Abstract
Novel influenza A (2009/H1N1) is a new influenza virus of swine origin. The epidemiology, morbidity, and mortality are different from those of seasonal influenza viruses, with this new strain affecting younger patients. We describe the risk factors and outcome associated with 2009/H1N1 Influenza A (herein H1N1) infection in patients with hematological malignancies.
Among the first 69 cases of H1N1 infections in cancer patients occurring between April 17 to October 21, 2009, 54 (78%) had hematological malignancies (leukemia or lymphoma) and 22 (41%) had undergone hematopoietic stem cell transplantation. H1N1 strain was the only influenza A virus reported in Texas during the study period.
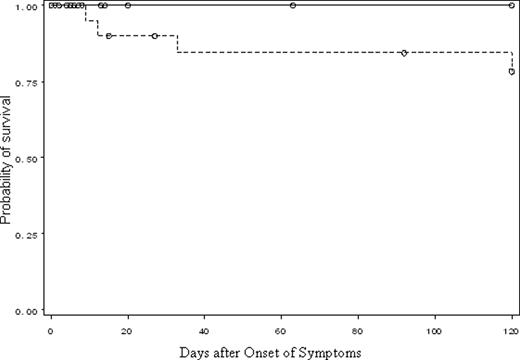
Most of the patients with H1N1 and hematological malignancy were old (50% were 50 years of age or older), moderately to severely immunosuppressed (67%) and obese (39%), High rates of hospitalization (48%), pneumonia (24%), and death (11%) were encountered in our patients. The majority of patients who developed pneumonia due to H1N1 were on immunosuppressive therapy at the time of diagnosis (11 (85%) of 13 cases with pneumonia vs. 20 (49%) of 41 with upper respiratory infection [URI], p=0.02). Delay in initiation of oseltamivir from onset of symptoms was the only significant predictor of progression to pneumonia (7 days in patients with pneumonia vs. 1 day in patients with URI, p=0.002) and of mortality (9 days in patients who died vs. 1 day in those who survived, p=0.003). The development of H1N1 pneumonia was significantly associated with higher mortality (p<0.001). Starting antiviral therapy within 72 hours of onset of symptoms was significantly associated with better survival (p=0.05, Figure 1) and with a trend towards less progression to pneumonia (p=0.07).
Novel influenza A (2009/H1N1) infection affected older, obese, and moderately to severely immunosuppressed patients with hematologic malignancies. Early initiation of antiviral therapy may impact outcome with less progression to pneumonia and may improve survival in this immunosuppressed patient population.
Kaplan-Meier Estimates of Survival of Hematological Malignancy Patients Infected with Influenza A (2009/H1N1) Virus who Received Antiviral Therapy ≤ 72 Hours after Onset of Symptoms Compared to those who Received it > 72 Hours Later (p=0.05 from a Log-Rank Test)
Kaplan-Meier Estimates of Survival of Hematological Malignancy Patients Infected with Influenza A (2009/H1N1) Virus who Received Antiviral Therapy ≤ 72 Hours after Onset of Symptoms Compared to those who Received it > 72 Hours Later (p=0.05 from a Log-Rank Test)
———— Patients who received antiviral therapy ≤ 72 hours after onset of symptoms
——–—- Patients who received antiviral therapy >72 hours after onset of symptoms
○ ○ ○ ○ ○ ○ Censored observations
No relevant conflicts of interest to declare.
This icon denotes an abstract that is clinically relevant.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal