Abstract
Abstract 349
Failure to collect enough CD34+ cells following poor hematopoietic progenitor cell (HPC) mobilization is generally thought to be due to marrow damage following previous therapies. However, marrow damage alone cannot account for all poor mobilizations since there are donors who have good marrow cellularity but fail to adequately mobilize. In addition, the relationship between CD34+ cell dose and engraftment rate is not linear, suggesting other factors play a role during engraftment. Previous animal studies suggest CD8+ T cells are important for CD34+ cell homing to the marrow. We hypothesized that CD8+ T cells have dual roles by inhibiting CD34+ cell mobilization and facilitating engraftment.
To test our hypothesis, we followed 192 autologous HPC donors to assess the role of CD8+ T cells in HPC mobilization and engraftment. The median donor age was 58.5 years (range 18 – 76), and 110 of the 192 patients were males. Donors were diagnosed with plasma cell disease, primarily multiple myeloma, or non-Hodgkin's lymphoma. CD34+ and CD8+ T cell content in the patients' apheresis products were enumerated using flow cytometry, quantitative RT-PCR (qPCR) and immunohistochemistry. The median CD34+ cell/kg was 4.7 × 106.
Patients were categorized as low, moderate or high mobilizers according to their total CD34+ cell collection quartile distribution: <3.7, 3.8 – 5.5 and ≥5.6 × 106/Kg respectively. We found that HPC products from low mobilizers contained significantly more CD8+ T cells (median 1.25 × 108/Kg) than HPC products from moderate and high mobilizers (Table 1 p = 0.04). Therefore, the ratio of CD8+ T cells to CD34+ cells was 4 – 8 folds higher in low mobilizers than in moderate and high mobilizers. Moreover, CD8 antigen expression measured using qPCR, normalized with 18s ribosomal RNA, was significantly higher in low mobilizers than in moderate or high mobilizers. Similar analysis with CD4+ T cells did not demonstrate significant correlation with mobilization.
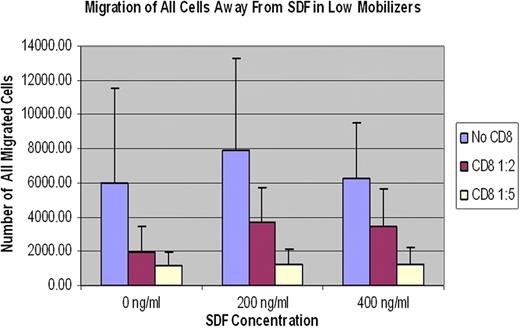
To assess the role of CD8+ T cells in HPC mobilization, we purified CD8+ T and CD34+ cells from both low and moderate or high mobilizers. Using chemotaxis assay, we assessed the effect of CD8+ T cells on CD34+ cell migration away from high stromal cell derived factor (SDF1-a) concentrations. Purified CD34+ cells were plated in bone marrow epithelial cell coated trans-wells and co-cultured with purified CD8+ T cells at 1:2 and 1:5 ratios in the presence of SDF-1a at 0, 200 and 400ng/ml concentrations. Cells that migrated through the micro-pores of the trans-well into the lower wells were enumerated and analyzed. Our studies showed CD8+ T cells inhibit migration of CD34+ cells in-vitro independent of SDF1-a concentration. (Figure 1) The inhibitory properties of CD8+ T cells were more pronounced on CD34+ cells from low mobilizers than on CD34+ cells from moderate or high mobilizers.
We also assessed if CD8+ cell content was associated with days to neutrophil engraftment. Our analysis showed the higher the CD8+ T cell content per unit CD34+ cell, the faster the rate of neutrophil engraftment.
In summary, HPC products from low mobilizers contained significantly higher numbers of CD8+ T cells. CD8+ T cells, independent of SDF1-a concentration, significantly inhibited migration of purified CD34+ cells. In addition, CD8+ T cell content per unit CD34+ cell was inversely proportional to the time to neutrophil engraftment. These findings suggest CD8+ T cells may play dual roles by inhibiting CD34+ cell mobilization and facilitating its homing and engraftment.
Association of CD8+ T cell number with CD34+ cell mobilization capacity
| Mobilization Quartiles . | Low . | Moderate . | High . | p-value . |
|---|---|---|---|---|
| Median CD34+ Cell × 106/Kg | 3.03 | 4.55 | 6.73 | <0.001 |
| Median CD8+ T cell × 108/Kg | 1.25 | 0.50 | 0.33 | 0.04 |
| CD8+ T: CD34+ cell ratio | 41 | 11 | 5 |
| Mobilization Quartiles . | Low . | Moderate . | High . | p-value . |
|---|---|---|---|---|
| Median CD34+ Cell × 106/Kg | 3.03 | 4.55 | 6.73 | <0.001 |
| Median CD8+ T cell × 108/Kg | 1.25 | 0.50 | 0.33 | 0.04 |
| CD8+ T: CD34+ cell ratio | 41 | 11 | 5 |
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal