Abstract
Abstract 2717
LR11 (also called SorLA or SORL1) is a type I membrane protein, from which a large extracellular part, soluble LR11 (sLR11), is released by proteolytic shedding. LR11 plays a key role in the migration of undifferentiated vascular smooth muscle cells, and circulating sLR11 is known to be a biomarker of carotid intima-media thickness. Along with the fact that circulating sLR11 levels represent the accumulation of vascular immature cells, human CD34+CD38− immature hematopoietic precursors have been reported to express high levels of LR11 mRNA. We have recently found that LR11 is specifically and highly expressed on cell surface of acute leukemia cells in addition to normal leukocytes (unpublished data). These facts prompted us to evaluate the serum sLR11 level in patients with acute leukemia and other hematological malignancies to validate sLR11 as a novel circulating marker for treatment outcome and prognosis.
Serum sLR11 levels were measured by ELISA method in 139 patients with acute leukemia and other hematological malignancies treated at a single institution from 1999 to 2010. Patients' laboratory data and treatment outcome were collected retrospectively in 43 acute myeloid leukemia (AML) and 23 acute lymphoblastic leukemia (ALL) patients.
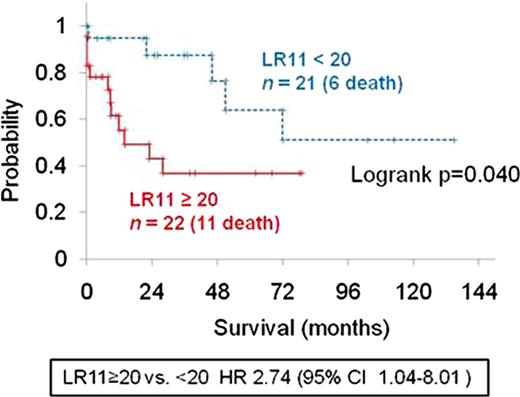
sLR11 levels of acute leukemia patients were significantly increased [ALL, 73.5±93.5 ng mld−1 (range, 5.7–407.0), P<0.0001; AML, 26.8±29.1 ng ml−1 (range, 5.0–157.5), P<0.0001] in comparison to the control subjects (9.2±3.3 ng ml−1), while sLR11 levels in patients with chronic myeloid leukemia (17.9±11.1 ng ml−1), chronic lymphocytic leukemia (12.7±11.6 ng ml−1), multiple myeloma (10.5±4.8 ng ml−1), and POEMS syndrome (9.0±2.7 ng ml−1) were not significantly different from controls. sLR11 levels were significantly higher in ALL than those in other leukemias. Paired sample analysis of patients with AML and ALL at complete remission (CR) after chemotherapy showed significantly decreased sLR11 levels compared to the time of diagnosis (AML: 30.9±37.5 ng ml−1 vs. 10.4±4.3 ng ml−1, P=0.015, ALL: 39.1±126.0 ng ml−1 vs. 11.2±5.0 ng ml−1, P=0.0029). The multiple stepwise liner regression analysis showed that the peripheral blast proportion in both ALL and AML patients were independently associated with sLR11 at diagnosis (AML: r2= 0.21, P=0.0026, ALL: r2= 0.34, P=0.0043). Among 42 AML patients, sLR11 levels of subjects in the highest tertile of peripheral blast proportion (>67.5% of WBC) were 2.44- and 3.05-fold higher than those in the middle (23.0-64.0% of WBC) and lowest tertiles (<20.0% of WBC), respectively. Twenty out of 21 AML patients with <20 ng ml−1 sLR11 at diagnosis achieved CR after induction chemotherapy, and the CR rate was significantly higher in patients with <20 ng ml−1 sLR11 than in patients with ≥20 ng ml−1 (95.2% vs 65.5%, P=0.02). The probability of overall 5-year survival was significantly lower in AML patients with ≥20 ng ml−1 sLR11 at diagnosis than in those with <20 ng ml−1 [Figure1, 36.8% vs 63.7%, P = 0.04; hazard ratio (HR): 2.74; 95% confidence interval (CI): 1.04–8.01].
Serum sLR11 levels in patients with acute leukemia were significantly elevated and were associated with the peripheral blast population but not in other chronic proliferative hematological malignancies. These findings suggest that the serum sLR11 levels are predictive for pathogenic properties of immature blasts, including their migration and attachment activities, rather than simply associating with proliferating cell numbers. Especially in AML patients, serum sLR11 levels at diagnosis significantly affect CR rate and OS. Although larger scale studies including karyotype or FAB classification would be required for its patho-clinical significance, serum sLR11 is a promising novel biomarker for acute leukemia and it could play an important role as prognostic factor.
Kaplan-Meier plots of overall survival of AML patients
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal