Abstract
Abstract 1325
While some studies associated an increased relapse rate and poorer progression-free survival with lower donor T-cell chimerism after reduced-intensity conditioning (RIC) allogeneic hematopoietic stem cell transplantation (HSCT); early total donor cell chimerism, despite its use as a clinical trial endpoint, is not established as a predictor of survival. In part this may reflect its non-uniform impact across hematologic malignancies. Clarifying the survival impact of early donor chimerism could help identify patients who may benefit from additional interventions; and also define its value as a disease-specific endpoint.
We undertook a retrospective analysis of adult patients undergoing 8/8 HLA-matched RIC HSCT from 2002–2008 with available day 30 total donor cell chimerism (range, 20–50 days; PB or marrow). All received fludarabine/busulfan-based conditioning with sirolimus- and/or tacrolimus-based GVHD prophylaxis. Umbilical cord blood transplants; ex-/in-vivo T-cell depletion (e.g. ATG); or malignant relapse/progression at or prior to day 30 chimerism were excluded. 414 patients with a median age of 58 years (range, 41–69) and median follow-up among survivors of 34 months (range, 6–80) were analyzed. Patient characteristics: 211 myeloid (116 AML, 63 MDS, 20 CML, 12 MPD); 203 lymphoid (70 NHL, 66 CLL, 39 HD, 21 MM, 6 ALL); 343 (83%) with high-risk disease; 265 (64%) were males; 404 (98%) received peripheral blood stem cells; 234 (57%) had matched-unrelated donors; and 132 (32%) had prior transplants.
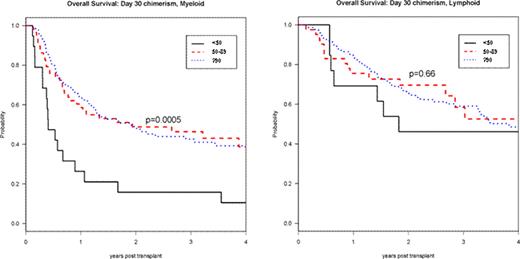
Day 30 total donor cell chimerism was <50% in 32 patients (8%); 50–89% in 99 patients (24%); and ≥90% in 283 patients (68%). Factors predictive of lower day 30 chimerism were high-risk disease (p<0.01), no prior transplant (p<0.01), male sex (p=0.02) and myeloid diagnosis (p=0.03). In univariable analyses patients with lower day 30 chimerism had worse 3-year progression-free and overall survival (PFS, OS) (p<0.0001; p=0.003). The cumulative incidence of chronic GVHD correlated with the degree of day 30 chimerism (p=0.049). Stratified by diagnosis, lower day 30 chimerism was associated with poorer 3-year PFS and OS in myeloid (p<0.0001; p<0.001) but not in lymphoid disorders (p=0.09; p=0.66) (Figure ). For both myeloid and lymphoid disorders, lower day 30 chimerism was associated with increased incidence of relapse (p<0.0001; p=0.02) but not of non-relapse mortality (p=0.24; p=0.41).
In multivariable Cox-models, day 30 chimerism <50% was associated with lower PFS and OS (HR 3.13, 95% CI 2.04–5, p<0.0001; HR 2.27, 95% CI 1.41–3.57, p<0.001). For myeloid disorders, day 30 chimerism <50% was associated with poorer PFS and OS (HR 6.25, 95% CI 3.23–11.11, p<0.0001; HR 3.33, 95% CI 1.82–6.25, p=0.0001). For lymphoid disorders, day 30 chimerism <50% was associated with poorer PFS (HR 2.13, 95% CI 1.09–4.17, p=0.03) but not OS (HR 1.44, 95% CI 0.65–3.23, p=0.36), that may reflect in-part longer survival after lymphoid compared to myeloid relapse. However, there may also be disease-specific differences. For instance, low day 30 chimerism was highly associated with poorer PFS and OS in AML/MDS (HR 4.54, 95% CI 2.13–10, p<0.0001; HR 3.33, 95% CI 1.67–10, p=0.001); had a trend of PFS impairment in CLL/NHL (HR 2.17, 95% CI 0.97–5, p=0.06); but had no impact in HD or myeloma.
Day 30 Total Donor Chimerism and RIC HSCT Overall Survival: by Diagnosis
No relevant conflicts of interest to declare.
This icon denotes an abstract that is clinically relevant.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal