Abstract
Abstract 4102
ET is an acquired clonal myeloproliferative neoplasm rarely transforming into AML or MDS. Such transformations have been reported to occur in patients treated with alkylating agents primarily. Recently, concern has arisen about the long term safety of the 2 mainstream cytoreductive therapies, i.e. hydroxyurea (HU) and anagrelide (AG), in terms of leukemogenesis and myelofibrosis. This large series of 17 cases of transformed ET aims to compare clinicopathologic features and outcomes of MDS or AML that developed in patients treated with HU or AG.
Cases of secondary MDS or AML evolved from ET from January 2000 to February 2010 were retrieved from institutional databases.
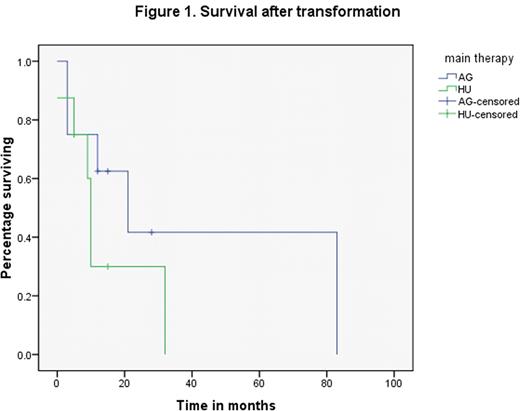
Eighteen patients with MDS or AML transformed from ET in our institute were evaluated. One patient who was previously treated with an alkylating agent was removed from the study. General characteristics and clinical outcomes of 17 cases are summarized in Table 1. Ten out of 17 patients were diagnosed with AML at the time of transformation while 7 were diagnosed as high grade MDS. Results showed that the median time to MDS/AML development in 17 cases was 87 months; the median survival from the time of ET diagnosis was 124 months and the median survival after transformation was 12 months. There were 16 patients that were mainly treated with long term HU or AG alone. One patient treated with a combination of HU and AG, was excluded from the comparative studies. All patients were founded to have moderate (3/16) to severe (13/16) reticulin fibrosis (2+ to 3+), regardless of treatment. Dyspoiesis including MDS or AML with myelodysplasia related changes (AML-MRC) were more commonly noted in patients treated with AG than in those with HU (6/8 vs. 2/8, p=0.046). The time to transformation was similar in the 2 groups: 76 months (AG) vs. 82 months (HU). The survival since ET diagnosis also was not significantly different, with median survival of 146 months in AG group vs. 96 months in HU group (p= 0.51). However, although not statistically significant (p=0.24), the AG group had a median survival after transformation of 21 months vs. 10 months in the HU group (Figure 1). Thirteen of 14 available karyotypes were abnormal. Complex cytogenetic abnormalities were found in 8/14 cases (5 in AG group vs. 3 in HU group). The common abnormalities, listed in the order of their frequencies, were: -20 or 20q- (5), 5q-(4), 17p- (2), -7 (2), 15q-(2),-18(2), +8(1).
There were no differences in degree of fibrosis or time to onset of secondary MDS and/or AML between HU and AG therapy in ET. There was a trend that the median survival after transformation was longer in AG group, which was thought to be related to a predominance of MDS at the time of transformation in AG group. We attributed the shorter median duration of survival from the time of ET diagnosis in our study, in comparison to the literature, to our focus on selection of transformed cases. Complex cytogenetic abnormalities with loss of chromosomal materials in 20, 5, 7, and 17, could play a critical role in disease transformation and progression independent of the specific drug use (HU or AG) in ET.
Clinical Features and Follow up in Patients with ET developing MDS or AML.
| ID . | age/sex . | main therapy for ET . | type of transformation . | degree of fibrosis (0-3+) . | time to MDS or AML(mo) . | treatment after transformation . | survival from event (mo) . | clinical status . | cytogenetics at MDS or AML . |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 56F | AG | MDS | 3 | 121 | Thalidomide | 3 | D-OD | 5q-, +9q, +13q, +6p, t(2;15), t(17;17), t(3;18) |
| 2 | 60F | AG | MDS | 3 | 96 | HA | 28 | A-WD | 20-,+22q, 19p-, |
| 3 | 64M | AG | MDS | 3 | 60 | HA | 83 | D-OD | 20q- |
| 4 | 34M | AG | MDS to AML-MRC | 2 | 322 | SCT | 3 | D-SCT | 20q- |
| 5 | 61F | AG | AML-MRC | 3 | 76 | Chemo | 15 | A-WD | t(3;9), +8, t(1;5) |
| 6 | 60F | AG | AML-MRC | 3 | 92 | HA | 12 | A-WD | 46,XX |
| 7 | 66F | AG | AML (M7) | 3 | 61 | Supportive care | 0 | D-OD | NA |
| 8 | 56M | AG | AML | 3 | 27 | Unknown | 21 | D-OD | NA |
| 9 | 68F | HU | MDS | 2 | 48 | HA | 10 | A-WD | 5q-, 13-, 15q-, +20q, 21-, +3, t(6;9), t(X;17) |
| 10 | 59M | HU | MDS to AML-MRC | 3 | 68 | HA | 32 | D-OD | 5q-, +2q, +5q, -7, -13, -15, -17q |
| 11 | 66F | HU | AML | NA | 82 | Chemo | 5 | D-OD | NA |
| 12 | 26M | HU | AML | 3 | 247 | HA->SCT | 5 | NED-SCT | +3q, -12, -15q |
| 13 | 36M | HU | AML | 2 | 228 | Chemo->SCT | 15 | NED-SCT | +6p, -13, -14, -16, -18, -20, +21q |
| 14 | 65M | HU | AML | 3 | 87 | HA | 9 | D-OD | 11q- |
| 15 | 55M | HU | AML | 3 | 119 | SCT | 10 | D-OD-SCT | 5q- |
| 16 | 55M | HU | AML | 3 | 51 | Chemo | 0.5 | D-OD | -7 |
| 17 | 58M | HU + AG | AML-MRC | 3 | 97 | Supportive care | 4 | D-OD | 3p-, -4, -9, +12p, -17p, -18, -20 |
| ID . | age/sex . | main therapy for ET . | type of transformation . | degree of fibrosis (0-3+) . | time to MDS or AML(mo) . | treatment after transformation . | survival from event (mo) . | clinical status . | cytogenetics at MDS or AML . |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 56F | AG | MDS | 3 | 121 | Thalidomide | 3 | D-OD | 5q-, +9q, +13q, +6p, t(2;15), t(17;17), t(3;18) |
| 2 | 60F | AG | MDS | 3 | 96 | HA | 28 | A-WD | 20-,+22q, 19p-, |
| 3 | 64M | AG | MDS | 3 | 60 | HA | 83 | D-OD | 20q- |
| 4 | 34M | AG | MDS to AML-MRC | 2 | 322 | SCT | 3 | D-SCT | 20q- |
| 5 | 61F | AG | AML-MRC | 3 | 76 | Chemo | 15 | A-WD | t(3;9), +8, t(1;5) |
| 6 | 60F | AG | AML-MRC | 3 | 92 | HA | 12 | A-WD | 46,XX |
| 7 | 66F | AG | AML (M7) | 3 | 61 | Supportive care | 0 | D-OD | NA |
| 8 | 56M | AG | AML | 3 | 27 | Unknown | 21 | D-OD | NA |
| 9 | 68F | HU | MDS | 2 | 48 | HA | 10 | A-WD | 5q-, 13-, 15q-, +20q, 21-, +3, t(6;9), t(X;17) |
| 10 | 59M | HU | MDS to AML-MRC | 3 | 68 | HA | 32 | D-OD | 5q-, +2q, +5q, -7, -13, -15, -17q |
| 11 | 66F | HU | AML | NA | 82 | Chemo | 5 | D-OD | NA |
| 12 | 26M | HU | AML | 3 | 247 | HA->SCT | 5 | NED-SCT | +3q, -12, -15q |
| 13 | 36M | HU | AML | 2 | 228 | Chemo->SCT | 15 | NED-SCT | +6p, -13, -14, -16, -18, -20, +21q |
| 14 | 65M | HU | AML | 3 | 87 | HA | 9 | D-OD | 11q- |
| 15 | 55M | HU | AML | 3 | 119 | SCT | 10 | D-OD-SCT | 5q- |
| 16 | 55M | HU | AML | 3 | 51 | Chemo | 0.5 | D-OD | -7 |
| 17 | 58M | HU + AG | AML-MRC | 3 | 97 | Supportive care | 4 | D-OD | 3p-, -4, -9, +12p, -17p, -18, -20 |
D-OD: died of disease; A-WD: alive with disease; D-SCT: died of stem cell transplant (SCT) complication; NED-SCT: no evidence of disease after SCT; HA: hypomethylating agents; Chemo: intensive chemotherapy.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal