Abstract
Abstract 1269
Influenza is a potentially serious infection after hematopoietic stem cell transplantation (HSCT). Prolonged immunosuppression leads to impaired immunity to infectious agents that contributes to the poor outcome after HSCT. Vaccination is the main prophylactic approach in individuals at an increased risk for severe influenza disease, but it is less effective in immunocompromised patients. Nevertheless, annual vaccination against influenza is recommended for HSCT recipients, starting at 6 months after transplant. In 2009, due to the emergence of a pandemic influenza A (H1N1)v virus, the development of safe and effective vaccines was a public health priority. Some oil-in-water-emulsion adjuvants were used in some 2009 influenza A (H1N1) vaccines to increase their immunogenicity. In France, the use of 2 doses of such vaccines was recommended for HSCT recipients.
This study, conducted by the Société de Greffe de Moelle et de Thérapie Cellulaire, has evaluated the safety and the efficacy of an adjuvanted monovalent influenza A (H1N1)v vaccine in allogeneic HSCT recipients. Patients between the age of 18 and 65 years who were vaccinated from 3 months to 5 years post-transplant were included in the study. Patients in relapse of their hematological disease or receiving immunoglobulins were excluded. Patients were separated into two groups: patients with graft-versus-host-disease (GVHD) treated by immunosuppressive drugs (G1) and those without GVHD or immunosuppressive therapy (G2). Antibody responses were measured by means of a hemagglutination-inhibition assay on days 0, 21 and 42 after injection of the first dose of vaccine.
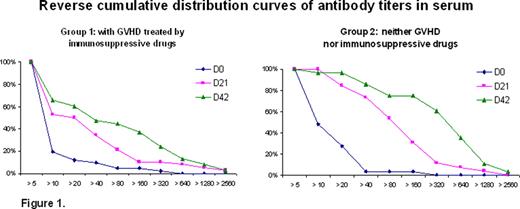
Seventy nine patients were included and 70 who received the 2 doses of adjuvanted vaccine (at day 0 and 21) were analyzed: 41 in G1, 29 in G2. Median patient age was 53 yr (range 21–65). Median interval between transplant and vaccination was11 months in G1 and 21months in G2. Fifty five % of patients received stem cells from peripheral blood and 44% received a myeloablative conditioning regimen. No severe post-vaccination side effect was observed, except in 2 patients who presented with an aggravation of their GVHD. No case of influenza A (H1N1)v infection was observed. At day 21 after the first dose, antibody titers, expressed as geometric means (GMT), were 20 and 69 in G1 and G2, respectively, whereas, they were 43 and 223, respectively, at day 42 after the second dose. By day 21, antibody titers of 1:40 or more were observed in 34% of patients in G1 and 73% in G2. By day 42, after the second dose of vaccine, antibody titers of 1:40 or more were observed in 47 % of patients in G1 and 86% in G2. The reverse cumulative distribution curves of antibody titers in serum at D0, D21 and D42 are shown in Figure 1.
These data show that adjuvanted vaccine is safe in recipients of allogeneic HSCT. The humoral response was improved by the second dose of vaccine. The use of 2 doses of adjuvanted vaccine allows a seroprotection in almost all recipients without GVHD and immunosuppressive therapy and in half of the patients with GVHD and treated by immunosuppressive drugs. These data also suggest that addition of an adjuvant to improve the efficacy of a vaccine offers an advantage in recipients of HSCT.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal