Abstract
Abstract 417
Trials enrolling patients with limited stage Hodgkin lymphoma conducted by European cooperative groups including EORTC, GELA and the GHSG frequently include patients with stage II B or stage II bulky (≥ 10 cm) disease in either “favorable” or “unfavorable” subgroups. Similar patients are usually excluded from North American trials of limited stage disease and rather are included in trials of advanced stage lymphoma. We sought to clarify the appropriateness of these approaches.
All adult (age > 15 but < 66 y) HIV antibody negative patients with stage II A bulky, II B non-bulky or II B bulky Hodgkin lymphoma treated in British Columbia since 1981, when ABVD-type chemotherapy became standard, were identified using the BC Cancer Agency Lymphoid Cancer Database. Characteristics: n=416; male 54%; age range 16–64 y (median = 30); histologic subtype nodular sclerosis 86%, mixed cellularity 5%, not subclassifiable 6%, other 3%; subdiaphragmatic 3%; stage II A bulky 32%, II B non-bulky 32%, II B bulky 36%; localized extranodal extension (E lesion) 35%; largest mass diameter non-bulky range 1–9 cm (median 6 cm), bulky range 10–25 cm (median 12 cm); IPFP prognostic score 0, 9%; 1, 33%; 2, 32%; 3, 19%; 4, 6%; 5, 1% (data missing 36%).
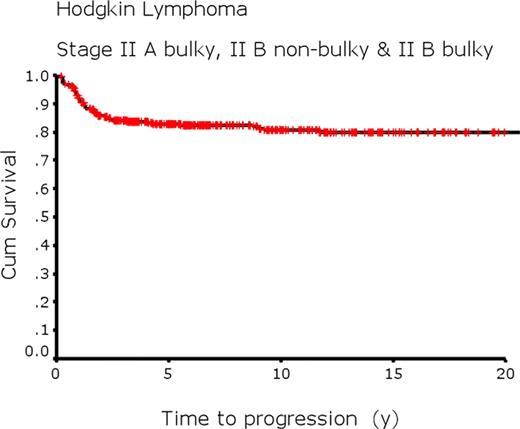
Planned treatment consisted of 6–8 cycles of ABVD-type chemotherapy followed by radiation for a persistent residual mass (only if PET positive since 2005). 50% of patients received radiation (extended field 24%, involved field 76%). Thus, patients were treated with the same approach that is used for stage III or IV. Patients with bulky disease were much more likely to receive radiation: II A bulky 72%; II B non-bulky 11%; II B bulky 65%. For all 416 patients, with a median follow-up of 8 y, 5 and 10 y progression free survivals (PFS) were 81% and 76%; time-to-progression (TTP) estimates 83% and 81% (Fig. 1); and overall survivals (OS) were 94% and 90%. Although there is some variation, survivals were very similar across the three stage groups.
| Stage group . | n . | PFS% . | TTP% . | OS% . | |||
|---|---|---|---|---|---|---|---|
| 5-y . | 10-y . | 5-y . | 10-y . | 5-y . | 10-y . | ||
| II A bulky | 135 | 85 | 85 | 85 | 85 | 94 | 93 |
| II B non-bulky | 134 | 80 | 70 | 80 | 77 | 95 | 88 |
| II B bulky | 147 | 78 | 72 | 82 | 79 | 94 | 90 |
| All patients | 416 | 81 | 76 | 83 | 81 | 94 | 90 |
| Stage group . | n . | PFS% . | TTP% . | OS% . | |||
|---|---|---|---|---|---|---|---|
| 5-y . | 10-y . | 5-y . | 10-y . | 5-y . | 10-y . | ||
| II A bulky | 135 | 85 | 85 | 85 | 85 | 94 | 93 |
| II B non-bulky | 134 | 80 | 70 | 80 | 77 | 95 | 88 |
| II B bulky | 147 | 78 | 72 | 82 | 79 | 94 | 90 |
| All patients | 416 | 81 | 76 | 83 | 81 | 94 | 90 |
This experience is instructive and indicates that when patients with stage II B bulky or non-bulky or stage II A bulky Hodgkin lymphoma are treated with the same approach as is used for advanced stage disease at least 20% of patients relapse.
It is inappropriate to include patients with stage II B or stage II A bulky Hodgkin lymphoma on trials designed for limited stage disease. With an estimated relapse rate of at least 20% inclusion of these patients can grossly distort outcomes and compromise interpretation of the results of the trial since the expectation for the rest of the patients (stage I A and II A, low bulk) is that no more than 5% of patients will relapse. Patients with stage II B or stage II A bulky Hodgkin lymphoma should be included in trials for patients with advanced stage disease.
Klasa:Seattle Genetics, Inc.: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal