Abstract
Abstract 1892
Primary or light chain amyloidosis (AL) is a clonal plasma cell disorder characterized by a relatively low plasma cell burden and multi-organ deposition of immunoglobulin light chain derived amyloid fibrils. The survival of patients with amyloidosis is quite variable with median survival of 12–18 months in different series, and largely dependent on the number and severity of organs involved. In addition to new drugs, the combination of melphalan and dexamethasone is an effective regimen for AL and risk adapted approaches to SCT has decreased treatment related mortality. It is not clear if the recent progress in risk stratification and treatment approaches have translated into improved survival for these patients.
We studied two separate cohorts of patients; the first cohort consisting of 2118 patients with AL seen at Mayo Clinic over a 40-year period between November 1967 and August 2006 was used to examine the trends in overall survival from diagnosis during this time period. The second cohort of 443 patients seen between September 2006 and August 2009 were examined only for the changes in early mortality and for validation of a model for predicting early mortality developed using the first cohort of patients.
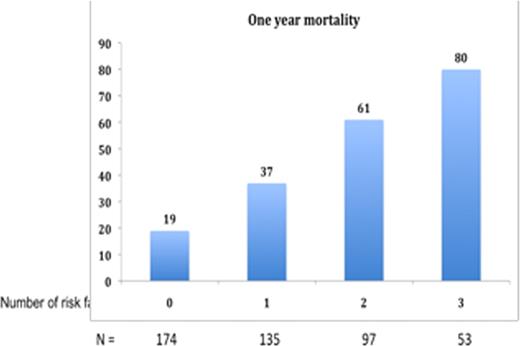
The estimated median follow up for the entire group was 7.8 years (95% CI; 7.1, 8.5). The patients were divided into 4 cohorts based on date of diagnosis; 1966–1976 (n=121), 1977–1986 (n=343), 1987–1996 (n=636) and 1997–2006 (n=1017). The median OS from diagnosis for the four cohorts were 0.9, 1.2, 1.2 and 1.5 years respectively, P < 0.001 (Figure 1 ). More importantly there was steady improvement in the long-term survival among these patients with 4-year survival estimates of 16%, 21%, 24% and 33% respectively. Next, we specifically looked at the survival trends within the last cohort (1996-2006), dividing the patients into three time periods; 1996–1999 (n=263), 2000–2003 (n=291) and 2003–2006 (n=463). While the OS from diagnosis was relatively unchanged between the first and second time periods (28 vs. 30% 4 year survival), there was significant improvement in the last three year period (42% survival at 4 years); P =0.02. Given the high early mortality in this disease, we specifically examined the one-year mortality among the entire patient cohort and found no clear improvement in terms of one-year mortality. Using logistic regression analysis, we identified cTnT > 0.01, NT-ProBNP > 4200 and Uric Acid > 8.0 mg/dL to be the best predictors of death within one year of diagnosis. The probability of death within one year for those with none, 1, 2, and 3 risk factors was 19, 38, 61, and 78% respectively, P < 0.01 (Figure 2 ). This model was further validated among patients seen during the most recent three years, September 2006 and August 2009.
Survival in AL amyloidosis has improved over time, particularly in the recent years and likely reflects the recent improvements in therapy. However, early mortality remains a problem and baseline factors can be used to identify patients at risk for early mortality, who then can be targeted for risk adapted therapy approaches in clinical trials.
Kumar:Celgene: Consultancy, Research Funding; Millennium: Research Funding; Merck: Consultancy, Research Funding; Novartis: Research Funding; Genzyme: Consultancy, Research Funding; Cephalon: Research Funding. Off Label Use: Lenalidomide for treatment of newly diagnosed myeloma. Dispenzieri:Celgene: Honoraria, Research Funding; Binding Site: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal