Abstract
Abstract 1143
Microparticles (MP) exhibit procoagulant activity by exposure of tissue factor (TF) and consecutive factor (F) × activation via binding to activated FVII (FVIIa). We have shown that TF-negative MP derived from endothelial cells (EMP) after exposure to cisplatin induce thrombin generation in vitro in a TF-independent manner (Lechner et al., J. Thromb. Haemost. 2007). The procoagulant properties of TF-negative MP are not well characterized. We therefore aimed to investigate the mechanisms of coagulation activation by TF-negative EMP (obtained from human pulmonary microvascular endothelial cells - HMVEC-L) in comparison to TF-abundant MP derived from a cancer cell line (A431-MP).
MP were obtained by ultracentrifugation (100,000 × g for 1 h) of cell culture supernatant. The procoagulant activity was measured in normal plasma and in plasmas deficient in coagulation factors VII, VIII, IX, X, XI, and XII, respectively, by an in vitro thrombin generation assay (Technothrombin TGA, Technoclone, Austria), by activated partial thromboplastin (aPTT), prothrombin time (PT) and a chromogenic FIX activation assay. Antibodies to TF and FVII (both American Diagnostica, USA) were used. Plasmas used in the thrombin generation assay were MP-depleted by ultracentrifugation before use.
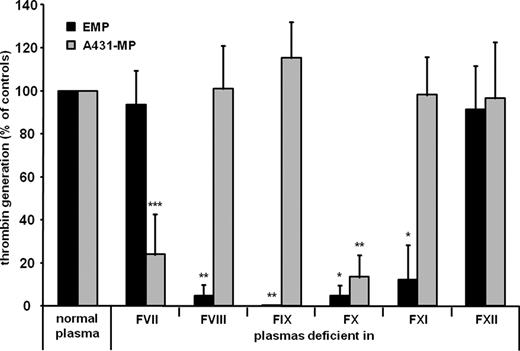
EMP and A431-MP induced in vitro thrombin generation in normal plasma. In vitro thrombin generation induced by EMP was abolished in plasmas deficient in FVIII or FIX, and was markedly reduced in FXI-deficient plasma. In FVII-deficient plasma, normal thrombin generation after addition of EMP was seen. In contrast, A431-MP-triggered thrombin generation was abolished in FVII-deficient plasma, but was not influenced by depletion of FVIII, FIX or FXI. In FX-deficient plasma, thrombin generation could not be triggered by addition of either EMP or A431-MP. Thrombin generation was normal after addition of EMP or A431-MP to FXII-deficient plasma (Figure). In a modified aPTT system using kaolin as surface activator, EMP and A431-MP induced clot formation in normal plasma. In a modified PT system only A431-MP but not EMP induced clot formation. We then investigated whether MP can directly activate FIX. In a plasma-free environment FIX activation by A431-MP was much more pronounced than by EMP (14-fold vs. 2.4 fold increase compared to a control experiment with cell culture medium only). FIX activation by A431-MP was blocked by the addition of antibodies to TF and FVII, while no such effect was seen after addition to EMP.
Our findings show that both TF-positive and TF-negative MP exhibit procoagulant activity. If TF is expressed (A431-MP), coagulation activation is triggered via FVII/FVIIa, which is abolished in FVII-deficient plasma or by the addition of antibodies to TF or FVII. TF-negative EMP induce thrombin generation via the intrinsic coagulation pathway by activating FXI and FIX, as shown by the generation of activated FIX in a FIX activation assay. EMP are also able to induce clot formation in an aPTT based system. We surmise that the procoagulatory effect of EMP may be due to their high phospholipid content or to a specific phospholipid surface composition. The results of our FIX activation assay also confirm a direct FIX activation by TF/FVIIa, which can be abolished by addition of antibodies to TF or FVII.
Comparison of thrombin generation induced by two different microparticle species: endothelial microparticles (EMP; black columns) versus microparticles derived from a cancer cell line (A431-MP; gray columns). Thrombin generation was measured in plasmas deficient in coagulation factors (F) VII, VIII, IX, X, XI, or XII. Values are expressed as percentage of thrombin amounts measured in normal plasma (*P < 0.05, **P < 0.01, and ***P < 0.001).
Comparison of thrombin generation induced by two different microparticle species: endothelial microparticles (EMP; black columns) versus microparticles derived from a cancer cell line (A431-MP; gray columns). Thrombin generation was measured in plasmas deficient in coagulation factors (F) VII, VIII, IX, X, XI, or XII. Values are expressed as percentage of thrombin amounts measured in normal plasma (*P < 0.05, **P < 0.01, and ***P < 0.001).
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal