Abstract
Abstract 3998
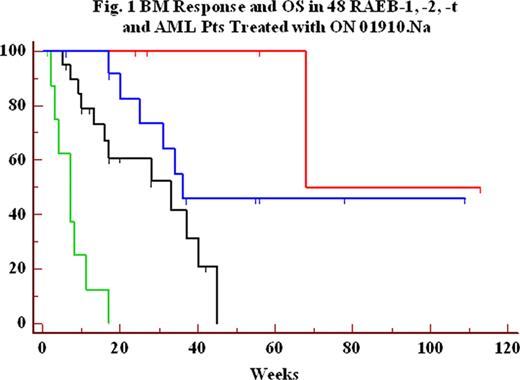
We analyzed bone marrow (BM) response and overall survival (OS) in 48 patients (pts) with a myelodysplastic syndrome (MDS) and WHO/FAB subtypes of refractory anemia with excess blasts (RAEB) -1,- 2 or -t and acute myeloid leukemia (AML), enrolled in 4 independent ongoing clinical trials of the novel small molecule ON 01910.Na. Pts received ON 01910.Na administered as a continuous intravenous infusion (CIV) from 2 to 6 days weekly or every other week with BM response initially assessed per protocol by week 4 or 8. A BM complete response (CR) (>50% decrease from baseline BM blast and decrease below 5% for at least 4 weeks, per MDS IWG 2006 criteria) or an initial 50% decrease of BM blasts by week 4 to 8 was documented in 19/48 (40%) treated pts and was associated with a significant increase in overall survival (OS) (p = 0.0001) by the method of Kaplan-Meier (Figure 1 and Table 1). This relationship was still significant when excluding AML patients (p = 0.008). Six pts (3 RAEB-1, 3 RAEB-2) had complete BM response. Five of these six pts previously failed to respond or relapsed after azacitidine/decitabine and five out of six are alive (17 to 113 weeks follow-up; one death at 68 weeks). Eleven pts (5 AML, 3 RAEB-t, 2 RAEB-2 and 1 RAEB-1) could not be assessed at 4–8 weeks with follow-up BM evaluation; their median OS was 7.5 weeks; 9 of these 11 pts previously failed to respond or relapsed after azacitidine/decitabine. Among 10 pts with trisomy 8 cytogenetics (4 had an initial BM response), median survival was 25 weeks. FAB/WHO classification of all 48 pts was also significantly correlated with survival (Table 2, p=0.003). Overall, ON 01910.Na infusions were well tolerated. Eight pts had hematological improvements at various time points after starting therapy. Median OS was 33 weeks in the subset of 29 RAEB-1,-2,-t pts refractory or relapsing after azacitidine/decitabine, and a significant association (p = 0.056) between BM response and OS was also found in these pts (Table 1). The median survival of MDS pts who had failed to respond to prior treatment with decitabine has been reported to be approximately 17 weeks (Jabbour et al, Cancer 2010, in press). These results suggest a strong correlation between BM blast response and OS and the predictive value of BM response to ON 01910.Na for estimating overall survival of higher risk MDS or AML pts.
Overall Survival of Pts Treated with ON 01910.Na by BM CR and Initial (4-8 Weeks) Blast Response
| 4–8 Week BM Blast Reduction (%) . | BM CR . | > 50% Initial Response . | < 50% Initial Response . | Not Assessed . | P value Logrank test . |
|---|---|---|---|---|---|
| Figure Legend | Red | Blue | Black | Green | |
| RAEB-1, RAEB-2, RAEB-t, & AML pts, N = 48 | 6 | 13 | 18 | 11 | |
| Median Survival (weeks) | Not reached | 36 | 28 | 7.5 | P = 0.0001 |
| RAEB-1, RAEB-2, RAEB-t pts relapsed/refractory to azacitidine/decitabine, N = 29 | 5 | 6 | 12 | 6 | |
| Median Survival (weeks) | 68 | Not reached | 33 | 3.5 | P = 0.056 |
| 4–8 Week BM Blast Reduction (%) . | BM CR . | > 50% Initial Response . | < 50% Initial Response . | Not Assessed . | P value Logrank test . |
|---|---|---|---|---|---|
| Figure Legend | Red | Blue | Black | Green | |
| RAEB-1, RAEB-2, RAEB-t, & AML pts, N = 48 | 6 | 13 | 18 | 11 | |
| Median Survival (weeks) | Not reached | 36 | 28 | 7.5 | P = 0.0001 |
| RAEB-1, RAEB-2, RAEB-t pts relapsed/refractory to azacitidine/decitabine, N = 29 | 5 | 6 | 12 | 6 | |
| Median Survival (weeks) | 68 | Not reached | 33 | 3.5 | P = 0.056 |
Overall Survival by FAB/WHO Classification of 48 RAEB and AML Pts Treated with ON 01910.Na
| FAB/WHO Classification . | RAEB-1 . | RAEB-2 . | RAEB-t . | AML (>30% BM blasts) . | P value Logrank test . |
|---|---|---|---|---|---|
| N pts | 11 | 18 | 10 | 9 | |
| Median Survival (weeks) | Not reached | 28 | 20 | 11 | P = 0.003 |
| FAB/WHO Classification . | RAEB-1 . | RAEB-2 . | RAEB-t . | AML (>30% BM blasts) . | P value Logrank test . |
|---|---|---|---|---|---|
| N pts | 11 | 18 | 10 | 9 | |
| Median Survival (weeks) | Not reached | 28 | 20 | 11 | P = 0.003 |
Silverman:Onconova Therapeutics Inc: Research Funding, Research support of Clinical Trial. Raza:Onconova Therapeutics Inc: Research Funding, Research support for clinical trial. Greenberg:Onconova funding of clinical trial: Research Funding, Research support from Onconova for performance of this trial. Wilhelm:Onconova Therapeutics Inc.: Employment, Equity Ownership.
This icon denotes a clinically relevant abstract
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal