Abstract
Abstract 3247
Ph+ALL is a rare subgroup of pediatric ALL with very high risk of treatment failure and reported long-term overall survival (OS) of only 30–40%. Standard treatment includes allogeneic SCT in first complete remission (CR1). Imatinib has been lately incorporated to first-line treatment regimens. In a recent study with pediatric Ph+ALL patients, imatinib given continuously at a dose of 340 mg/m2/day in combination with chemotherapy resulted in a significant improvement in early outcome (Schultz KR et al, J Clin Oncol 2009).
to describe the Spanish Cooperative Group SHOP results in Ph+ALL with imatinib given continuously at an intermediate dose in combination with intensive chemotherapy and compare the toxicities and outcome of patients treated in the imatinib cohort -SHOP 05- with those treated without imatinib -SHOP 94 and SHOP 99- (historical controls).
children with Ph+ALL aged 1–18 years treated in 39 institutions in Spain were enrolled in three consecutive SHOP-ALL trials (SHOP 94/SHOP 99/SHOP 05) from February-1994 to April-2010. Imatinib, at a dose of 260 mg/m2/d was added to an intensive chemotherapy regimen since day-15 of induction treatment in SHOP 05. Allogeneic SCT from matched sibling donor (MSD) or matched unrelated donor (MUD) was scheduled in CR1 in all trials. Toxicities were graded following WHO criteria. Survival analysis was determined by the Kaplan-Meier estimate.
a total of 47 patients out of 1436 pediatric ALL were enrolled during the study period (SHOP 94: 8, SHOP 99: 23, SHOP 05: 16). Four patients enrolled in SHOP 99 trial were excluded from the analysis for protocol violation (imatinib treatment in CR1 in a pre-imatinib protocol). Among 43 evaluable patients, 16 were treated with continuous intermediate-dose imatinib as per SHOP 05 protocol whereas 27 received a similar chemotherapy backbone treatment without imatinib. There were 22 boys (51.2%) and median age was 6.8 years, range 1.2–15. Median WBC (109/L) was 41, range 2.8–481.2. Three patients (7.3%) had CNS involvement at diagnosis. Main clinical characteristics at presentation were comparable among the three trials.
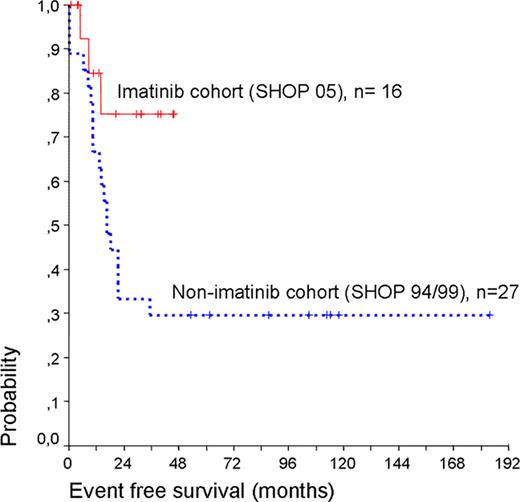
All patients in the imatinib cohort (SHOP 05) and 24 out of 27 patients in the non-imatinib cohort (SHOP 94/99) achieved CR1 after induction treatment, with 3 refractory patients in the non-imatinib cohort. Imatinib in combination with intensive chemotherapy was in general well tolerated. There were no induction deaths in our series. One patient in the imatinib group died in CR1 due to an infection prior to SCT. There were more grade III-IV transaminase (ALT) elevations in consolidation in patients receiving imatinib (7 out of 16) than in those not receiving it (2 out of 19). Hepatic failure or bilirrubin elevation greater than grade I were not observed in these cases and ALT elevations were transient without imatinib discontinuation or dose-adjustment. Hematological toxicities were similar in patients treated with or without imatinib, and no unexpected treatment delay was observed. No pleural effusions were reported. Thirty patients underwent an allogeneic SCT in first CR, 17 out of 27 (63%) patients in trials SHOP 94/99 (non-imatinib cohort) and 13 out of 16 (81%) patients in trial SHOP 05 (imatinib cohort). Median time to SCT was 6.4 months (range 3–14.8). Transplant related mortality was 19.5%. Ten patients relapsed (non imatinib cohort: 9, imatinib cohort: 1), at a median time of 17.6 months (range 8.3–35.2). Median follow-up of the trials SHOP 94/99 (pre-imatinib) and SHOP 05 (imatinib) was 113 and 30 months respectively. Event-free survival (EFS) at 30 months was 33.3% (+/−9.1%) and 75.2 % (+/−12.6) respectively (p = 0.032) and overall survival (OS) at 30 months was 38.46% (+/−9.5%) and 84.6 (+/−10%) respectively (p=0.029).
Intermediate dose of imatinib (260 mg/m2/day) given concomitantly with chemotherapy and followed by allogeneic SCT (MSD or MUD) had an acceptable toxicity profile and markedly improved early EFS and OS in pediatric Ph+ALL. Longer follow-up is needed to assess the effect of imatinib on long-term EFS.
Off Label Use: Imatinib is a tyrosine-kinase inhibitor approved for the treatment of chronic myeloid leukemia. Its use in acute lymphoblastic leukemia Philadelphia positive has not been approved yet although preliminar results show promising results.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal