Abstract
Abstract 3027
Bortezomib (Velcade®) is effective and well tolerated in patients with relapsed/refractory multiple myeloma (MM). Although it is often administered in combination with dexamethasone (VelDex) in the relapsed setting, there are little data on VelDex in this patient population. The international, non-interventional, Electronic Velcade Observational Study (eVOBS) is an ongoing observational study to assess the clinical and health economic outcomes in MM patients treated with bortezomib in the clinical-practice setting. Here, we compared response and long-term outcomes in relapsed/refractory MM patients who received bortezomib monotherapy or VelDex throughout the treatment period.
Adult patients scheduled to receive bortezomib for relapsed/refractory MM were eligible for inclusion in eVOBS; patients from Belgium, France, Greece, Spain, Sweden, Turkey, and Brazil were included in this analysis. All bortezomib doses and concomitant treatments (except investigational therapies) were permitted; dose adjustments and cycle delays were documented. Patients are being followed for up to 3 years from their last cycle of bortezomib to document long-term outcomes. Due to the non-interventional nature of the study, no predefined response criteria were mandated; response criteria were investigator-defined and were based on European Group for Blood and Marrow Transplantation (EBMT), Southwest Oncology Group (SWOG), or M-protein criteria. Patients who received bortezomib monotherapy or VelDex throughout the treatment period were included in this analysis. Endpoints included response, progression-free survival (PFS), time to progression (TTP), and overall survival (OS). Outcomes were analyzed using Kaplan-Meier methodology; unadjusted analyses and adjusted analyses, accounting for differences in baseline characteristics, were performed. Adjusted analyses were performed using propensity score-based inverse probability weighting (IPW), matched cohorts and multivariate proportional hazards regression. IPW results are shown; similar results were obtained for all three adjusted analyses.
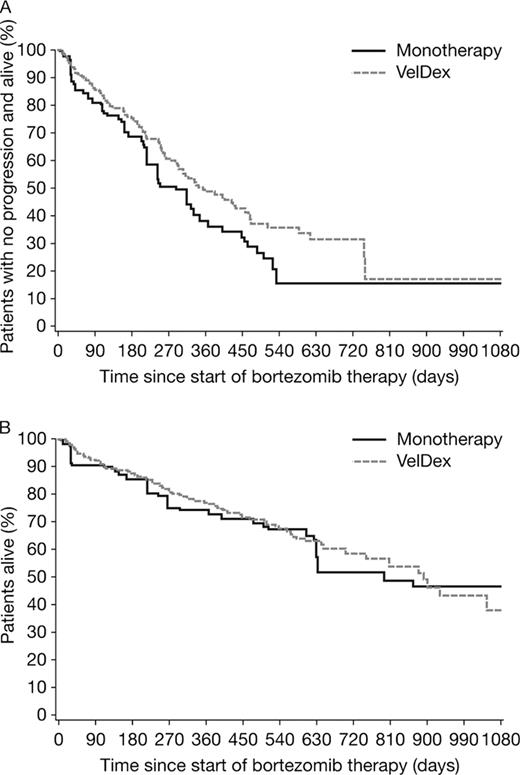
A total of 432 patients were included; 106 received bortezomib monotherapy and 326 received VelDex throughout the treatment period. Baseline characteristics were similar between the two groups, with some differences in age, time since diagnosis, and gender: monotherapy patients tended to be older (mean 67.1 vs 62.9 years, p=0.0003) and have a longer disease history (mean 3.1 vs 2.5 years since diagnosis, p=0.049), and the VelDex arm included more male patients (61.7% vs 50.9%, p=0.051). The overall response rate (ORR; at least partial response [PR]; best response) was 72.7% in the VelDex group and 68.4% in the monotherapy group. The complete response (CR) rate was 24.1% and 8.2% in the two groups, respectively. Median follow-up was 11.5 and 8.6 months for the VelDex and monotherapy groups, respectively. As shown by Kaplan-Meier analysis of time to CR, the median time to CR was approximately 3.2 months and approximately 3.6 months for the VelDex and monotherapy groups (unadjusted: HR=3.51, p=0.0071; IPW adjusted: HR=2.42, p=0.0272); at 3 months, approximately 8% and 3% of patients in the VelDex and monotherapy groups had achieved CR, respectively. There was a trend to prolonged PFS with VelDex but the differences were not significant (unadjusted: median PFS was approximately 345 vs 330 days, HR=0.80, p=0.2195; IPW adjusted: median PFS was approximately 345 vs 270 days, HR=0.73; p=0.0758) (Figure). A similar trend was seen in TTP (unadjusted: HR=0.82, p=0.3261; IPW adjusted: HR=0.82; p=0.3393). There was no apparent difference in OS between the two groups (unadjusted: HR=1.15, p=0.5342; IPW adjusted: HR=0.93; p=0.7127) (Figure).
The addition of dexamethasone to bortezomib monotherapy appears to increase the CR rate in relapsed/refractory MM patients. Consistent with the higher CR rate, addition of dexamethasone was associated with trends to improved PFS and TTP, but the differences were not significant in both unadjusted and adjusted analyses. Although more data from a larger patient population may be needed to confirm these results, the addition of dexamethasone to bortezomib did not appear to increase OS in these relapsed/refractory patients.
Progression-free (A) and overall survival (B) by IPW adjusted Kaplan-Meier analysis
Progression-free (A) and overall survival (B) by IPW adjusted Kaplan-Meier analysis
Dimopoulos:Millennium: Consultancy; Ortho-Biotech: Consultancy, Honoraria. De Samblanx:Millennium Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees. Hulin:Janssen-Cilag: Honoraria; Celgene: Honoraria; Amgen: Honoraria. De La Rubia:Janssen-Cilag: Consultancy, Honoraria; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees. Ganguly:Johnson & Johnson: Employment, Equity Ownership. Diels:Johnson & Johnson: Employment, Equity Ownership. van de Velde:Johnson & Johnson: Employment, Equity Ownership. Dhawan:Johnson & Johnson: Employment, Equity Ownership. Spencer:Janssen-Cilag: Employment. Delforge:Celgene: Consultancy, Honoraria, Speakers Bureau; Ortho-Biotech: Consultancy, Honoraria, Speakers Bureau; Novartis: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal