Abstract
Abstract 1344
In September 2007 we published the results of a prospective randomized trial comparing in 100 AL amyloidosis patients, enrolled between January 2000 and January 2005, high dose melphalan with ASCT and the oral regimen M-Dex (melphalan 10 mg per square meter of body-surface area and dexamethasone 40 mg per day, on days 1 to 4). With a median follow-up of 3 years the median survival was better in the M-Dex arm (56.9 months) than in the ASCT arm (22.2) months (p=0.04). The hematological responses were not statistically different between the 2 arms and the higher toxicity of the ASCT arm was responsible for the shorter median survival.
This study has been criticised because of the high treatment related mortality (TRM) in the ASCT arm but a landmark analysis of patients who survived for at least 6 months and who received their assigned treatment, did not show any difference in survival. A second frequent criticism was that too severe patients, who were not able to go through the high dose procedure, have been included. A separate analysis done within the 59 good risk patients showed a nonsignificant difference between the two groups in overall survival at 3 years (58% in the group assigned to receive ASCT vs. 80% in the group assigned to receive M-Dex; P = 0.13).
A third concern was related to the duration of response, should high dose treatment, giving slightly more complete responses, results in more sustained responses and, with a prolonged follow-up, in a better long term survival ?
To answer this question we extended the follow-up of the surviving patients. The new cut off date was August 1st, 2010, more than 5 and a half years after the last inclusion. Only 1 patient has been lost to follow-up. We did again the landmark analysis with the longer follow-up and we looked, in this population of 65 patients with 100% feasibility and 0 % TRM, at survival and remission duration. As the follow-up was very long and the biologic surveillance not planned after 2006 we took unequivocal events as censor points for the event-free survival analysis: deaths and second line treatment.
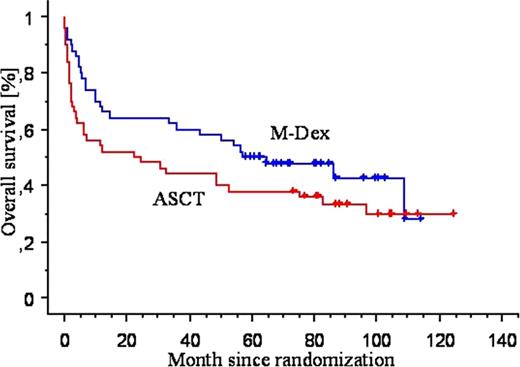
At the first cutoff date, in 2006, 49 patients were alive, 30 in the M-Dex arm and 19 in the HDT arm. At the new cutoff date in 2010, 38 patients are alive, 22 in the M-Dex arm and 16 in the intensive arm, with a median follow-up of 49 months for the entire cohort and 86 months for surviving patients (figure 1). The majority of late deaths were amyloid related, but 3 patients in the M-Dex arm died of unrelated lung and digestive cancer.
The median survival in the 2 arms has not been modified (56.9 month in the M-Dex arm and 22.2 month in ASCT arm, p=0.15). For the 65 patients included in the landmark analysis the median survival is not different in the 2 arms (103 month in the M-Dex arm and 97 month in ASCT arm) and the median event free survival is 56 months in the M-Dex arm and 26 months in the intensive arm (p=0.3, figure 2). Eleven surviving patients in the M-Dex arm and 6 in the intensive have not received a second treatment, 9 of these patients in the M-Dex arm and 5 in the ASCT arm have normal free light chain measurement at their last visit. Only 1 patient, assigned to receive ASCT, has been diagnosed with myelodysplasia.
Event-Free Survival According to Treatment Group in the Landmark Analysis
Leblond:roche: Consultancy, Membership on an entity's Board of Directors or advisory committees; mundipharma: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; genzyme: Membership on an entity's Board of Directors or advisory committees; celgene: Membership on an entity's Board of Directors or advisory committees; jansen: Membership on an entity's Board of Directors or advisory committees. Leleu:Celgene: Consultancy, Research Funding; Janssen Cilag: Consultancy, Research Funding; Leo Pharma: Consultancy; Amgen: Consultancy; Chugai: Research Funding; Roche: Consultancy, Research Funding; Novartis: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal