
Thrombospondin-1 prevents PGE1-mediated inhibition of platelet aggregation and platelet accrual under flow
In the first instance, we tested the possibility that TSP-1 could modulate PGE1-mediated inhibition of platelet aggregation. PGE1 (0.1μM) reduced collagen (5 μg/mL)–induced aggregation from 78% ± 3% to 25% ± 6% (P < .05; Figure 1A-B). Importantly, preincubation of platelets with TSP-1 (0.1-10 μg/mL) before PGE1 caused a concentration-dependent reversal of platelet inhibition. Maximal effects were observed with TSP-1 (10 μg/mL), where aggregation was increased from 25% ± 6% to 59% ± 8% (P < .05, compared with PGE1 alone; Figure 1B). The ability of TSP-1 to modulate the inhibitory effect of PGE1 was time-dependent, requiring 15 minutes incubation to exert optimal effects. In control experiments, the addition of either fibrinogen or albumin (not shown) failed to influence PGE1 inhibition of aggregation (Figure 1A), indicating that moderation of PGE1-mediated effects was specific for TSP-1. Moreover, TSP-1 alone did not stimulate aggregation or potentiate that induced by collagen (Figure 1C), suggesting that the increased aggregation was due to an inhibition of PGE1 signaling, rather than direct platelet activation by TSP-1 alone.
TSP-1 blocks the inhibitory actions of PGE1 on platelet aggregation. Platelets (3 × 108/mL) were preincubated with TSP-1 (10 μg/mL) or fibrinogen (1 mg/mL) for 15 minutes, followed by addition of PGE1 (0.1μM) for 1 minute and then stimulated with collagen (5 μg/mL) for 4 minutes under stirring conditions. (A) Shown are representative aggregation traces. (B) As in panel A, except platelets were incubated with TSP-1 (0.1-10 μg/mL) for 1-15 minutes before addition of PGE1 (0.1μM). Data are expressed as mean ± SEM from 4 independent experiments performed in duplicate. §P < .05 compared with absence of PGE1; *P < .05 compared with PGE1 in the absence of TSP-1. (C) Platelets were incubated with TSP-1 (10 μg/mL) or vehicle for 15 minutes and then stimulated with collagen (0.25-2 μg/mL) and aggregation measured. (i) Representative traces of experiments performed with collagen at either 0.75 or 2 μg/mL. (ii) Collated data of 3 independent experiments expressed as mean ± SEM of aggregation. Black bars indicate the presence of TSP-1; gray bars, absence of TSP-1.
TSP-1 blocks the inhibitory actions of PGE1 on platelet aggregation. Platelets (3 × 108/mL) were preincubated with TSP-1 (10 μg/mL) or fibrinogen (1 mg/mL) for 15 minutes, followed by addition of PGE1 (0.1μM) for 1 minute and then stimulated with collagen (5 μg/mL) for 4 minutes under stirring conditions. (A) Shown are representative aggregation traces. (B) As in panel A, except platelets were incubated with TSP-1 (0.1-10 μg/mL) for 1-15 minutes before addition of PGE1 (0.1μM). Data are expressed as mean ± SEM from 4 independent experiments performed in duplicate. §P < .05 compared with absence of PGE1; *P < .05 compared with PGE1 in the absence of TSP-1. (C) Platelets were incubated with TSP-1 (10 μg/mL) or vehicle for 15 minutes and then stimulated with collagen (0.25-2 μg/mL) and aggregation measured. (i) Representative traces of experiments performed with collagen at either 0.75 or 2 μg/mL. (ii) Collated data of 3 independent experiments expressed as mean ± SEM of aggregation. Black bars indicate the presence of TSP-1; gray bars, absence of TSP-1.
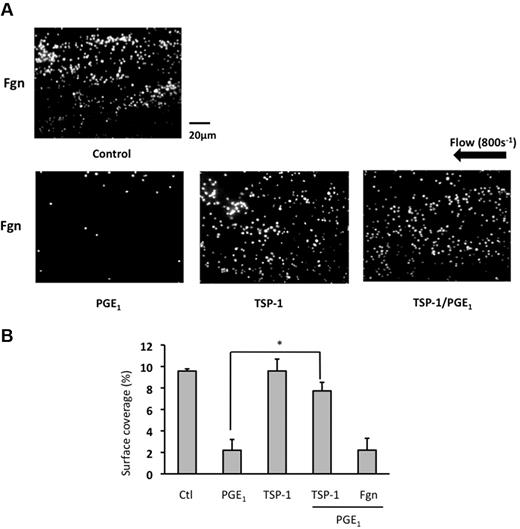
To determine whether TSP-1 influenced platelet function under physiologic conditions we examined platelet aggregation under flow. Under arterial shear (800s−1), immobilized fibrinogen (1 mg/mL) supported adhesion of numerous small aggregates that covered 9.5% ± 0.2% of the fibrinogen-coated surface (Figure 2A-B and supplemental Video 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). This was reduced to 2.2% ± 1.0% (P < .05) by the presence of PGE1 (10μM), demonstrating that PGE1 inhibits platelet aggregation under physiologic conditions of flow (Figure 2A-B and supplemental Video 2). Treatment of platelets with TSP-1 alone did not influence platelet adhesion under flow (Figure 2 and supplemental Video 3). Incubation of platelets with TSP-1 (10 μg/mL) for 15 minutes before PGE1, conditions that caused maximal effects in aggregation experiments, reversed the inhibitory effects of PGE1, with the level of surface coverage increasing to 7.7% ± 0.8% (P < .05 compared with PGE1 alone) similar to that of control (Figure 2A-B and supplemental Video 4). These data indicate that TSP-1 can increase platelet adhesion and aggregation by modulating the inhibitory actions of PGE1.
TSP-1 modulates PGE1-mediated inhibition of platelet arrest under conditions of flow. Platelets were incubated with either TSP-1 (10 μg/mL) alone for 15 minutes, PGE1 (10μM) alone for 2 minutes, or TSP-1 (10 μg/mL) for 15 minutes followed by PGE1 for 2 minutes. Platelets were recombined with autologous red blood cells, and the reconstituted blood was flowed over immobilized fibrinogen (1 mg/mL), for 2 minutes at 800s−1, and resulting platelet aggregates were viewed by fluorescent microscopy. Shown are (A) representative images from 4 independent experiments and (B) percent surface-area coverage. Data are shown as percent area coverage and are the mean ± SEM of 4 experiments. *P < .01 surface-area coverage when platelets with treated with PGE1 alone are compared with those pretreated with TSP-1 before PGE1.
TSP-1 modulates PGE1-mediated inhibition of platelet arrest under conditions of flow. Platelets were incubated with either TSP-1 (10 μg/mL) alone for 15 minutes, PGE1 (10μM) alone for 2 minutes, or TSP-1 (10 μg/mL) for 15 minutes followed by PGE1 for 2 minutes. Platelets were recombined with autologous red blood cells, and the reconstituted blood was flowed over immobilized fibrinogen (1 mg/mL), for 2 minutes at 800s−1, and resulting platelet aggregates were viewed by fluorescent microscopy. Shown are (A) representative images from 4 independent experiments and (B) percent surface-area coverage. Data are shown as percent area coverage and are the mean ± SEM of 4 experiments. *P < .01 surface-area coverage when platelets with treated with PGE1 alone are compared with those pretreated with TSP-1 before PGE1.
TSP-1 prevents accumulation of cAMP and PKA signaling in platelets
PGE1 exerts its antiplatelet actions through the activation of AC and elevations in cAMP.16 Increased cAMP binds to cAMP-dependent kinase (PKA) in a cooperative manner leading to its activation, which in turn blunts platelet function through the phosphorylation of several key proteins.2 To determine the mechanism of action of TSP-1 we examined its effects on PGE1-induced activation of the cAMP signaling cascade. PGE1 (0.1μM) stimulated a rapid rise in platelet cAMP, which peaked at 30 seconds before declining back to basal (not shown). At the 30-second time point, cAMP was elevated 5-fold from 249 ± 25 to 1334 ± 95 fmol/1 × 107 platelets by PGE1 (P < .01; Figure 3A). Pretreatment of platelets with TSP-1 (10 μg/mL) for 15 minutes reduced PGE1-mediated cAMP accrual to 496 ± 59 fmol/1 × 107 platelets (P < .01; Figure 3A). Under the same conditions, fibrinogen did not influence the capacity for PGE1 to increase cAMP formation. The level of cAMP in the cell reflects a balance between its synthesis by AC and hydrolysis by phosphodiesterase 3A.17 In order to dissect mechanisms underpinning the changes in cAMP concentration induced by TSP-1, the experiments were repeated in the presence the PDE3A inhibitor milrinone. We reasoned that if TSP-1 inhibited AC activity directly, then PGE1-induced cAMP accumulation would still be reduced by TSP-1 under conditions where PDE activity is blocked. In the presence of milrinone (10μM), cAMP levels induced by PGE1 were increased to 3025 ± 591 fmol/1 × 107 platelets; significantly greater than in the absence of the PDE3 inhibitor and confirming a key role of this PDE in shaping platelet cAMP responses. However, under these conditions, TSP-1 had no effect on PGE1-induced cAMP formation since the cAMP levels remained elevated at 3266 ± 668 fmol/1 × 107 platelets (Figure 3A). The inability of TSP-1 to prevent cAMP accrual when PDE3A activity was blocked indicated that TSP-1 regulates cAMP concentrations through a mechanism that involves increased hydrolysis of cAMP by PDE3A rather than targeting AC and modulating synthesis.
TSP-1 blunts PGE1-mediated cAMP production and PKA signaling. (A) Platelets (2 × 108/mL) treated with or without milrinone were preincubated with TSP-1 (10 μg/mL) for 15 minutes, followed by treatment with PGE1 (0.1μM) for 30 seconds. In some cases platelets were treated with fibrinogen (10 μg/mL) instead of TSP-1, before addition of PGE1. cAMP levels were measured per manufacturer's instructions and are expressed as fmol cAMP/1 × 107 platelets. Data are presented as mean ± SEM of 10 individual experiments. §P < .05 PGE1 treatment compared with basal cAMP; *P < .05 PGE1 stimulated cAMP compared with the presence of TSP-1. **P < .01 PGE1 treatment compared with basal cAMP. (B) Platelets (3 × 108/mL) were preincubated with TSP-1 (0-10 μg/mL) for 1-15 minutes before treatment with PGE1 (0.1μM) for 1 minute. Platelets were lysed, separated by SDS-PAGE and immunoblotted for phosphoVASP-ser157, followed by reprobing for β-tubulin. (C) As in panel B, except platelets were incubated with TSP-1 (10 μg/mL) for up to 60 minutes before addition of PGE1 for 1 minute. (D) As in panel B, except platelets were incubated with fibrinogen (0.001-10 μg/mL) before addition of PGE1. Immunoblots for panels A-D are representative of 4 independent experiments. (E) Platelets (3 × 108/mL) were preincubated with TSP-1 (10 μg/mL) for 15 minutes, followed by the addition of PGE1 (0.1μM) for 1 minute. Platelets were lysed, separated by SDS-PAGE, and immunoblotted for phospho-PKA substrates, followed by reprobing for β-tubulin. Shown is a representative blot of 3 independent experiments.
TSP-1 blunts PGE1-mediated cAMP production and PKA signaling. (A) Platelets (2 × 108/mL) treated with or without milrinone were preincubated with TSP-1 (10 μg/mL) for 15 minutes, followed by treatment with PGE1 (0.1μM) for 30 seconds. In some cases platelets were treated with fibrinogen (10 μg/mL) instead of TSP-1, before addition of PGE1. cAMP levels were measured per manufacturer's instructions and are expressed as fmol cAMP/1 × 107 platelets. Data are presented as mean ± SEM of 10 individual experiments. §P < .05 PGE1 treatment compared with basal cAMP; *P < .05 PGE1 stimulated cAMP compared with the presence of TSP-1. **P < .01 PGE1 treatment compared with basal cAMP. (B) Platelets (3 × 108/mL) were preincubated with TSP-1 (0-10 μg/mL) for 1-15 minutes before treatment with PGE1 (0.1μM) for 1 minute. Platelets were lysed, separated by SDS-PAGE and immunoblotted for phosphoVASP-ser157, followed by reprobing for β-tubulin. (C) As in panel B, except platelets were incubated with TSP-1 (10 μg/mL) for up to 60 minutes before addition of PGE1 for 1 minute. (D) As in panel B, except platelets were incubated with fibrinogen (0.001-10 μg/mL) before addition of PGE1. Immunoblots for panels A-D are representative of 4 independent experiments. (E) Platelets (3 × 108/mL) were preincubated with TSP-1 (10 μg/mL) for 15 minutes, followed by the addition of PGE1 (0.1μM) for 1 minute. Platelets were lysed, separated by SDS-PAGE, and immunoblotted for phospho-PKA substrates, followed by reprobing for β-tubulin. Shown is a representative blot of 3 independent experiments.
To examine if reduced cytosolic cAMP concentrations resulted in diminished activation of PKA, we used phosphorylation of the cytoskeletal protein VASP at serine157 as a well-characterized marker of PKA signaling.18 Treatment of platelets with PGE1 (0.1μM) induced robust phosphorylation of VASP, which was prevented by the presence of TSP-1 (Figure 3B). After a 1-minute incubation, TSP-1, regardless of concentration, had little effect on PKA signaling. However, after a 5-minute preincubation, TSP-1 caused a concentration-dependent decrease in phosphoVASP157, with a detectable reduction observed with TSP-1 (1 μg/mL). However, maximal effects were observed using TSP-1 (10 μg/mL) incubated with platelets for 15 minutes, where PGE1-mediated VASP phosphorylation was ablated (Figure 3B). The ability of TSP-1 to block cAMP/PKA signaling was maintained for at least 60 minutes (longest time tested; Figure 3C). At these time points and concentrations, neither fibrinogen (Figure 3D) nor albumin (not shown) affected VASP phosphorylation.
To examine if the effects of TSP-1 were specific for PKA-mediated phosphorylation of VASP or caused a more general depression in activity, we used an antibody that recognizes phosphorylated PKA substrates.19 Using this approach we found that proteins with apparent molecular weights 46, 50, and 160 kDa were mildly phosphorylated under basal conditions. Stimulation of AC with PGE1 (0.1μM) induced a robust increase in phosphorylation of PKA substrates with apparent molecular weights of 22, 26, 30 35, 46, 50, 70, 90, and 160 kDa, consistent with proteins previously shown to be phosphorylated in response to cAMP.20 In the presence of TSP-1 (10 μg/mL), the phosphorylation of these proteins was reduced to basal levels, with the exception of the 160-kDa protein, although its phosphorylation was also reduced substantially (Figure 3E). These data indicate that TSP-1 causes a general and a broad depression in PKA signaling events consistent with the reduced bioavailability of intracellular cAMP.
TSP-1 requires CD36 to inhibit PGE1 signaling
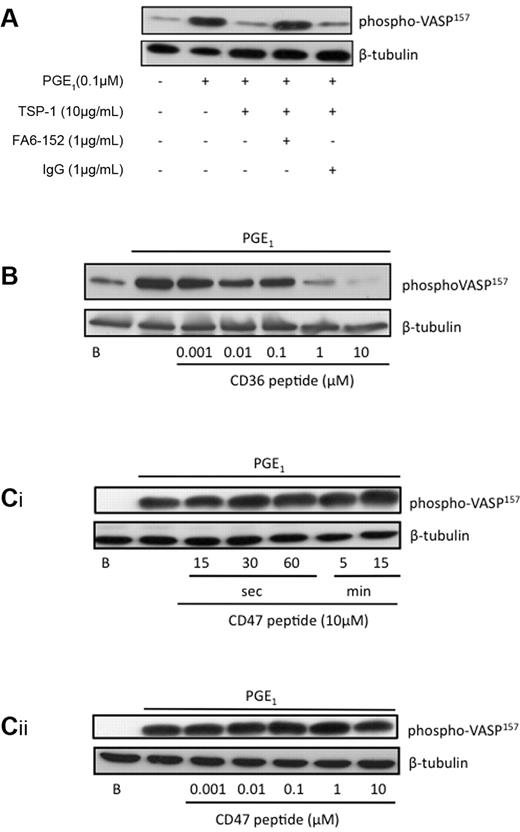
TSP-1 is an established ligand for the membrane protein CD36.4 We therefore examined the role of CD36 as a potential receptor mediating the actions of TSP-1 on cAMP/PKA signaling. Consistent with earlier experiments TSP-1 (10 μg/mL) prevented PGE1-induced phosphorylation of VASP157, which remained at basal levels (Figure 4A). Treatment of the platelets with the well-characterized CD36 blocking antibody FA6-152 (1 μg/mL)21 abolished the ability of TSP-1 to influence phosphoVASP157. In contrast, TSP-1 was still able to block phosphoVASP157 in the presence a nonspecific IgG (Figure 4A). Since TSP-1 can also bind to CD47 and αIIbβ3 on the platelet surface,5 it was important to examine the potential role of these receptors in the modulation of cAMP/PKA signaling. To define the domains of TSP-1 and the potential receptors that modulate cAMP/PKA signaling in platelets, we used peptides corresponding to CD36 and CD47 binding domains within TSP-1. Incubation of platelets with a CD36-binding peptide based on type 1 repeats of TSP1 (488VTAGGGVQKRSRL500)13,14 for 15 minutes prevented PGE1-stimulated VASP ser157 phosphorylation in a concentration-dependent manner, with phosphorylation ablated at 10μM (Figure 4B). A scrambled peptide had no effect on VASP phosphorylation (not shown). In contrast, the peptide corresponding to a section of TSP-1 that binds to CD47 (1102FIRVVMYEGKK1112; p7N3)5 and fibrinogen were without effect on VASP phosphorylation (Figures 3D,4Ci-ii), discounting roles for CD47 and αIIbβ3. Since FA6-152 prevents TSP-1 from modulating PKA activity, and the peptide based on the CD36 binding sequence of TSP-1 mimics the effects of TSP-1, our data suggests that engagement of CD36 can reduce cAMP/PKA signaling in platelets.
TSP-1 and CD36-binding peptides inhibit PKA signaling. (A) Platelets (3 × 108/mL) were treated PGE1 (0.1μM) alone for 1 minute, or TSP-1 (10 μg/mL) for 15 minutes followed by PGE1 for 1 minute in the presence and absence of FA6-152 (1 μg/mL) or control IgG. Platelets were lysed, separated by SDS-PAGE, and blotted for phosphoVASP-ser157, followed by reprobing for β-tubulin. Immunoblots are representative of 3 independent experiments. (B) Platelets (3 × 108/mL) were treated with a CD36 binding peptide (0.001-10 μg/mL) for 15 minutes, then with PGE1 for 1 minute, and then with PGE1 for 1 minute. Platelets were then processed for immunoblotting as in panel A. (Ci) Platelets (3 × 108/mL) were treated with a CD47 binding peptide (10 μg/mL) for 0-15 minutes followed by PGE1 for 1 minute; platelets were then processed as in panel A. (Cii) Platelets (3 × 108/mL) were treated with a CD47 binding peptide (0.001-10 μg/mL) for 15 minutes by PGE1 for 1 minute, platelets were then processed as in panel A. All immunoblots are representative of 3 to 5 independent experiments.
TSP-1 and CD36-binding peptides inhibit PKA signaling. (A) Platelets (3 × 108/mL) were treated PGE1 (0.1μM) alone for 1 minute, or TSP-1 (10 μg/mL) for 15 minutes followed by PGE1 for 1 minute in the presence and absence of FA6-152 (1 μg/mL) or control IgG. Platelets were lysed, separated by SDS-PAGE, and blotted for phosphoVASP-ser157, followed by reprobing for β-tubulin. Immunoblots are representative of 3 independent experiments. (B) Platelets (3 × 108/mL) were treated with a CD36 binding peptide (0.001-10 μg/mL) for 15 minutes, then with PGE1 for 1 minute, and then with PGE1 for 1 minute. Platelets were then processed for immunoblotting as in panel A. (Ci) Platelets (3 × 108/mL) were treated with a CD47 binding peptide (10 μg/mL) for 0-15 minutes followed by PGE1 for 1 minute; platelets were then processed as in panel A. (Cii) Platelets (3 × 108/mL) were treated with a CD47 binding peptide (0.001-10 μg/mL) for 15 minutes by PGE1 for 1 minute, platelets were then processed as in panel A. All immunoblots are representative of 3 to 5 independent experiments.
TSP-1 inhibits activation of PKA independently of changes in cAMP concentrations
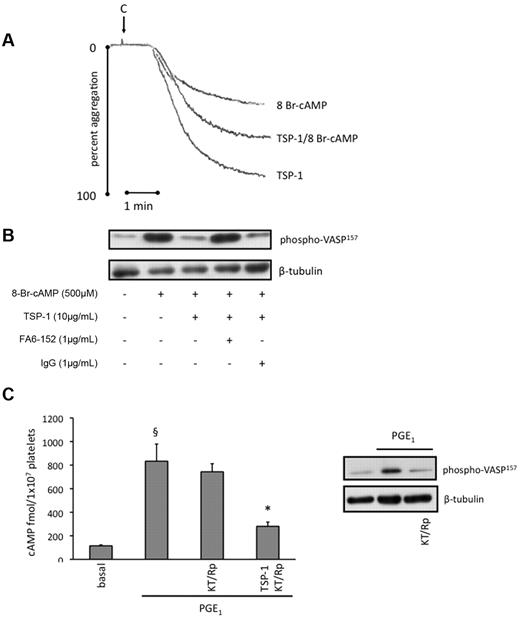
It was possible that TSP-1 modulated PKA activity independently of its effect on cAMP synthesis. To examine this possibility, we used the nonhydrolyzable cAMP analog, 8-Br-cAMP, to bypass AC and activate PKA directly.22 Treatment of platelets with 8-Br-cAMP (500μM) induced a strong inhibition of collagen-induced platelet aggregation, which recovered by 50% in the presence of TSP-1 (10 μg/mL; Figure 5B). As expected, 8-Br-cAMP (500μM) induced a robust phosphorylation of VASP-ser157, consistent with its ability to activate PKA (Figure 5B). However, phosphorylation was prevented when platelets were preincubated with TSP-1 (10 μg/mL) for 15 minutes before addition of the cyclic nucleotide. We also confirmed that CD36 was required for TSP-1 to modulate cAMP-dependent activation of PKA. FA6-152 (1 μg/mL) abolished the ability of TSP-1 to modulate VASP phosphorylation, while the control IgG was without effects (Figure 5B). These data suggest that TSP-1 can also modulate the activation of PKA by cAMP, at concentrations and time points consistent with its ability to modulate PGE1-mediated inhibition of platelet aggregation.
TSP-1 inhibits activation of PKA independently of changes in cAMP concentrations. (A) Platelets (3 × 108/mL) were preincubated with TSP-1 (10 μg/mL) for 15 minutes, followed by addition of 8-bromo-cAMP (500μM) for 15 minutes, and then stimulated with collagen (5 μg/mL) for 4 minutes under stirring conditions. Representative traces of 3 independent experiments. (B) Platelets (3 × 108/mL) were treated with 8-bromo-cAMP (500μM) alone for 15 minutes or TSP-1 (10 μg/mL) for 15 minutes followed by 8-bromo-cAMP for 15 minutes in the presence and absence of FA6-152 (1 μg/mL) or control IgG. Platelets were lysed, separated by SDS-PAGE and blotted for phosphoVASP-ser157, followed by reprobing for β-tubulin. Immunoblots are representative of 3 independent experiments. (C) Platelets (2 × 108/mL) were preincubated with PGE1 (0.1μM) alone for 30 seconds or with TSP-1 (10 mg/mL) for 15 minutes before PGE1, in the presence or absence KT-5720 (5μM) and Rp-cAMPS (0.5mM). cAMP levels were measured as per manufacturer's instructions and are expressed as fmol cAMP/1 × 107 platelets. Data are presented as mean ± SEM of 3 individual experiments. §P < .05, PGE1-stimulated cAMP compared with basal; *P < .05, PGE1-stimulated cAMP compared with the absence of TSP-1. Platelets (3 × 108/mL) were treated with PGE1 (0.1μM) for 30 seconds in the presence or absence KT-5720 (5μM) and Rp-cAMPS (0.5mM), and then immunoblotted for phosphoVASP-ser157 with a representative blot presented.
TSP-1 inhibits activation of PKA independently of changes in cAMP concentrations. (A) Platelets (3 × 108/mL) were preincubated with TSP-1 (10 μg/mL) for 15 minutes, followed by addition of 8-bromo-cAMP (500μM) for 15 minutes, and then stimulated with collagen (5 μg/mL) for 4 minutes under stirring conditions. Representative traces of 3 independent experiments. (B) Platelets (3 × 108/mL) were treated with 8-bromo-cAMP (500μM) alone for 15 minutes or TSP-1 (10 μg/mL) for 15 minutes followed by 8-bromo-cAMP for 15 minutes in the presence and absence of FA6-152 (1 μg/mL) or control IgG. Platelets were lysed, separated by SDS-PAGE and blotted for phosphoVASP-ser157, followed by reprobing for β-tubulin. Immunoblots are representative of 3 independent experiments. (C) Platelets (2 × 108/mL) were preincubated with PGE1 (0.1μM) alone for 30 seconds or with TSP-1 (10 mg/mL) for 15 minutes before PGE1, in the presence or absence KT-5720 (5μM) and Rp-cAMPS (0.5mM). cAMP levels were measured as per manufacturer's instructions and are expressed as fmol cAMP/1 × 107 platelets. Data are presented as mean ± SEM of 3 individual experiments. §P < .05, PGE1-stimulated cAMP compared with basal; *P < .05, PGE1-stimulated cAMP compared with the absence of TSP-1. Platelets (3 × 108/mL) were treated with PGE1 (0.1μM) for 30 seconds in the presence or absence KT-5720 (5μM) and Rp-cAMPS (0.5mM), and then immunoblotted for phosphoVASP-ser157 with a representative blot presented.
The ability of TSP-1 to prevent VASP phosphorylation in response to 8-Br-cAMP implied that TSP-1 signaling regulated PKA itself in addition to cAMP levels. This raised the possibility that the inhibition of PKA may account for the effects of TSP-1 on cAMP levels by activating a feedback inhibitory loop. To test this we examined whether inhibition of PKA using a combination of KT-5720 (5μM) and Rp-cAMPS (0.5mM), 2 structurally distinct PKA inhibitors previously used with platelets,23 influenced PGE1-stimulated cAMP formation. PGE1 increased cAMP from 115 ± 13 to 800 ± 173 fmol/L × 107 platelets, which remained at 743 ± 69 fmol/L × 107 platelets in the presence of KT-5720/Rp-cAMPS (Figure 5C). Importantly, TSP-1 (10 μg/mL) prevented PGE1-induced cAMP accumulation, which remained close to basal levels (280 ± 36 fmol/L × 107 platelets; P < .05 compared with PGE1 alone), despite the presence of PKA inhibitors. The PKA inhibitors completely abolished the ability of PGE1 to stimulate VASP phosphorylation at ser157, confirming the activity of the inhibitors (Figure 5D). These data indicate that TSP-1 signaling regulates cAMP concentrations independent of its potential effects on cAMP-mediated activation of PKA.
TSP-1 induces CD36 signaling in blood platelets
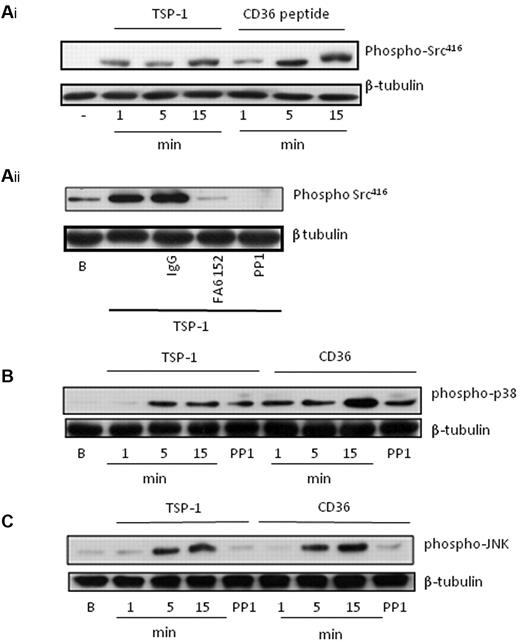
The ability of TSP-1 to modulate cAMP signaling suggested potential crosstalk between the CD36 and cAMP signaling cascades. In numerous cell types including platelets, CD36 signaling has been shown to involve caspase3, c-Jun N-terminal kinase (JNK), p38 MAPkinase, and Src family kinases.24,25 Since Src-family kinases are constitutively associated with CD3626 and lie upstream of signaling pathways diverging from the receptor, we explored their role in crosstalk between CD36 and cAMP/PKA signaling. To determine which signaling events were important for crosstalk between CD36 and cAMP/PKA signaling, we first examined potential signaling pathways activated by TSP-1. Under conditions that ablated the actions of ADP/TxA2 and outside-in signaling, we found that TSP-1 stimulated the phosphorylation of Src family kinases on Tyr416, consistent with their activation. These effects were time-dependent, with detectable changes observed after 1 minute, but maximal effects occurring after 15 minutes (Figure 6Ai). Importantly, the CD36-peptide (10 μg/mL) induced identical phosphorylation events at the same time points (Figure 6Ai). Using the CD36 blocking antibody FA6-152, we confirmed that TSP-1 activates Src kinases through a CD36-dependent mechanism (Figure 6Aii). TSP-1 and the CD36 peptide also stimulated the phosphorylation of p38 and JNK (Figure 6B-C), but not ERK (not shown). Importantly, the phosphorylation of JNK (Figure 6C), but not p38 (Figure 6B), was Src kinase-dependent as it was inhibited by PP1 (20 μM) and indicating that JNK lies downstream of Src kinases, while p38 represented a distinct pathway.25 Our data suggests that TSP-1 activates CD36-dependent pathways that include Src kinase-dependent activation of JNK and Src kinase-independent activation of p38.
TSP-1 and CD36-binding peptides induce activatory signaling in platelets. (A) (i) Platelets (3 × 108/mL) were incubated with either TSP-1 (10 μg/mL) or CD36 binding peptide (10μM) for 1-15 minutes. After lysis, platelet proteins were separated by SDS-PAGE and immunoblotted for phosphoSrc-Tyr418, followed by stripping and reprobing for β-tubulin. (ii) Platelets (3 × 108/mL) were treated with TSP-1 (10 μg/mL) in the presence of either PP1 (20μM), FA6-152 (1 μg/mL), or IgG control (1 μg/mL) and processed as described in panel A. (B) As in panel A, except membranes were probed for phospho-p38. (C) As in panel A, except membranes were probed for phosphoJNK. All immunoblots are representative of 4 to 6 independent experiments.
TSP-1 and CD36-binding peptides induce activatory signaling in platelets. (A) (i) Platelets (3 × 108/mL) were incubated with either TSP-1 (10 μg/mL) or CD36 binding peptide (10μM) for 1-15 minutes. After lysis, platelet proteins were separated by SDS-PAGE and immunoblotted for phosphoSrc-Tyr418, followed by stripping and reprobing for β-tubulin. (ii) Platelets (3 × 108/mL) were treated with TSP-1 (10 μg/mL) in the presence of either PP1 (20μM), FA6-152 (1 μg/mL), or IgG control (1 μg/mL) and processed as described in panel A. (B) As in panel A, except membranes were probed for phospho-p38. (C) As in panel A, except membranes were probed for phosphoJNK. All immunoblots are representative of 4 to 6 independent experiments.
The role of Src kinases in modulating cAMP signaling
To determine which of these signaling molecules were involved in the crosstalk with cAMP signaling events, we used a series of kinase inhibitors. In the first instance, we examined their roles in the regulation of cAMP levels by TSP-1. Strikingly, the Src tyrosine kinase inhibitor, PP1 (20μM), completely inhibited the ability of TSP-1 to affect PGE1-stimulated cAMP accrual. In these experiments, PGE1 treatment increased cAMP to 1334 ± 240, which remained at 590 ± 50 fmol/1 × 107 platelets (P < .05, compared with PGE1 alone) in the presence of TSP-1 (10 μg/mL). However, when the platelets were preincubated with PP1 (20μM) before the addition of TSP-1, the PGE1 stimulated increase in cAMP remained elevated at 1360 ± 390 fmol/1 × 107 platelets (Figure 7A). Almost identical results were produced when CD36 peptide (10 μg/mL) was substituted for TSP-1 (Figure 7A). In both cases PP3, the inactive analog of PP1 did not affect cAMP formation. The p38 inhibitor, SB202190 (10μM), or JNK inhibitor 2 (10μM) had no effect on PGE1-induced cAMP formation either in the presence or absence of TSP-1 (not shown). Fibrinogen used as a control for TSP-1 also failed to influence PGE1-stimulated cAMP formation (Figure 7A).
Src kinases mediate TSP-1 inhibition of AC and PKA activity. (A) Platelets (2 × 108/mL), incubated with either PP1 or PP3 (20μM), were preincubated with TSP-1 (10 μg/mL) or CD36 peptide (10μM) for 15 minutes followed by treatment with PGE1 (0.1μM) for 30 seconds. cAMP levels were measured as per manufacturer's instructions and are expressed as fmol cAMP/1 × 107 platelets. Data are presented as mean ± SEM of 4 individual experiments. §P < .05 for cAMP levels induced by PGE1 alone compared with the presence of TSP-1 or CD36 peptide. *P < .05 for cAMP levels induced by PGE1 in the presence TSP-1 or CD36 peptide, where platelets had been pretreated with PP1. (B) Platelets (3 × 108/mL), incubated with either PP1 (20μM), PP3 (20μM), JNK peptide inhibitor 1 (10μM), or SB202190 (10μM), were preincubated with TSP-1 (10 μg/mL) for 15 minutes followed by treatment with PGE1 (0.1μM) for 1 minute. Samples were then lysed and phosphorVASPser157 was analyzed. (C) As in panel B, but the CD36 stimulatory peptide (10μM) was used in the place of TSP-1. All immunoblots are representative of 4 independent experiments.
Src kinases mediate TSP-1 inhibition of AC and PKA activity. (A) Platelets (2 × 108/mL), incubated with either PP1 or PP3 (20μM), were preincubated with TSP-1 (10 μg/mL) or CD36 peptide (10μM) for 15 minutes followed by treatment with PGE1 (0.1μM) for 30 seconds. cAMP levels were measured as per manufacturer's instructions and are expressed as fmol cAMP/1 × 107 platelets. Data are presented as mean ± SEM of 4 individual experiments. §P < .05 for cAMP levels induced by PGE1 alone compared with the presence of TSP-1 or CD36 peptide. *P < .05 for cAMP levels induced by PGE1 in the presence TSP-1 or CD36 peptide, where platelets had been pretreated with PP1. (B) Platelets (3 × 108/mL), incubated with either PP1 (20μM), PP3 (20μM), JNK peptide inhibitor 1 (10μM), or SB202190 (10μM), were preincubated with TSP-1 (10 μg/mL) for 15 minutes followed by treatment with PGE1 (0.1μM) for 1 minute. Samples were then lysed and phosphorVASPser157 was analyzed. (C) As in panel B, but the CD36 stimulatory peptide (10μM) was used in the place of TSP-1. All immunoblots are representative of 4 independent experiments.
We next examined the downstream effects of these kinase inhibitors on TSP-1 modulation of the cAMP-PKA signaling cascade through measurement of phosphoVASP-ser157. Consistent with experiments measuring cAMP concentrations, we found that inhibition of Src kinases with PP1 blocked the ability of TSP-1 to modulate PKA activity in response to PGE1. TSP-1 prevents PGE1-stimulated increases in phospho-VASP, but its ability to impede phosphorylation was lost in the presence of PP1 (Figure 7B). In contrast, the ability of TSP-1 to prevent VASP phosphorylation was maintained in the presence of either SB202190 or JNK inhibitor 2. To confirm that these events were restricted to CD36, we then repeated the experiments using the CD36 peptide and found that similar to TSP-1, its ability to moderate VASP phosphorylation was blocked by PP1, but maintained in the presence of SB202190 or JNK inhibitor 1 (Figure 7C). Thus, these data indicate that TSP-1 negatively regulates cAMP-dependent signaling through a CD36 and tyrosine kinase-dependent mechanism.
Discussion
The cAMP-PKA signaling cascade regulates several platelet responses including Ca2+ mobilization,16,27,28 shape change,29 and secretion,30 which are associated with reduced agonist-induced platelet aggregation31 and the accumulation of platelets at sites of vascular injury.32 Platelet activation by several agonists involves suppression of this pathway through activation of Gαi leading to inhibition of AC.33-35 Here, we present evidence of a potentially new mechanism by which platelets regulate cAMP-dependent signaling. Our data indicate that the platelet α-granule protein TSP-1 reduces platelet sensitivity to the cAMP/PKA signaling cascade through a tyrosine kinase-dependent mechanism that results in increased platelet aggregation. While the AC agonist PGE1 inhibited platelet aggregation, its actions were blunted by the presence of physiologic concentrations of TSP-1 (Figure 1A-B).4 Importantly, TSP-1 also prevented the inhibitory effects of PGE1 on platelet adhesion under flow, indicating that TSP-1 promotes platelet adhesion in addition to aggregation (Figure 2 and supplemental Video 2). Since we found that TSP-1 alone did not induce aggregation or increase platelet arrest under flow, our data suggested that this protein-enhanced platelet aggregation by modulating the inhibitory effects of the cAMP/PKA signaling cascade. Consistent with this, TSP-1 blocked the ability of PGE1 to increase cAMP levels (Figure 3A) and using the phosphorylation of the cytoskeletal protein VASP on Ser157, an established target for PKA,18 we demonstrate that TSP-1–mediated platelet cAMP reduction results in diminished activity of PKA. These effects were maintained for more than 60 minutes, indicating a long-term inhibition of cAMP signaling.
The concentrations of cAMP in the cell at any one time represent a balance between synthesis through AC and degradation by PDE3A.17 To explore which of these 2 targets TSP-1 signaling was directed at, we used the PDE3A inhibitor milrinone. PGE1-stimulated cAMP concentrations were increased by the presence of the inhibitor confirming the role of PDE3A in controlling platelet cAMP levels. However, under conditions that blocked the hydrolytic activity of PDE3A, TSP-1 failed to affect cAMP levels in response to PGE1 (Figure 3F), suggesting that TSP-1 diminishes PKA activation primarily by triggering cAMP degradation without affecting AC activity. These data are consistent with recent report of platelet agonists such as thrombin and U44619 activating PDE3A in platelets.36,37 The mechanism by which TSP-1 signaling activates PDE3A in platelets requires further study.
Interestingly, TSP-1 also prevented PGE1-mediated inhibition of aggregation and VASP-ser157 phosphorylation when the nonhydrolyzable cAMP-analog 8-bromo cAMP was used to activate PKA, indicating that TSP-1 may also modulate cAMP activation of PKA (Figure 5). PKA is found in both particulate and soluble platelet fractions, leading to spatial phosphorylation of several protein targets.20 The ability of TSP-1 to broadly reduce PKA substrate phosphorylation (Figure 3D) is consistent with TSP-1 reducing availability of cAMP rather than targeting individual PKA-substrate interactions. Thus, our data suggests that TSP-1 primarily modulates cAMP-PKA signaling by regulating cellular cAMP concentrations, leading to diminished PKA activity.
Several structurally and functionally distinct receptors for TSP-1 have been reported on platelets including integrins αvβ3 and αIIbβ3, CD36, and integrin-associated protein (IAP or CD47).5,7,38 In order to characterize which receptor was responsible for the effect of TSP-1 on cAMP signaling, we used a multipronged approach. Firstly, we demonstrated that the ability of TSP-1 to block both PGE1-induced cAMP synthesis and VASP phosphorylation was maintained in the presence of apyrase, indomethacin, EGTA, and RGDS. Therefore, TSP-1 does not require secondary mediators ADP and TxA2 or integrin-mediated signaling to exert its effects of cAMP/PKA pathway. However, CD36 and CD47 remained 2 potential candidates, particularly since elegant studies by Isenberg et al had demonstrated that these receptors could modulate the cGMP-PKG pathway in response to TSP-1.13 We found that the established CD36 blocking antibody FA6-152, but not an IgG control, abolished the ability of TSP-1 to modulate PGE1 induced phosphorylation of VASP (Figure 4A). Unfortunately, these antibodies interfered with the cAMP assay preventing the examination of CD36 blockade on cAMP levels. As an alternative approach, we used a peptide based on the CD36 binding sequence in TSP-1 protein.13 This peptide, when used under the same conditions as TSP-1, reduced PGE1-induced cAMP accrual leading to diminished PKA activity (Figure 4B). Using a similar approach we also tested a peptide based on a C-terminal domain sequence of TSP-1, known as 7N3 (FIRVVMYEGKK) that interacts with CD47 and modulates the activity of soluble guanylyl cyclase.14 Under our experimental conditions, the CD47 peptide, 7N3, had no effects on cAMP levels or PKA activity (Figure 4C). Previously, 4NIK1, a CD47 binding peptide, but not the full TSP-1 protein, has been reported to reduce platelet cAMP levels through a CD47-mediated interaction with Gαi proteins.39 However, we have been unable to demonstrate an effect of CD47 binding peptide on either basal or PGE1-stimulated cAMP levels (not shown) or PKA activity (Figure 4C). The reasons for this are unclear, but could indicate that CD36 and CD47 regulate cAMP-PKA signaling by separate pathways. Our current data, along with that of Isenberg and colleagues,13 suggest that TSP-1 has the capacity to regulate both major cyclic nucleotide pathways in platelets. However, there is a divergence in the mechanism by which TSP-1 induces its effects since regulation of cAMP-PKA signaling occurs exclusively by CD36, while the modulation of cGMP-PKG pathway requires CD36 and CD47.
In endothelial cells, TSP-1 initiates the recruitment of Src kinase Fyn to CD36, leading to caspase3-dependent activation of p38MAPkinase.24 Since our data indicated the central importance of CD36 to the regulation of cAMP/PKA signaling, we investigated the activation of signaling enzymes downstream of CD36 engagement in platelets. For the first time we demonstrate that TSP-1 induces phosphorylation of Src family kinases, p38 MAPkinase, and JNK, but not ERK in platelets. These data are consistent with the activation of Src family kinases bound to CD36 in response to both thrombin26 and oxLDL,25 and indicate that Src kinases play a key role in transducing signals after CD36 ligation. Our data provide a new understanding of the potential role of Src kinases associated with CD36, as we show that they may facilitate a novel crosstalk with the cAMP-PKA pathway. In the presence of a pan Src family kinase inhibitor, the ability of both TSP-1 and the CD36 peptide to modulate PGE1-stimulated increases in cAMP were abolished, indicating firstly that TSP-1–mediated effects were Src kinase–dependent and secondly that they lay downstream of CD36 (Figure 7A). The inability of TSP-1 to modulate cAMP levels under conditions of Src kinase inhibition was also reflected by a recovery of PKA activity under these conditions (Figure 7B-C). In contrast, inhibition of p38MAPkinse or JNK had no effect on either cAMP levels or PKA activation. Interestingly, our experiments with TSP-1 demonstrate that JNK lies downstream of Src family kinases, since JNK phosphorylation was blocked by PP1, which is consistent with other studies demonstrating that oxLDL activates a CD36-Src family kinase-JNK pathway in platelets.25,40 However, our data suggests a divergence in the mechanisms through which Src kinases regulate cAMP/PKA and JNK. It is possible that activation of Src family kinases downstream of CD36 ligation could therefore reduce platelet sensitivity to inhibition by the cAMP/PKA pathway but also increase platelet activation through phosphorylation of JNK.25
The roles of both TSP-1 and CD36 in platelet biology have remained unclear, but our current data describe a novel role for TSP-1 in the modulating platelet sensitivity to cAMP/PKA that could represent an entirely undefined key hemostatic mechanism, which ensures platelet activation at sites of vascular damage. It is possible that under physiologic conditions the release of TSP-1 upon platelet activation blunts inhibitory cyclic nucleotide pathways, both cAMP- and cGMP-mediated, which in turn reduces the threshold for platelet activation, facilitating the rapid capture of platelets at sites of vascular injury. Indeed, the augmented surface expression of CD36 after platelet activation by thrombin may facilitate this process by increasing the availability of TSP-1 binding sites.41 It is possible that plasma and secreted TSP-1, in concert with ADP, could act cooperatively to prevent cAMP-mediated inhibition of platelets through inhibition of cAMP signaling and synthesis. However, since CD36 is a promiscuous ligand, this pathway could potentially have pathophysiologic consequences. We show for the first time that TSP-1 stimulates CD36-mediated signaling events in platelets without inducing aggregation, which is consistent with recent studies demonstrating CD36-dependent stimulation of MAPKinases by oxLDL, without causing platelet aggregation.25,40 Together, these data suggest CD36-mediated signaling in plays a secondary rather than primary role in platelet function. Based on our data, it is possible that previous studies highlighting the activatory and potentiating effects of CD36 ligands such as TSP-derived peptides,7,8 oxLDL,24 and VLDL25,42 on platelet function could be due, at least in part, to their ability to modulate cAMP-PKA signaling events.
In conclusion we present new data to suggest that TSP-1 plays a novel and entirely undefined role in platelet function that involves modulating platelet sensitivity to cAMP. The ligation of CD36 by TSP-1 leads to the activation of a Src family kinase-dependent pathway that modulates cAMP generation and subsequent PKA signaling resulting in increased platelet aggregation and arrest under flow. The potential pathophysiologic importance of this pathway in mediating platelet hyperactivity found in numerous disease states43 requires investigation, but inhibition of CD36 may represent a novel strategy in controlling unwanted platelet activation.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgment
This study was funded by a grant from The British Heart Foundation.
Authorship
Contribution: W.R. performed experiments and analyzed and interpreted data; S.M. performed experiments and analyzed data; A.A. performed experiments; and K.M.N. designed research, analyzed and interpreted data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Khalid M. Naseem, Daisy Research Laboratories, Castle Hill Hospital, Castle Road, Cottingham HU16 1DP, United Kingdom; e-mail: khalid.naseem@hyms.ac.uk.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal