Abstract
Abstract 3996
AZA significantly improves OS in higher-risk MDS (including RAEB-t/AML) compared to conventional treatments (AZA 001 trial, Lancet Onc, 2009), but prognostic factors of response and OS to AZA remain largely unknown. We designed a prognostic score for OS in a cohort of AZA treated higher-risk MDS in a patient-named compassionate program (French ATU), and validated it in patients from the AZA 001 trial.
Between Sept 2004 and Jan 2009, prior to AZA approval in Europe, IPSS int-2/high risk MDS (including RAEB-t) not previously treated with intensive chemotherapy (IC), allo SCT, or a hypomethylating agent were included in a compassionate program (ATU), and received AZA (planned schedule 75 mg/m2/d ×7 d every 28 d for ≥4 cycles). Independent prognostic factors of OS were individualized in a Cox model. A prognostic score was then developed based on those factors. After validation of the score as a continuous variable, pts were grouped in three distinct risk categories. We subsequently tried to validate this score in the 175 higher risk MDS pts treated with AZA at the same schedule in AZA 001 trial (4 of the 179 pts randomized to AZA in that trial did not start AZA).
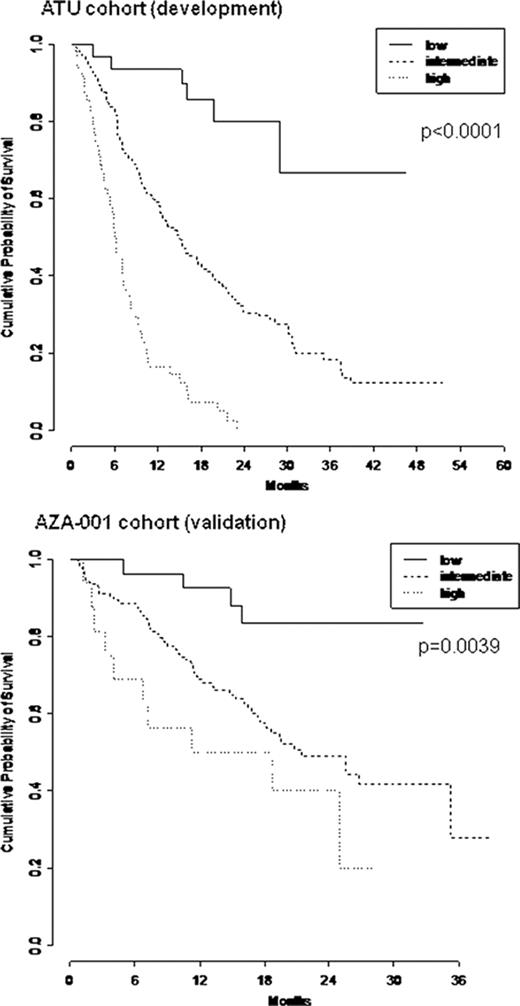
The ATU cohort included 282 pts with de novo (74%) or therapy related (t) (26%) higher-risk MDS (IPSS int-2 in 54% high in 43%, at least int-2 in 2%). ECOG PS ≥2, RBC transfusion dependence ≥4 units/8 weeks and circulating blasts were present in 21%, 46% and 46% of pts respectively (resp). Cytogenetic risk was good, int, and poor in 31%, 17% and 47% (unknown in 5%). 10% pts had previously been treated with LD araC for their MDS. Multivariate analysis of survival retained PS ≥2 (HR= 2.0 [95% CI: 1.4–2.9]), RBC transfusion dependence ≥4 units/8 weeks (HR=1.9 [1.4-2.6]), presence of circulating blasts (HR=2.0 [1.5-2.7]), and IPSS cytogenetic risk (intermediate: HR=1.4 [0.8-2.3], poor: HR=3.0 [2.0-4.3]) as independent prognostic factors (all p<10-4). We designed a simple prognostic score attributing 1 and 2 points for intermediate and poor risk cytogenetics, resp, and 1 point for each other adverse prognostic factor. As a continuous variable, an increasing score was associated to poorer OS (HR=1.9 [1.6-2.1], p<10-4). Patients were grouped as “low risk” (score=0), intermediate risk (score=1-3) and high risk (score=4-5). Due to missing values, a prognostic group could be attributed to 269 of the 282 pts: 30 (11%), 191 (71%), and 48 (18%) pts were considered low, intermediate and high risk resp. With a median follow-up of 26 months, median OS were not reached, 15.0 and 6.1 months in patients with low, intermediate, and high risk score respectively (p<10-4).
The validation cohort included 175 pts of the AZA-001 trial with higher-risk MDS. Apart from the presence of 5 (3%) int-1 pts in AZA-001 cohort (their removal did not affect conclusions), IPSS (int-2 in 42% high in 46%, at least int-2 in 9% vs 54%, 43%, 2% resp., p=0.15) was comparable to the ATU cohort, as well as RBC transfusion dependence ≥4 units/8 weeks (46 vs 45%, p=0.8), presence of circulating blasts (51 vs 45%, p=0.4), whereas all AZA-001 pts were de novo (vs 74% in the ATU cohort), had not been pretreated (vs 10% pretreated by LD AraC in the ATU cohort), had better PS (7% PS ≥2 vs 21% in the ATU cohort, p<10-4) and better cytogenetics (47%, 21% and 27% 5%, good, int, poor, unknown cytogenetics vs 31%, 17%, 47%, 5% resp., p<10-4). The score was validated as a continuous variable on OS (HR=1.4 [1.2-1.7], p=0.0005). Due to missing values, a prognostic group could be attributed to 166 (95%) of pts of the AZA 001 cohort: 27 (16%), 123 (74%), and 16 (10%) pts were classified in the low, intermediate and high risk respectively. Median follow-up was 21 months. Median OS were not reached, 21.4 and 14.3 months respectively in patients with low, intermediate, and high risk score (p=0.004).
Our compassionate cohort scoring system based on conventional prognostic factors (ECOG PS, RBC transfusion requirement, circulating blasts and karyotype) was prognostic for survival. Additionally, it was successfully validated in a more selected cohort of higher-risk MDS from a prospective multicenter trial. Prognostic factors of outcome with AZA based on more refined biological studies (including gene methylation status) will probably emerge. In particular, we found in a more recent patient cohort (Itzykson, abstract also submitted to ASH 2010), that TET2 mutations may predict better response to AZA in higher risk MDS.
Beach:Celgene: Employment. Fenaux:Celgene: Honoraria, Research Funding; Novartis: Honoraria, Research Funding; Janssen Cilag: Honoraria, Research Funding; ROCHE: Honoraria, Research Funding; AMGEN: Honoraria, Research Funding; GSK: Honoraria, Research Funding; Merck: Honoraria, Research Funding; Cephalon: Honoraria, Research Funding.

This icon denotes a clinically relevant abstract
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal