Abstract
Abstract 1357
High dose chemotherapy (HDT) with autologous stem cell transplantation (ASCT) is historically effective treatment for patients with relapsed follicular lymphoma (FL) but relapse is common possibly due to contaminated stem cell grafts or inadequate eradication of primary disease. The addition of immunotherapy to HDT/ASCT may augment “in vivo” graft purging and achieve more effective eradication of minimal residual disease (MRD) post ASCT. This may lead to improved PFS and OS.
We conducted 3 sequential prospective phase 2 trials of HDT/ASCT combined with immunotherapy in patients with relapsed FL from 1997–2010. Protocol 1 (n=13) used a-interferon 3 MU/m2 SC TIW for 2 years post ASCT. Protocol 2 (n=23) used a single infusion of rituximab (R) 375 mg/m2 for ‘in vivo’ purging 3–5 days pre-stem cell collection and 2 sets of 4 weekly R at 2 and 6 months post ASCT respectively. Protocol 3 (n=32) used 3 infusions of R 375 mg/m2 pre stem cell collection, followed by a-interferon 3 MU/m2 SC TIW × 2 years + R 375mg/m2 × 6 weekly infusions post ASCT. Eligibility included adult patients age 18–65 with stage III or IV follicular lymphoma grades 1–3a in first or second relapse. Salvage chemotherapy was either CHOP or DHAP depending on prior anthracycline exposure. High dose therapy was cyclophosphamide, carmustine, and etoposide (CBV). Minimal residual disease (MRD) assessment of t(14; 18) by quantitative PCR was serially performed in blood, PBSC and marrow. Studies were REB approved and all patients gave informed consent.
Sixty-eight patients from the 3 trials are included. Median age at study enrolment was 47 (30-71) and patients were a median of 31 mo (9-197) from diagnosis. 54% were male. Median FLIPI score was 2. Median # of prior chemotherapies was 1. Median number of cycles of salvage chemotherapy was 4 (2 - 10). 63 patients proceeded to ASCT. 5 patients were not transplanted either do to disease progression (2), or failed stem cell mobilization (3). Baseline characteristics of the patients enrolled in the three trials were similar, with the exception of rituximab(R) exposure in 22% of the patients in Protocol 3. 4 (6%) patients were lost to follow up.
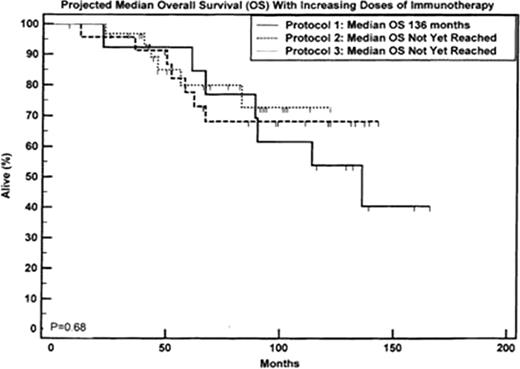
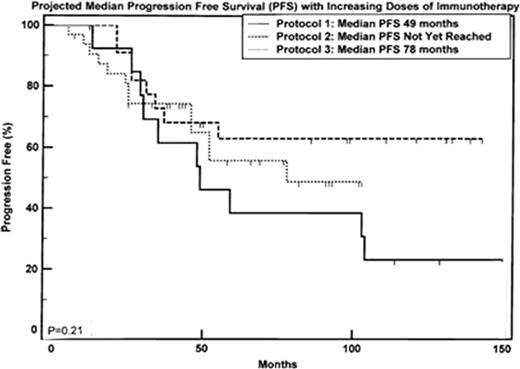
Median time to death or last follow was 84.5 mo (7-166). 32 patients (47%) relapsed and 20 (29%) have died. Median follow up times for each trial were 116, 99 and 57 mo. Actuarial median OS for Protocol 1 was 136 mo from enrolment and has not yet been reached for Protocols 2 and 3. Actuarial median PFS for Protocols 1 and 3 are 49 and 78 mo respectively and has not yet been reached for Protocol 2.
MRD assessment by PCR was collected on 48 patients. Compared with study 1, the increased use of R pre SC collection improved in vivo purging by 2 logs. In Protocols 1 and 2, most still had detectable disease in the graft to a sensitivity of 1 cell/100,000. In Protocol 3, 56% had MRD negative apheresis products. 91% of 46 evaluable patients achieved molecular remission post stem cell transplant.
Thirteen patients (19%) developed secondary malignancies post ASCT with a median time to malignancy of 88.5 mo (39-144). 1 patient developed 2 distinct malignancies. Secondary malignancies present were: MDS (4), AML (1), ALL (1), basal cell carcinoma (2), squamous cell carcinoma (3), thyroid cancer (1), non small cell lung cancer (1) and adenocarcinoma of unknown origin (1). Five (7%) patients transformed to DLBCL, with a median time to transformation post ASCT of 43.5 mo (17-142).
The majority of patients who received rituximab immunotherapy pre and post ASCT developed persistent hypogammaglobulinemia. 17 patients (26%) developed interstitial pneumonitis or >2 respiratory complications post rituximab. 4 patients (6%) require monthly IVIG to treat recurrent respiratory infections. 10 patients (15%) developed herpes zoster <90 days post ASCT.
15% and 30% of patients in Protocols 1 and 3 did not complete the 2 years of alpha-IFN post ASCT secondary to one or more of depression, fatigue or cytopenias.
HDT + ASCT combined with R +/− a-IFN produces durable PFS and molecular remissions but is associated with prolonged hypogammaglobulinemia and higher rates of interstitial pneumonitis. Three infusions of R pre SC collection achieve higher rates of molecular remission. Compliance with 2 years of a-IFN added to R post ASCT is poor and may not add clinical benefits to R alone. The additional role of HDT/ASCT in the era of R-chemotherapy for FL needs to be explored.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal