Abstract
Abstract 3287
Dinaciclib is a potent and selective inhibitor of the CDKs 1, 2, 5, and 9 that has demonstrated anti-tumor activity against both myeloid and lymphoid leukemia cell lines in vitro and human tumor xenografts in vivo.
A randomized, multicenter, open-label phase 2 study of dinaciclib 50 mg/m2 administered by 2-hour i.v. infusion once every 21 days was initiated with the goal of assessing its efficacy and safety in patients (pts) with advanced acute myeloid (AML, ≥60 years old) or lymphoid (ALL, ≥18 years old) leukemia. AML pts were randomized between dinaciclib and gemtuzumab ozogamicin (GO) with cross-over to dinaciclib if no response to GO, while ALL pts only received dinaciclib. Intra-patient dose escalation of dinaciclib to 70 mg/m2 in cycle 2 was allowed. Twenty-six pts were treated on study (20 AML, 6 ALL). Data on 14 AML (2 cross-over from GO) and 6 ALL pts treated with dinaciclib are presented. Their median age was 70 (range 38–76) years and 70% were male. Sixteen pts were refractory and 4 pts had relapsed after a median of one (range 1–4) chemotherapy regimens. Four AML pts had complex karyotypes (≥3 abnormalities), 2 monosomy 7, 2 trisomy 8, 1 der (1:7)(q10;p10), 1 trisomy 21, 1 deletion 9q, and 3 had normal karyotype. Two ALL pts had t(9;22).
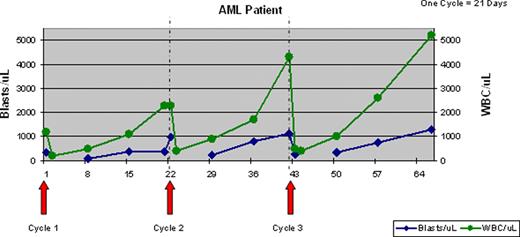
Response: Anti-leukemia activity was observed in 60% of pts. Ten of 13 pts with circulating blasts (7/7 AML and 3/6 ALL) had >50% and 6 pts (4 AML, 2 ALL) >80% decrease in the absolute blast count (ABC) within 24 hours of the first dinaciclib dose. An additional pt had a 29% decrease in ABC. The median pre-treatment ABC was 1085 (range 220–9975) and the median ABC nadir was 169 (range 0–1350). The median duration of blast nadir was 6 days (range 2–23). A representative graph from an AML patient (below) shows a rapid decrease of circulating blasts and WBC after treatment, followed by a gradual recovery. Two patients had >50% reduction of marrow blasts (35% on d1 to 17% on d 42 in an AML pt; 81% on d1 to 27% on d 21 in an ALL pt). However, no objective responses by International Working Group criteria were observed. The median number of treatment cycles was 1 (range 1–5), with 10 pts receiving more than one cycle of treatment. Eight pts were treated with dinaciclib 70 mg/m2 starting in cycle 2.
Toxicity: Treatment related AE's occurring in >30% of pts included diarrhea, nausea, vomiting, anemia, elevated AST, fatigue, leukopenia, hypocalcemia, and hypotension. The most common CTCAE v3 treatment-related grade 3 and 4 toxicities, occurring in 3 or more pts, were anemia, leukopenia, febrile neutropenia, thrombocytopenia, fatigue, increased AST, and tumor lysis syndrome (TLS). Laboratory evidence of tumor lysis in cycle 1, using the Cairo-Bishop criteria, was seen in 6 pts in addition to 3 pts with clinical TLS (JCO 2008;26:2767). Hyperacute TLS requiring hemodialysis occurred in one pt with AML, who died of acute renal failure. Subsequently, all pts were aggressively managed to prevent and treat TLS (hospitalization, hydration, allopurinol, rasburicase, oral phosphate binder administration, and early management of hyperkalemia). An additional 9 pts died on study, 8 pts from leukemia progression and 1 pt from intracranial bleed due to disease-related thrombocytopenia.
Pharmacodynamics: Pre-treatment, 4 and 24 hrs post end-of-infusion samples of circulating leukemic blasts were obtained from 1 AML and 3 ALL pts. By Western blot, post-treatment decrease in Mcl-1 and increase in PARP cleavage were seen in all 4 pts at 4 hrs post-treatment, confirming that in vivo inhibition of CDKs was achieved, but recovery of Mcl-1 at 24 hrs was observed in all 4 pts, suggesting that inhibition was lost at 24 hrs. Decline in p-Rb was observed in 1 pt, while 2 pts had almost undetectable p-Rb levels at baseline.
Dinaciclib showed anti-leukemia activity in this heavily pre-treated patient population. TLS was a notable toxicity, but was manageable in most pts with aggressive prophylaxis, monitoring and treatment. Early blast recovery and short duration of nadir observed on this study, combined with PK data showing a short t1/2 (1.5-3.3 hours) for dinaciclib and PD data demonstrating rapid reexpression of Mcl-1, support either use of longer infusion schedules (currently explored in solid tumors) or more frequent drug administration. Further exploration of dinaciclib dose and schedules in AML and ALL is planned.
Gojo:Merck & Co.: Research Funding. Off Label Use: SCH 727965 (dinaciclib) is an investigational drug. Padmanabhan:Schering-Plough: Consultancy; Merck & Co.: Research Funding. Small:Merck & Co.: Employment, Equity Ownership. Zhang:Merck & Co.: Employment. Sadowska:Merck & Co.: Research Funding. Bannerji:Merck & Co.: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal