Abstract
Abstract 1804
Advanced stage follicular lymphoma (FL) cannot be cured by current conventional therapies. Treatment with idiotype cancer vaccines has thus far been disappointing, and is expensive and labor intensive. Hence, simpler principles for vaccinations that do not require production of patient-specific tumor antigens are warranted. We designed a novel strategy involving intratumoral injections of low-dose anti-CD20 antibody (Rituximab) and autologous dendritic cells (DC) in irradiated lymph nodes. The aim was to facilitate antigen up-take and presentation by DCs to T cells to achieve systemic anti-tumor responses in patients with FL.
Ten untreated patients with stage III/IV follicular lymphoma not in need of conventional therapy underwent standard work-up, including CT-scans, FDG PET/CT and bone marrow samples. Single tumor cells were frozen for later immunoassays. Apheresis was performed for isolation of monocytes subsequently cultured with IL-4 and GM-CSF for 5 days to generate immature dendritic cells (DC). The first 6 patients received a single 8 Gy dose of radiotherapy against an enlarged node on day 1, followed by intratumoral injections of DCs (1 × 108) and GM-CSF (50 μg) on days 3 and 4. The treatment was repeated against a different enlarged node after 6 weeks. Thereafter, the protocol was changed, and patients received intratumoral injections of a small dose of rituximab (5 mg) on days 1 and 3 in order to enhance ADCC, radiotherapy (8 Gy) on day 2 and intratumoral injections of DCs and GM-CSF sc on days 4 and 5. This therapy was then repeated after 2 and 4 weeks, targeting other lymph nodes. Follow-up included repeated PET/CT-scans, bone marrow sampling and preparation of peripheral blood mononuclear cells (PBMC) for later immune studies. Tumor-specific helper and cytotoxic T cell responses were monitored by flow cytometry after a 5-day co-culture of single tumor cells with autologous PBMC sampled before, and 2 and 4 months after, treatment. Proliferation was studied by incorporation of CFSE, whereas degranulation (CD107a/b) and interferon gamma release was measured following re-stimulation with tumor cells for 5h.
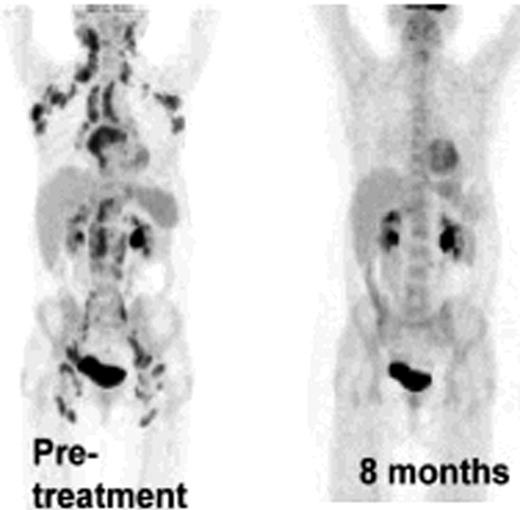
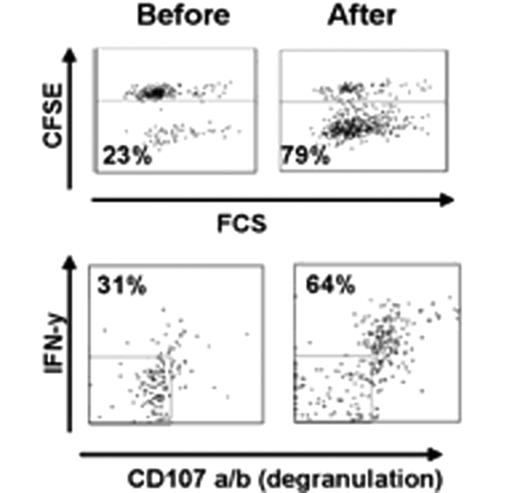
Ten patients with FL of a total of 20 planned for this study were treated so far. No adverse events were observed. Among the first 6 patients treated by the original strategy, two showed a systemic metabolic response by PET/CT and one of these had a good response by regular CT not qualifying for PR. The protocol was then modified as described above. The first patient treated by the new strategy that included intratumoral rituximab, had a systemic metabolic response by PET/CT after 2 and 4 months and was PET negative with a normal CT by 8 months (Figure 1). Another patient showed a systemic PET response at 2 and 4 months. Analysis of T cell responses against autologous tumor cells has been initiated and we have so far demonstrated a vigorous CD8 dominated T cell response, as assayed by proliferation, degranulation and cytokine production, in the patient who achieved a negative PET-scan at 8 months (Figure 2). In parallel with the metabolic response, the immune response became stronger during follow-up after treatment. One patient without clinical response was also analysed, and no immune response was demonstrated. Further analysis of immune responses in the other patients is on-going.
The present strategy is a novel principle to generate T cell responses and clinical anti-tumor responses detected by PET/CT in FL.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal